Cost-effectiveness of risk-tailored screening strategy for colorectal cancer: A systematic review
Declaration of conflict of interest: The authors declare no competing interests. Data are available from the corresponding author on request.
Financial support: This work was supported by Health Science and Technology Project of Zhejiang Province (2021KY586) and National Natural Science Foundation of China (12071032).
Abstract
Background and Aim
Though one-size-fits-all age-based screening for colorectal cancer (CRC) is effective in reducing the incidence and mortality, the evidence regarding on personized screening based on individual risk factors has been growing. The study aimed to perform a systematic review to synthesize economic evidence of risk-tailored CRC screening strategies.
Methods
This systematic review was conducted in EMBASE, Web of Science, PubMed, Cochrane Library, Econlit, and National Institute for Health Research Economic Evaluation Database from inception to June 30, 2021. We calculated the incremental cost-effectiveness ratio (ICER) of cost per life year or quality-adjusted life year gained for the risk-tailored screening compared with no screening or uniform screening. A strategy was cost-effective with less cost and equal or more effectiveness than the comparator along with lower ICER than the willingness-to-pay threshold.
Results
Our review finally comprised seven studies. Five studies reported the results of comparisons of risk-tailored CRC screening with no screening, and supported that risk-tailored screening was cost-effective. All of seven studies reported the ICERs of risk-tailored screening and age-based screening. Disparities in the discrimination of risk-prediction tool, accuracy of adopted techniques, uptake rate of screening and cost estimation impacted the cost-effectiveness.
Conclusions
Studies on the economic evaluation of risk-tailored CRC screening are limited, and current evidence is not sufficient to support the replacement of risk-tailored screening for traditional age-based screening.
Introduction
Colorectal cancer (CRC) is one of the most common diagnosed cancer in the world, with 1 880 725 new cases and 915 880 death cases occurring in 2020.1 For many countries in Asia, Eastern Europe, and South America, the CRC burden have been increasing in the past decades.2 Because of relatively definite natural history from adenoma to cancer and its long preclinical phase for early detection, screening has been demonstrated effective in reducing the incidence and mortality of CRC.3
Quite a few countries have implemented population-based or opportunistic CRC screening programs for several decades, and main screening techniques included colonoscopy, sigmoidoscopy, fecal immunochemical test (FIT) and guaiac-based fecal occult blood test (FOBT).4 Basically, all CRC screening programs determine the target population solely based on the age (mostly 50–74 years). However, except for age, several risk factors have continuously been identified and quantified, including epidemiological factors (family history of CRC, diet, lifestyle, previous history of disease), blood and stool biomarkers, genetic variations like single nucleotide polymorphisms (SNPs).5, 6 Plentiful studies have published more than 50 risk prediction models for CRC,7-9 by which the average-risk population could be divided into different risk groups like high-risk, medium-risk, and low-risk.7-9 Risk-tailored screening, which was defined as proposing different screening strategies in terms of age to initiate and terminate screening, screening techniques and screening intervals for different risk groups, has the potential in improving the screening yield and decreasing the waste of healthcare resources.10-12
Currently, there is limited evidence in the real-world setting on the effectiveness and efficacy of risk-tailored CRC screening strategies. One study reported the protocol of a risk-tailored CRC screening for population aged from 20 to 50 years in Germany,13 and one study from China aimed to compare the effectiveness of a risk-tailored screening scenario by using an established CRC risk scoring system with colonoscopy and FIT screening for population aged 50 to 74,14 and its baseline results showed that the risk-tailored screening scenario detected more advanced neoplasm of CRC than that of the FIT scenario.15 Furthermore, researchers demonstrated that the predictive value for advanced neoplasm was significantly improved after updating the risk prediction model by incorporating more lifestyle factors and polygenic SNPs, which can be potentially used for developing personalized CRC screening strategies.16
Despite of limited evidence regarding on epidemiological effectiveness of risk-tailored CRC screening strategies, a few studies have reported the potential cost-effectiveness of risk-tailored screening by using simulation models compared with no screening or age-based screening. However, current evidence is inconsistent, and to our knowledge there is no systematic review. Thus, this study aims to summarize the current evidence on the cost-effectiveness of risk-tailored CRC screening compared with no screening or traditional one-size-fits-all age-based screening.
Methods
This systematic review was performed followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.17 The database search, data extraction and synthesis, and quality assessment were performed by two authors (L. W. and C. L.) independently. Disagreements were resolved by consensus or by consulting one or other review authors (Y. W. and L. D.).
Search strategy
This systematic review was conducted in four general databases, containing Embase (Table S1), Web of Science (Table S2), PubMed (Table S3), and The Cochran Library (Table S4), from inception to June 30, 2021, by combination of Mesh terms and keywords related to “colorectal neoplasms,” “screening,” “risk-tailored or individualized, or personalized,” and “economic evaluations.” Furthermore, literature search was further conducted in database specific to health economy, including the Econlit (Table S5) and Center for Reviews and Dissemination (Table S6). Additionally, a forward and backward snowballing search method was used.
Inclusion and exclusion criteria
The primary objective of this study was to summarize current evidence regarding risk-based or risk-tailored screening compared with no screening or uniform age-based screening in average-risk population. Eligible studies were screened by title, abstract and full-text according to the predefined inclusion criteria, including (i) study design: randomized controlled trail, cross-sectional study, prospective cohort study, or model-based study; (ii) study population: participants who are eligible for CRC screening; (iii) intervention: at least one screening technique for CRC, including risk-assessment, FIT, FOBT, sigmoidoscopy, colonoscopy; (iv) control: participants screened without risk-tailored strategies and no screening strategy; (v) outcomes: at least one indicator produced from cost-effectiveness analysis, cost-utility analysis, and cost–benefit analysis, containing cost to detect one CRC case, cost to avoid one CRC death, cost per life-year (LY) saved, cost per quality adjusted life year (QALY) gained or incremental cost-effectiveness ratio (ICER). Studies would be excluded if they met one of the following criteria: (i) duplicated studies; (ii) review, comments, or news; (iii) without language in English; (iv) without colorectal cancer screening; (v) without risk-tailored screening strategies compared with no screening nor age-based screening; (vi) incomplete data on economic evaluation.
Quality appraisal
The quality of all included studies was assessed by Drummond et al.'s checklist.18 Thirty-five items of the check list were scored using “Yes,” “Partially,” “No,” and “NA” if the criteria were fully met, partially met, not met, and not applicable, respectively. We assigned a score between 0 and 1 for each of the items (0 = no; 0.5 = partially and 1 = yes, without score for not applicable). Overall quality index for each study was equal to the proportions of the true score to the number of all applicable items. The corresponding quality of study was considered as one of the criteria for inclusion, with the threshold of 60% for inclusion.
Data extraction
A predefined form was specially designed, including the following information. (i) Basic information: first author, year of publication, country, over design, targeted population, evaluated screening strategies; (ii) methodology for economic evaluation: method, perspective, time horizon, cost and covering items, discount; (iii) screening strategies: evaluated risk-tailored strategy (definition for risk factors and validation of risk-tailored) and comparator; (iv) model details: type, structure, inputting parameters, and calibration or validation would be included; (v) evaluation results: cost to detect one CRC, cost to avoid one CRC death, cost per LY saved, cost per QALY gained, and the corresponding ICERs.
Data analysis
Cost values reported in the individual studies were converted to 2020 United States (US) dollars using purchasing power parity estimates from World Bank19 and US consumer price index.20 We calculated the ICERs in terms of cost per QALY or LY gained, by comparing each risk-tailored screening scenario with no screening and age-based screening for each study. A risk-tailored screening scenario is determined as cost-effective if its ICER is lower than the value of willingness-to-pay (WTP) reported by each study, which is also called a dominant scenario and vice versa. If the risk-tailored screening scenario gained less QALY or LY and costed more than the comparator, then the screening scenario is a dominated scenario. In addition, to better make comparisons from various studies with different WTPs, we summarized all ICREs in one figure by using the same WTPs at $25 000, $50 000, $75 000, and $100 000, respectively.
Results
Basic characteristics
The initial electronic search retrieved 2196 records. After removal of duplicates, two rounds of screening, and a snowball search, seven studies were included for analysis and quality appraisal (Fig. 1).
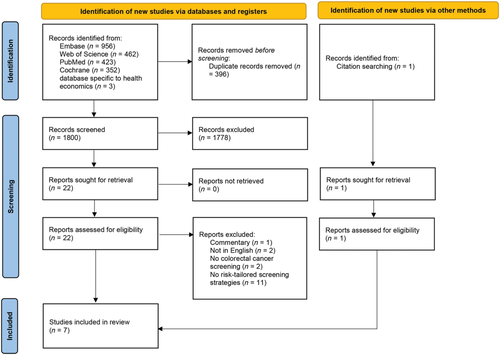
The qualities of included studies were good, with the 87% (median) for quality index of included studies (Table S7).
Four studies were from the USA,21-24 one study from the United Kingdom,25 Japan,26 and Australia,27 respectively (Table 1). Most studies used individual-level microsimulation models,21, 23-25, 27 while two studies used the cohort-level Markov models (Table S8).22, 26 Only two studies adopted the societal perspective,21, 24 which comprehensively reflects the costs of all stakeholders, while most studies adopted the payer's perspective.22, 23, 25-27 From the health outcomes, QALY was mostly used as the main outcomes, while two studies used only LY as the outcome.23, 24 Colonoscopy and FIT were two predominant screening techniques evaluated in all studies.
| Study | Country | Model type | Perspective | Time horizon | Discount rate | Outcome | Screening age | Screening techniques | No screening included | Quality appraisal |
|---|---|---|---|---|---|---|---|---|---|---|
| Thomas et al., 2021 | UK | Microsimulation | NHS | Lifetime | 3.5% | QALY | 40–74 | FIT | No | 76% |
| Sekiguchi et al., 2020 | Japan | Markov | Payer | Lifetime | 3% | QALY | 40–85 | Colonoscopy; FIT | Yes | 90% |
| Naber et al., 2019 | USA | Microsimulation | Societal | Lifetime | 3% | QALY and LY | 40–85 | Colonoscopy | Yes | 88% |
| Ladabaum et al., 2020 | USA | Markov | Payer | Lifetime | 3% | QALY | 50–80 | Colonoscopy; FIT | Yes | 87% |
| Cenin et al., 2020 | AUS | Microsimulation | Health system | Lifetime | 5% | QALY and LY | 40–74 | Colonoscopy; FIT | Yes | 87% |
| Subramanian et al., 2017 | USA | Microsimulation | Payer | Lifetime | 3% | LY | 20–74 | Colonoscopy; FIT | Yes | 64% |
| Ramsey et al., 2010 | USA | Microsimulation | Societal | Lifetime | 3% | LY | 40–80 | Colonoscopy | No | 88% |
- FIT, fecal immunochemical test; LY, life year; NHS, National Health Service; QALT, quality-adjusted life year; UK, United Kingdom; US, United States of America.
Risk stratification factors and screening strategies
Only one study stratified the CRC risk solely based on the first degree relative (FDR) family history of CRC,24 and six studies used the risk prediction tools,21-23, 25-27 which included both phenotypic, polygenic risk factors and potential biomarkers. The risk group categories varied among the studies: two risk groups,24 three risk groups,22, 26 five risk groups,23, 27 60 relative risk groups,21 and one study did not report the risk groups.25 (Table 2).
| Study | Risk factors | Risk stratification | RR | AUC | Risk-stratified screening strategies |
|---|---|---|---|---|---|
| Thomas et al., 2021 | Ma: BMI, smoking, alcohol, physical activity | Risk prediction tool | Not stated | 10-year: 0.559 |
Age at first FIT invite was calculated according to risk 1) biennial FIT (120 mg/g), mean screening start age of 60 2) biennial FIT (120 mg/g), mean screening start age of 50 3) biennial FIT (20 mg/g), mean screening start age of 60 |
| Jeon: 57 SNPs | 10-year: 0.577 | ||||
| MaJeon: Ma plus Jeon | 10-year: 0.660 | ||||
| Huyghe: 120 SNPs | 10-year: 0.678 | ||||
| Total risk: Ma plus Huyghe | 10-year: 0.720 | ||||
| Total risk plus sex | 10-year: 0.721 | ||||
| Sekiguchi et al., 2020 | Sex, age, CRC family history, BMI, smoking history |
8-point risk score: Low risk: ≥ 0 to < 3 Medium-risk: ≥ 3 to < 5 High-risk: ≥ 5 |
Not stated | Not stated |
High risk: 10-yearly colonoscopy Low-medium risk: annual FIT |
| Naber et al., 2020 | Polygenic factors | 60 relative risk groups |
0.0–0.1 0.1–0.2 5.8–5.9 > 5.9 |
0.60, 0.65, 0.70, 0.75, 0.80 | Colonoscopy, age for screening, and screening interval depend on the RR of polygenic risk |
| Ladabaum et al., 2019 | Risk prediction tool |
Three risk groups CRC risk at 50 Low risk: ≤ 3 Moderate risk: > 3 to < 12 High risk: ≥ 12 |
2.5%: 2.5 |
1) Tailored colonoscopy: High risk: 5-yearly colonoscopy Moderate risk: 10-yearly colonoscopy Low risk: once colonoscopy 2) Tailored FIT/colonoscopy: High risk: 5-yearly colonoscopy Low or moderate risk: annual FIT |
|
| Cenin, et al., 2020 | 45 SNPs and FDR family history of CRC | Five risk groups |
Very low: < 0.5 Low: 0.5 to 0.9 Average: 0.9 to 1.2 High: 1.2 to 1.8 Very high: > 1.8 |
39 screening scenarios | |
| Subramanian et al., 2017 | Demographics, family history, and risk factors such as diet, smoking history, and aspirin use | Five risk groups |
High: > 50% Increased: 10–12% Medium: ~8% Decreased: ~3% Low: ~2% |
High risk: Biennial colonoscopy staring at age 20 Increased risk: 5-yearly colonoscopy staring at age 40 Medium risk: 10-yearly colonoscopy or annual FIT staring at age 50 Decreased risk: Colonoscopy at age 50 only, or biennial FIT staring at age 50 Low risk: Colonoscopy at age 50 only |
|
| Demographics, family history, and risk factors such as diet, smoking history, and aspirin use plus biomarkers | |||||
| Ramsey et al., 2010 | FDR family history of CRC |
Two risk groups: High: 1 FDR diagnosed with CRC < 60, or two FDRs diagnosed at any age |
3.8 (1.9–5.7) |
1) High-risk: 10-yearly colonoscopy staring at age 40Low-risk: 10-yearly colonoscopy staring at age 50 2) High-risk: 5-yearly colonoscopy staring at age 50Low-risk: 10-yearly colonoscopy staring at age 50 3) High-risk: 5-yearly colonoscopy staring at age 40Low-risk: 10-yearly colonoscopy staring at age 50 |
The risk-tailored screening scenarios differed greatly from age-based screening in several aspects, including the age for screening, screening techniques, and screening interval (Table 2), accuracy of screening techniques (Table S10) and costs (Table S11).
Cost-effectiveness of risk-tailored colorectal cancer screening compared with no screening
Five studies reported the results of comparisons of risk-tailored screening with no screening.21, 22, 24, 26, 27 Two studies reported that risk-tailored screening yielded more QALY or LY and cost less than no screening,22, 26 which were dominant, and two studies found that risk-tailored screening yielded more QALY or LY but cost more, but the ICERs were below the corresponding WTPs,24, 27 which were cost-effective. One studies which listed 39 risk-tailored screening scenarios demonstrated that 29 scenarios were cost-effective whereas 10 scenarios were not.21 Full results were presented in Table 3 and Figure 2.
| Study | Country | Currency | WTP | Compared with no screening | Compared with uniform screening | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Δcost per person | ΔLY per person | ΔQALY per person | Cost-saving | Δcost per person | ΔLY per person | ΔQALY per person | Cost-saving | ||||
| Thomas et al., 2021 | UK | Pound, 2018 | 20 000 or 30 000 |
1) −1.41 2) 0.78 3) −1.96 |
1) 0.0001 2) 0.0002 3) 0.0001 |
1) Yes 2) Yes 3) Yes |
|||||
|
1) −1.35 2) −0.34 3) −2.07 |
1) 0.0002 2) 0.0001 3) 0.0003 |
1) Yes 2) Yes 3) Yes |
|||||||||
|
1) −1.24 2) 3.46 3) −2.08 |
1) 0.0007 2) 0.0010 3) 0.0013 |
1) Yes 2) Yes 3) Yes |
|||||||||
|
1) 0.44 2) 2.35 3) −3.15 |
1) 0.0026 2) 0.0018 3) 0.0038 |
1) Yes 2) Yes 3) Yes |
|||||||||
|
1) 1.47 2) 3.15 3) 3.29 |
1) 0.0038 2) 0.0023 3) 0.0055 |
1) Yes 2) Yes 3) Yes |
|||||||||
|
1) 2.47 2) 3.82 3) −2.13 |
1) 0.0042 2) 0.0025 3) 0.0058 |
1) Yes 2) Yes 3) Yes |
|||||||||
| Sekiguchi et al., 2020 | Japan | USD, 2019 | 45 947 | −454 | 0.148 | Yes |
1) −24 2) 2 |
1) −0.04 2) 0.002 |
1) No 2) Yes |
||
| Naber et al., 2020 | USA | USD, 2014 | 69 000 | 1685 | 0.073 | Yes | 58 | −0.001 | 0 | No | |
| Ladabaum et al., 2020 | USA | USD, 2013 | 50 000 |
1) −107 2) −596 |
1) 0.0766 2) 0.0802 |
1) Yes 2) Yes |
1) −229 2) 61 |
1) −0.0015 2) 0.0012 |
1) D 2) No |
||
| Cenin, et al., 2020 | AUS | AUD, 2016 | 50 000 | 233.579 to 5330.249 | 0.002 to 0.051 | 0.003 to 0.058 |
29 Yes 10 No |
−166.594 to 4930.076 | −0.031 to 0.018 | −0.037 to 0.018 |
19 D 20 No |
| Subramanian et al., 2017 | USA | USD, Not stated | 15 9000 |
1) 5.39 2) 5.06 3) 4.02 |
1) 0.00294 2) 0.00211 3) −0.00068 |
1) Yes 2) Yes 3) D |
|||||
|
1) 15.43 2) 12.09 3) 30.61 |
1) 0.00227 2) 0.00242 3) 0.00154 |
1) Yes 2) Yes 3) Yes |
|||||||||
| Ramsey et al., 2010 | USA | USD, 2005 | 50 000 |
1) 1226.6 2) 1226.2 3) 1251.31 |
1) 0.06546 2) 0.06616 3) 0.06669 |
1) Yes 2) Yes 3) Yes |
1) 27.6 2) 37.6 3) 63.11 |
1) 0.0003 2) 0.0007 3) 0.00123 |
1) Yes 2) Yes 3) Yes |
||
- D, dominated, cost more and gain less; FIT, fecal immunochemical test; LY, life years; NHS, National Health Service; QALT, quality-adjusted life year; UK, United Kingdom; US, United States of America.
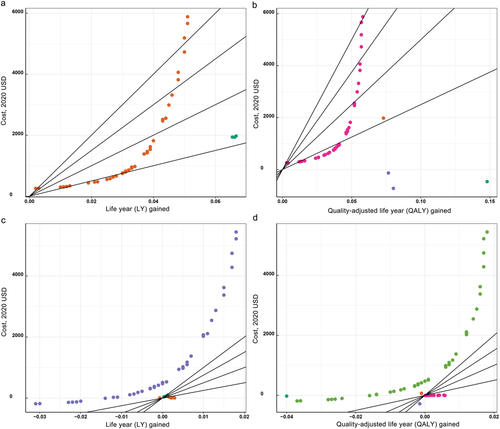
 , Sekiguchi et al., 2020;
, Sekiguchi et al., 2020;  , Naber et al., 2019;
, Naber et al., 2019;  , Ladabaum et al., 2020;
, Ladabaum et al., 2020;  , Ramsey et al., 2010;
, Ramsey et al., 2010;  , Subramanian et al., 2017;
, Subramanian et al., 2017;  , Tomas et al., 2021;
, Tomas et al., 2021;  , Cenin et al., 2020. [Color figure can be viewed at wileyonlinelibrary.com]
, Cenin et al., 2020. [Color figure can be viewed at wileyonlinelibrary.com]Cost-effectiveness of risk-tailored screening compared with age-based screening
All of seven studies compared the cost and health outcomes of risk-tailored screening with age-based screening (Table 3 and Fig. 2). Four studies presented that at least one risk-tailored screening scenario was more cost-effective compared with age-based screening,23-26 whereas the rest three studies totally denied.21, 22, 27
Epidemiological effectiveness of risk-tailored colorectal cancer screening
Five studies reported the reduction of incidence or mortality of CRC for risk-tailored screening compared with no screening (Table S9).21, 22, 24, 26, 27 The reduction for incident CRC cases ranged from 3 to 4165 per 100 000 simulated population, and the percent of reduction ranged from 3.6% to 67.9%. The reduction for CRC death cases ranged from 2 to 2000 per 100 000 simulated population, and the percent of reduction ranged from 6.9% to 79.3%.
Similarly, all of five studies with available data demonstrated the reduction of CRC incidence for at least one risk-tailored screening scenario compared with age-based screening,21, 22, 25-27 ranging from 2 per 1 000 000 to 347 per 100 000, and four in five studies demonstrated the reduction of CRC mortality for at least one risk-tailored screening scenario compared with age-based screening, ranging from 4 per 1 000 000 to 193 per 100 000.24-27 However, the extent of epidemiological benefit was accompanied with higher workload of colonoscopy examinations.
Discussions
This paper summarized the current status and results of economic evaluation of risk-tailored screening for CRC. Results from seven studies, though relatively limited, suggested that risk-tailored screening for CRC is cost-effective compared with no screening, whereas the conclusion of cost-effectiveness for risk-tailored CRC screening compared with age-based screening is still hard to be made due to few discrepancies across included studies, including the risk-prediction tools used, cost estimation, uptakes and screening strategies.
Epidemiological effectiveness is the premise of discussing the economic issue of screening program. Current evidence regarding the risk-tailored CRC screening in the real-world setting is sparse.12, 28 One study from China reported a randomized controlled trial to compare effectiveness and cost-effectiveness of the risk-tailored CRC screening by using a validated modified Asia-Pacific Colorectal Screening Score,29 with traditional one-time colonoscopy and annual FIT screening.14 Baseline results showed that risk-tailored screening had higher diagnostic yield of advanced neoplasm than that of FIT at a similar resource load of colonoscopy.15 Another risk-tailored screening program for CRC is ongoing, and the preliminary results are expected to be available in the near future.13 In addition, analytic models like seven studies in our study demonstrate the benefits of reducing the incidence and mortality of risk-tailored screening, though the magnitude of reduction increases at the cost of higher colonoscopy workload. In general, the risk-tailored screening is epidemiologically effective and is potentially cost-effective.
By using the risk-tailored tools of CRC, the general population can be divided into several risk groups in terms of low, medium and high risk, and suitable screening strategies are supposed to be proposed to each risk group. Herein, the accuracy and discrimination of risk-prediction tool is the principal determinant for the cost-effectiveness of risk-tailored colorectal cancer screening. Most prediction models based on demographics, family history of CRC, and well-established risk factors yielded low area under the curve (AUC) and substantial misclassification. The cost-effectiveness was attenuated due to increased cost from the overestimation of risk and reduced effectiveness due to underestimation of risk. Nowadays, to improve the accuracy, SNPs and potential biomarkers have been added into risk-prediction models, with AUC increasing to 0.78.9 Superior performance of risk prediction tool was promising for the cost-effectiveness of risk-tailored screening strategies. The study by Thomas et al found that when AUC was 0.65 onward, the risk-tailored screening was cost-effective.25 Admittedly, those prediction tools with more lab examination related to gene and biomarkers always accompanied with high cost and uncertain uptake.23, 25, 27
Cost plays an important role in the cost effectiveness for risk-tailored screening strategy. Cost of risk prediction tools varies substantially depending on included risk factors.21, 23, 24, 27 If risk factors were routinely collected in primary care or the screening programs, the cost would be reduced largely. But indirect costs in time for screening participants and field workers for the risk prediction were unavoidable. Furthermore, if additional factors like genetic testing are required, the cost for risk-prediction would increase to some extent. The relative cost-effectiveness of different risk prediction tools is likely to be different, contingent on their direct cost and administration cost as well as the gradient of benefits they produce. Thomas et al found that under high discrimination and risk stratification, cost per person for risk-prediction could be as high as £114 whilst still being cost-effective.25 Additionally, implementation of risk prediction in screening population might have potential impact on the risk exposure. If there were changes in relevant behavior, then the benefit would increase correspondingly,30 which might offset the cost. All the aforementioned factors should be taken into consideration when conducting comprehensive analysis on the cost-effectiveness of risk-tailored screening strategy.
Uptake rate, including for the risk prediction tool and subsequent colonoscopy or FIT for those who are at higher risk for CRC, is essential for the effectiveness and benefit of screening program. Risk prediction tool with simple and limited risk factors, like APCS, was well accepted by screening participants.15 But the corresponding AUC was relatively low and the potential benefit of risk stratified screening decreased. As we mentioned, when incorporated with more biomarkers or SNPs, though with high AUC, risk prediction is hard to be implemented at population level, which might lead to low uptake rate. Furthermore, complexity of risk prediction also impacts the uptake for subsequent colonoscopy. Prior study also showed that the increased complexity or low acceptability of a risk-tailored screening program resulted in a reduced colonoscopy uptake, then benefits of risk stratification can easily be offset.22
There is no denying that screening produces potential impact on disutility of screening participants, particularly in risk stratification. For those people who are higher risk for CRC, though there is psychological burden, early detection and diagnosis could bring in life gains. But the majority would be assessed as the low risk for CRC. The process for the risk prediction, especially for the invasive examination like gene test, must lead to burdensome. However, few studies took the negative impact of colonoscopy or colonoscopy complication into consideration.21, 27 None of the prior studies assumed the disutility caused by knowing the polygenic risk score or risk prediction model. To some content, lacking in disutility might lead to overestimation in the cost-effectiveness of risk-tailored screening strategy. In further research, quality of life and utility score should be adopted in the evaluation for risk-tailored screening.21-23, 25-27
Incremental cost-effectiveness ratio is the key parameter to assess the cost-effectiveness for evaluated risk-tailored screening. However, due to variations in study design and cost scope, it is hard to quantitatively synthesize all ICERs. WTP is the maximum price that the payer to pay for the unit gain in health outcome, which differs across countries.21-25, 27 When ICER is below WTP, the evaluated screening can be regarded as cost-effective. Thus, we present the differences in cost and gains in health outcome of evaluated risk-tailored screening when compared with no screening or age-based screening. Apparently, for countries with higher WTP, such as $10 000, there are a broad range of selections for cost-effective risk-tailored screening strategies when compared with no screening. However, along with the WTP decreasing, the choices in selecting cost-effective risk-tailored screening strategies become limited.
Limitations
Admittedly, limitations existed in this analysis. Firstly, though we identified the literatures based on the mostly common-used databases, databases specific to health economics, and manual search, there is possibility in the omission due to language. Secondly, the analysis was based on information for seven studies, though we searched the literatures from inception to June 30, 2021. Few available data might result in bias and only provide us with the preliminary conclusion at early stage. Most studies with well-design and elaborated parameters were from US, with model structure, screening technologies, relevant cost and utilities specific to US population. Extrapolation and generalization are limited and results interpretation should be cautious.
Conclusions
In summary, risk-tailored screening is promising for personalized cancer control and decreasing resource load. However, studies on the economic evaluation of risk-tailored CRC screening are limited, and current evidence is not sufficient to support the replacement of risk-tailored screening for traditional age-based screening. In such a risk-tailored strategy, we need to be informed that the risk-stratification tool is highly accurate and relatively less expensive with well acceptance in the screening population.




