Behavioural responses of fishes to anthropogenic disturbances: Adaptive value and ecological consequences
Abstract
Aquatic ecosystems are changing at an accelerating rate because of human activities. The changes alter the abundance and distribution of fishes, with potential consequences for ecosystem structure and function. Behavioural responses often underlie these changes in population dynamics, such as altered habitat choice or foraging activity. Here, we present a framework for understanding how and why behaviour is affected by human activities and how the behavioural responses in turn influence higher ecological levels. We further review the literature to assess the present state of the field and identify gaps in our knowledge. We begin with discussing the factors that determine how an individual responds to a change in the environment and whether the response is adaptive or not. In particular, we explain the importance of the evolutionary history of the species. We then search the literature to assess our current knowledge of the impact of human disturbances on the behaviour of fishes and the consequences for ecosystems. The search reveals that much attention has been directed to the impact of human activities on the behaviour of fishes, but that worryingly little is known about the consequences of these responses for populations, communities and ecosystems. Yet, behavioural responses can have profound ecological consequences given that behaviour underly many, if not most, species interactions. Thus, more attention should be paid to the mechanisms and pathways through which behavioural responses influence higher ecological levels. Such information is needed if we are to determine the ultimate effects of human activities on biodiversity and the function and stability of aquatic ecosystems.
1 INTRODUCTION
Humans are altering the abundance and distribution of fishes through a range of activities, from physically changing their habitat to causing climate change and rising water temperature. Underlying these alterations in population dynamics are often changes in the behaviour of individuals, such as habitat choice or foraging activity. These behavioural responses can influence the growth, survival and reproductive success of individuals, and hence the abundance and distribution of populations. Changes in population dynamics can in turn influence other species through species interactions, such as predator–prey and competitive interactions, and alter the composition of communities and associated ecological processes (Figure 1). Such effects of behavioural responses on ecosystem structure and function can be profound, given that species are linked to each other and to the abiotic environment through a multitude of interactions, forming an interaction network. Changes in any of these links can influence biodiversity and the functioning of the ecosystem (Hoover & Tylianakis, 2012). Thus, changes in the behaviour of fishes—because of human activities—can have far-reaching ecological consequences (Candolin & Wong, 2012; Wilson et al., 2020).
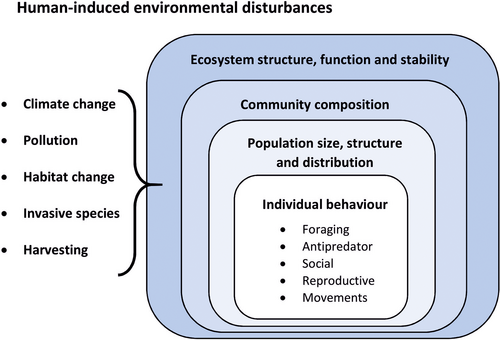
Evaluating the impact that human-induced environmental changes have on the behaviour of fishes and the consequences for populations, communities and ecosystems requires an understanding of the underlying mechanisms, pathways and influencing factors (both facilitating and constraining factors). Here, our aim is to present a conceptual framework that illustrates how human-induced environmental changes can influence the behaviour of fishes and thereby higher ecological levels, as well as to assess the current state of the field and reveal knowledge gaps. We begin with explaining how different factors can influence the behaviours of fishes and their adaptive value. We then review the current literature and discuss both the detected and expected effects of human activities on behaviour and impact on higher ecological levels. The review is not exhaustive but rather highlights the main pathways behind the effects and influencing factors, which are illustrated with one or a few examples. Finally, we point out gaps in our knowledge and the research needed to further advance our understanding of how human activities are influencing fishes and thereby biodiversity, and the structure and functioning of aquatic ecosystems.
2 MECHANISMS BEHIND BEHAVIOURAL RESPONSES
How an individual responds to a change in the environment depends on its traits and reaction norms. These have evolved under earlier environmental conditions, i.e., through past evolutionary processes, and can be adaptive or not (Sih, 2013; Tuomainen & Candolin, 2011; Wong & Candolin, 2015). Experiences during lifetime and learning can modify the responses, as may transgenerational effects (when the experience of parents, and possibly grandparents, influence the behaviour of offspring without altering their genetic makeup), but these modifications are also dependent on earlier evolved traits; (Bell & Hellmann, 2019). Thus, whether behavioural responses are adaptive or not depends on past environmental conditions and the degree to which these resemble the novel, altered conditions (Sih et al., 2011).
Behavioural response may evolve across generations and become better adapted to disturbed environmental conditions. However, evolution is generally a slow process and species with longer generation time, or those who lack the genetic make-up required for adaptive evolutionary changes, may not be able to keep pace with rapid environmental changes through evolutionary processes. Instead, these species have to rely on phenotypically plastic responses, such as behavioural responses (Chevin & Lande, 2010). These responses may in turn depend on other traits, such as physiological and morphological traits, and on plasticity in these. Thus, complex interactions among traits may occur, which can facilitate or constrain the adjustment of the species to the altered environmental conditions.
Behavioural adjustments can be direct responses to the environmental change or indirectly mediated by physiological responses, such as through increased stress levels or metabolic damage (Bailey et al., 2022; Buchanan & Partecke, 2012). Through the adjustments, individuals may remain in an optimal environment or take advantage of new opportunities, such as novel food sources, or avoid threatening situations. Alternatively, species may be passively affected, such as when toxic compounds alter the physiological processes that underlie behaviour.
Behavioural responses can be divided into three stages, although all of them may not occur: first an initial plastic response, which may be followed by a gradual change in behaviour through acclimatisation and possibly learning, and finally, the evolution of better adapted behaviours through genetic changes across generations (Sih, 2013) (Figure 2). In the next sections, we will discuss these different stages of responses and the factors that determine their occurrence.
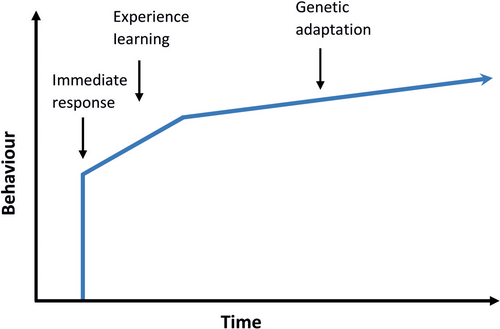
2.1 Immediate response
An immediate plastic response can be either a reaction to the environmental change, such as moving away from a habitat disturbed by humans, or a response to the reactions of other species, such as the disappearance of the preferred prey species. The responses can be mediated by physiological changes, such as increased stress levels or neurologic disorders. An example is ocean acidification, which can cause abnormal behaviour in fishes by interfering with the function of their neuroreceptors (Nilsson et al., 2012). Coral reef fish larvae, for instance, may select inappropriate settlement habitats when the pH of the water declines (Munday et al., 2012). A rise in water temperature can in turn reduce the amount of energy available to invest in behaviour by elevating metabolic rate. For instance, the coral reef trout, the grouper Plectropomus leopardus, saves energy when water temperature rises by spending more time resting motionless on the bottom (Johansen et al., 2014). Chemical pollution is another common cause of altered behaviour. Pharmaceuticals, in particular, can influence physiological states, such as anxiety, aggression and metabolism, and thereby behaviour (Salahinejad et al., 2022).
Behavioural responses to the reactions of other species are common in disturbed environments, as species are connected to each other through a complex web of species interactions, i.e., changes in one species influence other linked species (Wootton, 1994). Light pollution, for instance, causes aquatic invertebrates to aggregate in lit areas, which in turn attracts fishes that feed on them, which in turn may attract larger piscivorous fishes and hence influence their foraging behaviour and habitat choice (Becker et al., 2013).
2.2 Sensory limitations and evolutionary traps
How an individual responds to a change in the environment depends on its sensory system. This is the result of past selection and hence is tuned to past conditions. Species that are not able to perceive a change may consequently not respond, or the response may be beyond the control of the individual if mediated by physiological processes. Many chemical pollutants that influence physiology, such as antidepressants and other pharmaceuticals, are not perceived by fishes but still influence their behaviour, such as general activity and risk taking (Gould et al., 2021; Sumpter & Margiotta-Casaluci, 2022).
Environmental changes can also impair the ability of fishes to detect and evaluate cues that in the undisturbed environment conveyed important information about the environment. For instance, the ability of the larvae of the coral-reef damselfish Chromis viridis to judge habitat quality based on olfactory cues is impaired by pollution with red laterite soil, which causes the larvae to select dead corals instead of live ones as habitat (O'Connor et al., 2016). Another common cause of impaired perception is anthropogenic noise. Boat noise, for example, can mask conspecifics calls, as has been found for the meagre (Argyrosomus regius) during the breeding season (Vieira et al., 2021).
Cues that are used to evaluate environmental conditions can themselves become unreliable when the environment changes. Many fishes mistake plastic debris for food, with often fatal consequences (Azevedo-Santos et al., 2019; Markic et al., 2020; Savoca et al., 2021). Such cue-response mismatches, also termed evolutionary traps, occur when past selection has tuned the sensory system to respond to particular cues and these cues become unreliable under the altered conditions (Robertson & Chalfoun, 2016; Schlaepfer et al., 2002).
Species may also be unable to perceive new profitable opportunities, such as new food resources or habitats, because of sensory limitations. A further possibility is that altered habitats are perceived as dangerous, although in reality they are safe. This is often the case with habitats exposed to high levels of anthropogenic noise, such as oil extraction sites and areas with much waterway traffic (Dominoni et al., 2020; Shannon et al., 2016).
2.3 Learning
Many fishes have relatively well-developed cognitive abilities and can learn to adopt new behaviours, such as the selection of novel food or the avoidance of dangerous habitats (Brown et al., 2011). Thus, with time, populations may become better at coping with human-induced environmental changes. Individuals may learn new behaviours through experience, especially through trial and error, or through social interactions, including interactions with other species. For instance, group-living fishes may use publicly available social information to learn new behaviours, such as the use of novel food resources (Brown et al., 2011).
Environmental changes that influence the nervous system are especially likely to impair learning ability. For instance, ocean acidification has been found to influence sensory mechanisms essential for learning, such as vision, hearing and olfaction, in some species (Ashur et al., 2017; Chivers et al., 2021, 2014; Clements & Hunt, 2015; Ferrari et al., 2012), although not in others (Clements et al., 2022). Similarly, pollution with toxic compounds can damage the neurological processes required for learning. An example is the increase in the levels of selenium because of anthropogenic activities (such as mining, oil refining and agricultural runoff) that can impair social learning, as recently shown for the zebrafish (Danio rerio) (Attaran et al., 2020).
2.4 Transgenerational plasticity
Transgenerational plasticity occurs when parents, and possibly grandparents, influence the behaviour of offspring without involving genetic changes, i.e., nongenetic inheritance (Bonduriansky & Day, 2009). The effect can be transmitted through nutritional, somatic, cytoplasmic and epigenetic factors (DNA methylation, histones, small RNA). Thus, the environment experienced in one generation can affect the next generation (Donelson et al., 2018; Mousseau & Fox, 1998).
In fishes, transgenerational plasticity has been found to improve performance in a range of species (Donelson et al., 2018). However, the benefit of the effect depends on the ability of earlier generations to predict future conditions, or for the conditions to stay constant across generations, which may not always be the case. In the cinnamon anemone fish (Amphiprion melanopus), the exposure of parents to higher CO2 levels improves the escape performance of their offspring when these are exposed to similar CO2 levels, but the acclimation is not complete as the direction of the response (turning angle) is not restored (Allan et al., 2014).
In general, the degree to which transgenerational effects can save populations in changing environments is unclear, as it is unknown for how many generations transgenerational effects can be maintained. The exact mechanisms behind the effects are often also unknown, which is hampering the prediction of the effect on the future trajectory of fishes. Moreover, if conditions change from one generation to the other, transgenerational effects could amplify rather than moderate the negative effect of environmental change on species.
2.5 Adaptive or maladaptive responses?
How an individual responds to a change in the environment depends on the evolutionary history of the population and hence on past selection on traits, including reaction norms and sensory systems (Sih et al., 2011; Tuomainen & Candolin, 2011) (Figure 3). Responses can be adaptive and improve fitness if the environmental change only amplifies earlier encountered conditions, such as a slight rise in water temperature (Crozier & Hutchings, 2014). Thus, many species have been able to adjust their phenology to climate change, such as the timing of migration and reproductive activities, or to shift their distribution to remain in an optimal environment (Barrett & Armstrong, 2022; Woods et al., 2022). Sockeye salmon (Oncorhynchus nerka), for example, exploit thermal refuges such as tributary plumes and lake metalimnion when the temperature rises (Armstrong et al., 2016). Similarly, Atlantic cod (Gadus morhua) shift their vertical position within the water column when the temperature rises to remain at the optimal one (Claireaux et al., 1995).
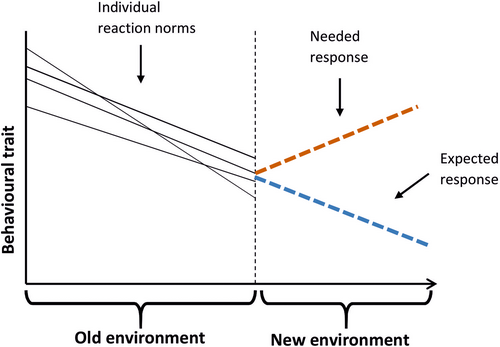
Such high plasticity and resilience to environmental change is typical for invasive species and has probably contributed to their success in invading disturbed environments (Pysek et al., 2020). An example is the round goby (Neogobius melanostomus)—one of the most impactful invasive fishes—which shows high resilience to changes in both temperature and salinity (Behrens et al., 2017; Christensen et al., 2021). Thus, the expansion of invasive species is likely to continue with the increased and sustained disturbance of environments by humans.
Behavioural responses to environmental change are not always enough to prevent fitness declines. This is particularly the case when the responses are counteracted by trade-offs with other traits. For instance, species that use thermal refuges to escape rising temperature may have to leave these to forage. The impact on fitness may then depend on the location of the thermal refuge relative to forage locations, as proposed for the brook trout (Salvelinus fontinalis) (White et al., 2019).
When conditions become extreme compared to earlier conditions, the probability increases of maladaptive responses. This is particularly the case when the extreme conditions have been rare in the past and selection for adaptive responses have been weak. For instance, a large proportion of male threespine stickleback (Gasterosteus aculeatus) respond maladaptively to reduced visibility under algal blooms; they reduce rather than increase their courtship activity, probably because the population has not been exposed to the current intensity and duration of algal blooms in its recent evolutionary past (Candolin et al., 2007; Candolin & Jensen, 2021).
Novel conditions are particularly likely to cause maladaptive responses, such as noise from boat traffic (Popper & Hawkins, 2019; Radford et al., 2014). For example, the exposure of the spiny chromis (Acanthochromis polyacanthus), a brooding coral reef fish, to motorboat-noise increases its defensive acts at other fish, which in turn reduces both feeding and parental care activity, resulting in lower offspring survival (Nedelec et al., 2017, 2022).
2.6 Genetic adaptation?
When plastic responses are not enough to maintain high fitness, genetic changes are needed to prevent population decline and possible extinction. Whether populations are able to genetically adapt to altered conditions depends on a range of factors, such as the generation time of the species, the presence of genetic variation in the direction of selection, the rate at which new mutations arise, gene flow and the size of the population (G. Bell, 2017; Hoffmann & Sgro, 2011). Species with longer generation time may not be able to adapt fast enough to prevent population decline, while species with shorter generation times are more likely to adapt, especially if combined with high genetic diversity (Chevin & Lande, 2010). For instance, the Atlantic killifish (Fundulus heteroclitus) has adapted to various toxins aided by their short generation time and high genetic diversity (Reid et al., 2016). Similarly, the creek chub (Semotilus atromaculatus) has evolved movements adapted to increased peak water velocities in urban streams (Kern & Langerhans, 2019).
Research on fisheries-induced selection demonstrate that behaviours can quickly evolve through the removal of individuals with less well-adapted behaviours. Guppies (Poecilia reticulata) rapidly evolve coordinated collective movements in response to artificial selection (Kotrschal et al., 2020), while zebrafish exposed to size-selective harvesting evolve either decreased vigilance or decreased attention, depending on whether small or large individuals are targeted (Sbragaglia et al., 2022).
While a growing body of literature demonstrates the possibility of genetic adaptation in fishes, little is still known about their ability to adapt to human-induced environmental changes. This is especially the case for behaviour which is usually determined by many genes and whose expression is dependent on many other traits (Bernatchez, 2016).
3 SYSTEMATIC SEARCH OF THE LITERATURE
To evaluate our knowledge of the effects of rapid human-induced environmental changes on the behaviour of fishes, whether the behavioural responses are adaptive and how they impact higher ecological levels, we searched for literature in Web of Science using the ‘All databases’ option, using the search string: (anthropogenic or human-induced or human-caused) and chang* and fish* and behavio*. We included papers published until 11 October 2022. The search resulted in 1092 publications that were screened for inclusion criteria: (a) test for effects of a human-induced environmental change on the behaviour of one or more fish species either in the field or the laboratory; (b) have a control in the form of before/after study or paired sites/treatments. This resulted in 158 papers, some of which had measured multiple behaviours resulting in 197 behavioural measures. The search is not exhaustive but gives an indication of the state of the field.
We categorised the papers depending on (a) the form of human disturbance (climate change, pollution, habitat alteration, invasion of non-native species, and harvesting), (b) the responding behaviour [antipredator (including boldness), foraging, habitat choice, movements (including activity), migration, reproductive and social (such as shoaling and aggression)], (c) whether the behaviour was adaptive, maladaptive or if adaptedness was unknown and (d) the ecological levels at which effects where recorded (individual, population, community or ecosystem).
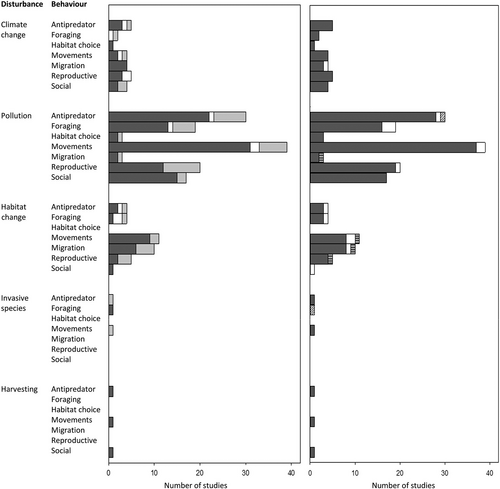
The results show that most attention has been directed to the impact of pollution on the behaviour of fishes, and that the impact of climate change and habitat choice are also well documented, while the impact of invasive species and harvesting has received less attention (Figure 4). The research is relatively well distributed among different behaviours and reflects the expected impact of different disturbances, such as changed habitat characteristics influencing movements and migration. Only habitat choice has curiously received less attention, but, on the other hand, can be imbedded in many other responses, such as movements, migration, antipredator and foraging behaviour.
 , unknown;
, unknown;  , adaptive;
, adaptive;  , maladaptive;
, maladaptive;  , individual;
, individual;  , population;
, population;  , community;
, community;  , ecosystem
, ecosystemThe search reveals that the adaptive value of behavioural responses is usually unknown, as are their consequences for higher ecological levels (population, community and ecosystem). Yet information on the adaptedness of behavioural responses is needed to evaluate their potential impact on populations. Similarly, knowledge of their consequences for higher ecological levels than the population is needed to evaluate their ultimate impact on ecosystems. Thus, the field needs to move from mainly measuring behavioural responses to assessing their impact on fitness and higher ecological levels. In the following, we will discuss how behavioural responses can influence higher ecological levels, drawing on examples from the literature as well as expected patterns based on ecological theory.
4 IMPACT OF BEHAVIOURAL RESPONSES ON POPULATIONS
When changes in behaviour influence the fitness of individuals—their survival, growth or reproductive success—or their spatial and temporal distribution, the dynamics of the population may also change, its size, structure and spatiotemporal distribution (Figure 1). The changes can be more or less immediate, such as when individuals move away from a disturbed habitat, or they may occur after a time lag, as is often the case when toxic compounds gradually accumulate in individuals (Pannetier et al., 2020).
Although behavioural responses are important determinants of population dynamics, few studies have linked behavioural responses of fishes to changes in their population dynamics, as revealed by our literature search (Figure 4). Most evidence is circumstantial, which indicates that more research is needed. For instance, the European whitefish (Coregonus lavaretus) reduces its specialization on specific prey when the number of prey species increases, which could improve its foraging rate and promote the growth of the population (Sanchez-Hernandez et al., 2021). A generalist species, the Eurasian perch (Perca fluviatilis), adopts again the opposite strategy when prey species number increases and enhances its specialization on the more abundant prey, which could similarly improve its feeding efficiency and the growth of the population (Sanchez-Hernandez et al., 2021).
The ability to move and avoid deteriorating habitats is often an advantage in a changing world. Thus, environmental changes may hit smaller species and less mobile ones hardest, and those restricted to smaller, isolated freshwater bodies (Barbarossa et al., 2021; Radinger et al., 2017; Raventos et al., 2021). Dispersal ability is, however, often limited by anthropogenic structures that form barriers to movements, such as levees in wetlands. Artificial constructions that facilitate dispersal, such as canals, can in turn increase exposure to predators (Hoch et al., 2022).
Reproductive behaviours are key determinants of population growth and often sensitive to environmental disturbances. Thus, changes in these behaviours are important links between human activities and altered population viability. For instance, pollution with pharmaceuticals decreases reproductive activities in a range of species, which can have negative effects on offspring production and population growth (Saaristo et al., 2018; Zala & Penn, 2004). Similarly, reduced visibility because of human-induced algal blooms can hamper mate detection and evaluation, which likewise can reduce offspring production and limit population growth (Candolin, 2019; Candolin et al., 2016).
5 PATHWAYS TO COMMUNITY CHANGE
Given that species are linked to each other via species interaction networks, changes in the behaviour of one species can influence several other species. The propagation of effects through this interaction network can then alter biodiversity and the structure of communities (Hoover & Tylianakis, 2012) (Figure 1). Complex feedbacks among species can cause further alterations to the community. For instance, the invasion of a new predator can cause native species to change their habitat or activity time, which in turn can alter the predation success of the invader (Sih et al., 2010).
Changes in trophic interactions are particularly likely to alter the structure of species communities (Bartley et al., 2019). One example is the current shift in food web structure towards the dominance of eurythermal fishes in European lakes; this is partly caused by climate change that alters prey selection and improves the foraging success of eurythermal species (Jeppesen et al., 2012; Tsavdaridou et al., 2022). Similarly, recent changes in the community structure of Baltic Sea fishes is related to changes in their foraging behaviour, which in turn is caused by various human-induced environmental changes, including eutrophication, climate change and the invasion of new species (Tornroos et al., 2019; Viitasalo & Bonsdorff, 2022).
Altered competitive interactions are common causes of modified community structure. Changes in the availability of food or other resources, such as territorial space or refuges, influence the strength of competition and thereby the abundance and distribution of species. For instance, the ongoing degradation of coral reefs reduces the availability of refuges for smaller fishes, which can cause weaker competitors to leave the reef (Hensel et al., 2019). A growing problem is the invasion of non-native species, as these are often strong competitors and can profoundly alter competitive interactions within communities (Cucherousset & Olden, 2011). An example is the invasion of the round goby into the Laurentian Great Lakes, which has intensified competition for resources with darter species, which in turn has reduced the abundance of the darters (McAllister et al., 2022).
Host–parasite interactions are particularly sensitive to changes in environmental conditions because of their high degree of specialization, i.e., the dependence of parasites on specific host species. Pollutants such as noise, artificial light and chemicals that increase stress levels and reduce body condition can impair the resistance of hosts to infections and hence influence the population dynamics of both the host and the parasite (Budria & Candolin, 2014; Byers, 2021; Masud et al., 2020). Changes in the habitat choice of hosts can in turn influence encounter rate between parasites and hosts, or alter their relative growth rate and the intensity of infections (Cable et al., 2017). For instance, infection of the threespine stickleback with the common parasite Schistocephalus solidus causes stickleback to search out warmer water where the parasite grows faster than the stickleback, with potentially negative effects on the abundance of the stickleback (Macnab & Barber, 2012).
On the positive side, behavioural responses can reduce the probability of community changes by conferring resistance to change. For instance, native species may consume non-native species, or outcompete them, or induce fear that reduces their foraging success. An example is native groupers on coral reefs that consume lionfish (Pterois sp.) that attempt to invade their habitat (Smith & Cote, 2021).
6 ECOLOGICAL RAMIFICATIONS
Changes in species interactions and community structure—because of behavioural responses to environmental disturbances—can influence ecological processes such as decomposition, nutrient cycling, carbon sequestering, primary production and the flow of energy to higher trophic levels (Hooper et al., 2005; Palkovacs & Dalton, 2012; Schmitz et al., 2008) (Figure 1). Changes in foraging behaviour and trophic interactions are particularly likely to influence ecosystem processes, given that they are the cornerstones of many ecological processes. For instance, changes in where to feed and what to consume can alter the composition of excreted wastes (Vanni, 2002), while changes in movements among habitats can alter the transfer of nutrients from one habitat to another (Manfrin et al., 2017; Martin et al., 2021). An example is changes in the spawning migration of salmon because of man-made obstructions that alter the spatial distribution of nutrients (Thorstad et al., 2008; Tiegs et al., 2009).
Changes in the behaviour of top predators can induce trophic cascades that alter the composition and behaviour of species at lower trophic levels, which in turn can alter their ecological functions, such as carbon sequestering, nutrient cycling and the modification of benthic substrates (Schmitz et al., 2004). Correspondingly, changes in the behaviour of their prey can influence higher trophic levels through bottom-up effects. For instance, reduced activity of prey can decrease the foraging success of their predators, which in turn can alter the transmission of matter and energy to higher trophic levels (Domenici et al., 2019). Altered behaviour of keystone species are especially likely to influence ecosystem processes. For example, altered distribution of the keystone fish Salminus brasiliensis in La Plata River Base in South America because of climate change is expected to influence the abundance and distribution of a range of other species and thereby their ecological functions (Ruaro et al., 2019).
The invasion of non-native species is a growing problem that frequently induces behavioural changes in native species with potential effects on ecological conditions (Didham et al., 2005; Pysek et al., 2020). For instance, the invasion of the shrimp Palaemon elegans into the Baltic Sea has increased competition for animal prey among mesopredators, particularly for grazers such as small crustaceans and molluscs, which has reduced the abundance of grazers and thereby promoted the growth of algae (Candolin et al., 2018).
7 CONCLUSIONS AND FUTURE OUTLOOK
Freshwater and marine environments are disturbed by a range of human activities. These modify habitat structure and connectivity, cause water pollution and eutrophication, and alter conditions such as temperature, dissolved oxygen concentration and pH. Such modifications are influencing the behaviour of fishes, including foraging, predator avoidance, reproduction, movements and habitat choice. Research has so far focussed on documenting behavioural responses of fishes to various human disturbances, while their fitness effects and consequences for higher ecological levels—populations, communities and ecosystems—are still poorly known. Considering that behaviour underlies many, if not most, species interactions and is a critical link between environment disturbances and ecosystem processes, more research should be directed to the consequences that behavioural responses of fishes have for ecosystems, from effects at the individual to the ecosystem level.
More information is also needed on the mechanisms behind behavioural responses and how these are influenced by previous environmental conditions and sensory limitations (i.e., earlier evolutionary processes). Similarly, information is needed on the potential of species to evolve better adapted behaviours in response to rapid environmental changes. Such information would improve our ability to predict which species will be able to cope with human-induced environmental changes and which will not. Most importantly, the information is needed for the development of strategies to mitigate the negative effects of human activities on fishes and thereby on aquatic ecosystems.
Finally, a topic in need of more research—which has not been touch on in this review but is still of high importance—is the interaction among disturbances in influencing the behaviour of fishes. Habitats are often disturbed by many factors, such as noise combined with rising temperature and declining pH. These disturbances can act additively or interactively (synergistically or antagonistically) and magnify or weaken the effect of each other. Yet little is known about such interactive effects.
To conclude, fishes are important components of aquatic ecosystems that are undergoing large changes in abundance and distribution because of human activities. The ultimate impact on ecosystem structure and function, as well as on the services aquatic ecosystems provide to humans, are poorly known. Research on the behavioural responses of fishes can reveal underlying causes and pathways behind the changes, as well as expected future trajectories of populations and communities, and hence should be an important component of research on the impact of human activities on aquatic ecosystems.
FUNDING INFORMATION
Svenska Kulturfonden in Finland (grant no 170152) and Jenny and Antti Wihuri Foundation (grant no 210290).




