Characterization of the First Fungal Glycosyl Hydrolase Family 19 Chitinase (NbchiA) from Nosema bombycis (Nb)
Abstract
Chitinases (EC 3.2.1.14), as one kind of glycosyl hydrolase, hydrolyze the β-(1,4) linkages of chitin. According to the sequence similarity, chitinases can be divided into glycoside hydrolase family 18 and family 19. Here, a chitinase from Nosema bombycis (NbchiA) was cloned and purified by metal affinity chromatography and molecular exclusion chromatography. Sequence analysis indicated that NbchiA belongs to glycoside hydrolase family 19 class IV chitinase. The optimal pH and temperature of NbchiA are 7.0 and 40 °C, respectively. This purified chitinase showed high activity toward soluble substrates such as ethylene glycol chitin and soluble chitosan. The degradation of chitin oligosaccharides (GlcNAc)2–5 detected by high-performance liquid chromatography showed that NbchiA hydrolyzed mainly the second glycosidic linkage from the reducing end of (GlcNAc)3-5. On the basis of structure-based multiple-sequence alignment, Glu51 and Glu60 are believed to be the key catalytic residues. The site-directed mutation analysis revealed that the enzymatic activity was decreased upon mutation of Glu60, whereas mutation of Glu51 totally abolished the enzymatic activity. This is the first report of a GH19 chitinase in fungi and in Microsporidia.
MICROSPORIDIA are unicellular eukaryotes that develop as obligate intracellular parasites. They are capable of infecting a wide variety of animals ranging from invertebrate to vertebrate, including humans and species of economic importance such as the silkworm and honey bee (Pasteur 1870; Wittner and Weiss 1999). Phylogenetic analysis suggest that microsporidia are related to fungi, either as a basal branch or sister group (Keeling 2003). Nosema bombycis (Nb), the earliest discovered microsporidian isolated and identified from silkworm in 1857, belongs to the class microsporea (Sprague and Vavra 1977). Mircosporidia possess a unique cell structure and infection apparatus including spore wall, sporoplasm, and polar tube (Weidner 1976). The spore wall, which plays a crucial role in both microsporidia infection and extracellular survival, consists of two layers: an electron-dense outer exospore layer, and an electron-lucent inner endospore layer. The endospore, which is composed predominantly of chitin, plays an important role in maintaining the cell structure of N. bombycis (Bhat et al. 2009; Chioralia et al. 1998; Rezaian and Krake 1987).
Chitin, which is found in insects, fungi, crustaceans, mollusks and algae, is an unbranched polysaccharide that is composed of β-(1,4)-linked N-acetyl-d-glucosamine (GlcNAc) (Synowiecki and Al-Khateeb 2003; Tharanathan and Kittur 2003). It is believed to be the second most abundant polysaccharide after cellulose in nature. During chitin catabolism in bacteria and fungi, chitin is degradated by chitinases (EC 3.2.1.14) through hydrolyzes of β-1,4-glycosidic bonds that often found in structural polysaccharides of cell walls (Bhattacharya et al. 2007; Duo-Chuan 2006). According to the CAZy database (http://www.cazy.org/), the chitinases are divided into two families, glycoside hydrolase families 18 (GH18) and 19 (GH19), based on sequence similarity, three-dimensional structure, and catalytic mechanism (Davies and Henrissat 1995). GH18 chitinases are widely distributed in a variety of organisms, including bacteria, fungi, animals and higher plants. By contrast, GH19 family chitinases are found predominantly in plants (Collinge et al. 1993; Graham and Sticklen 1994), and less frequently in bacteria (Itoh et al. 2002) and viruses (Yamada et al. 2010; Yoshida et al. 2008). In 1996, the first bacterial family 19 chitinase was described (Ohno et al. 1996). Since then several structures of GH19 chitinase have been reported, mainly from plants and bacteria (Hoell et al. 2006; Kezuka et al. 2006). The first structure of a GH19 chitinase from the seeds of Hordeum vulgare L. revealed an α-helix rich structure with three disulfide bonds. Structural analysis indicated a prominent elongated cleft presumably responsible for substrate binding and catalysis involving two conserved acidic residues (Andersen et al. 1997; Hart et al. 1993).
Recently, the complete genome sequence of N. bombycis was reported by Pan et al. (2013), from which a conceivable endochitinase gene NBO_41 g0043 was identified and classified as a member of GH19 family. In this study, we describe the cloning and characterization of the catalytic domain of this endochitinase (termed NbchiA). Structure-based multiple-sequence alignment together with 3D-modeling highlighted two glutamate residues Glu51 and Glu60 which are likely to represent the catalytic core. Enzymatic analysis and site-directed mutagenesis indicated that both Glu51 and Glu60 are essential for enzyme activity.
Materials and methods
Materials
Nosema bombycis isolate CQ1 was obtained from the China Veterinary Culture Collection Center. (GlcNAc)n (n = 1–5) oligosaccharides and N-Acetyl-d-glucosamine were purchased from Sigma (Springfield, MO). Ethylene glycol chitin and chitosan 100 were purchased from Wako (Oaska, Japan), powdery chitin and chitin beads were purchased from New England Biolabs (Ipswich, MA). Escherichia coli BL21-RIL (DE3) cells were purchased from Novagen (Darmstadt, Germany). The expression vector pET-28a was purchased from Novagen. The DNA polymerase (Easy pfu) was purchased from Takara (Dalian, China). All the buffered solutions were purchased from Sigma. All the reagents commercially available were of analytical grade.
Cloning, expression, and purification of NbchiA and mutants
Polymerase chain reaction (PCR) primers (5′-GGAATTCCATATGGTTAATGTCACCTCTGATCA-3′ and 5′-CCGCTCGAGTTAATAACAACCTCCTTCAT-3′ containing a NdeI and an XhoI restriction site, respectively) were designed to amplify the coding sequence of NbchiA from the genomic DNA of N. Bombycis by the PCR. The PCR product was cloned into a pET28a-derived expression vector with a hexahistidine tag added to the N-terminus of the recombinant protein, and transformed into E. coli strain BL21 (DE3) (Novagen) using 2 × YT culture medium (5 g of NaCl, 16 g of Bacto-Tryptone, and 10 g of yeast extract per liter). The transformed cells were grown at 37 °C in 2 × YT medium containing 30 μg/ml kanamycin (Sigma) until the OD600 nm reached about 0.6–0.8. Expression of the recombinant proteins was then induced with 0.2 mM isopropyl β-D-1-thiogalactopyranoside (Sigma) for another 20 h at 16 °C before harvesting. Cells were collected by centrifugation at 4,000 g for 20 min and resuspended in 40 ml lysis buffer (20 mM Tris-HCl, pH 8.0, and 100 mM NaCl). After 10 min of sonication and centrifugation at 12,000 g for 30 min, the supernatant containing the target protein was collected and loaded onto a Ni-NTA column (GE Healthcare, Uppsala, Sweden) equilibrated with the binding buffer (20 mM Tris-HCl, pH 8.0, and 200 mM NaCl). The target protein was eluted with 300 mM imidazole (Sigma), and further loaded onto a Superdex 75 column (GE Healthcare). Fractions containing the target protein were pooled and concentrated to appropriate concentration by ultrafiltration (Amicon, Billerica, MA, USA). The purity of protein was assessed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and the protein sample with 50% glycerin added was flash-frozen in liquid nitrogen and stored at −80 °C.
The recombinant plasmids with mutations were prepared using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The oligonucleotides used to introduce the mutations were E51A-F: 5′-CTGGTCATGCATCTGGTGGTTTTG-3′, E51A-R: 5′-CCACCAGATGCATGACCAGTTTGA-3′, E60A-F: 5′-ATATTGAAGCAATTGATTGTGCTG-3′ and E60A-R: 5′-CAATCAATTGCTTCAATATGTTCA-3′ (the mutation sites are underlined).
The mutants were expressed, purified, and stored in the same way as the wide type.
Structure-based sequence alignment and 3D-modeling of NbchiA
The amino acid sequence alignment was constructed in ESPript (http://espript.ibcp.fr/ESPript/ESPript/). The amino acid sequence of the NbchiA was aligned with other GH19 chitinase sequences for which X-ray structure have been analyzed. The modeled 3D structure of NbchiA was built by Swiss-Model (Arnold et al. 2006), which is a knowledge-based protein modeling tool, using the X-ray structure of Brassica juncea chitinase catalytic module (Bjchi3,PDB code: 2Z38) (Ubhayasekera et al. 2007) as the structure template. All figures of protein structure were prepared with PyMol (Bramucci et al. 2012) (http://www.pymol.org/).
Protein determinations were determined by the method of Bradford (1976) with bovine serum albumin (Beyotime, Shanghai, China) as the standard. For column chromatography, the protein concentration was determined by measuring the absorbance at 280 nm.
Enzyme assay
Both the wild-type chitinase and its mutants activity were determined by a modified Schales' procedure (standard assay) using glycolchitin as the substrate (Imoto and Yagishit 1971) with appropriate modification. 0.5 ml of 0.02 mg/ml chitinase was added to 1 ml of 0.5 mg/ml glycolchitin, both chitinase and glycolchitin were diluted in 0.1 M sodium acetate buffer (pH 4.5) (Sigma). After incubation at 37 °C for 30 min, 450 μl of the reaction mixture was added to 600 μl ferri-ferrocyanide reagent (Sigma), and the reducing power of the reaction mixture was measured in this way. The chitinase activity of each chromatographic fraction was determined by the absorbance of the reaction mixture at 420 nm (▵A420) using a U-3010 spectrophotometer (Hitachi, Tokyo, Japan). One unit of enzyme activity was defined as the amount of enzyme that liberated 1 μmol of reducing sugar per minute at 37 °C. To measure the activity against other substrates, colloidal chitin, soluble chitosan, powdery chitin, chitin beads, and the deproteinated chitin spore coats (DCSCs) were used instead of glycolchitin. DCSCs were acquired by boiling N. bombycis in 1M NaOH (Yang et al. 2014)
Optimization of pH and temperature of enzymatic reaction
The optimum temperature and pH were determined by performing the standard assay at the temperature range of 20–80 °C and the pH range of 3.0–11.0. To determine the effect of heat on the stability, experiment was carried out in 0.1 M sodium acetate buffer (pH 4.5) (Sigma), and the series of temperature values were prepared as follows: 20, 30, 40, 50, 60, 70, and 80 °C. The following series of buffered solutions were made to determine the effect of pH on enzyme stability: 0.1 M Na2HPO4–0.1 M sodium citrate buffer: pH 3.0, pH 4.0, pH 5.0, pH 6.0, pH 7.0, pH 8.0, and 0.1 M Na2CO3 – 0.1 M NaHCO3 buffer: pH 9.0, pH 10.0, and pH 11.0 (Sigma). The enzyme was preincubated in different temperature and pH for 30 min before adding substrate, then glycolchitin was added into enzyme solution, and incubated for another 30 min, the remaining activity of enzyme was determined by performing the standard assay.
High-performance liquid chromatography analysis of the reaction products
Analysis of the specific activity against chitooligosaccharides were performed in 10 μl reaction mixtures containing 15 nM purified NbchiA in 0.1 M sodium acetate buffer (pH 5.5), 1 mg/ml (GlcNAc)3, (GlcNAc)4, or (GlcNAc)5 (Sigma) and 0.1 mg/ml BSA. The reaction mixtures were incubated at 37 °C for various durations. The reaction was initiated by the addition of enzyme and terminated by mixing with an equal volume of 70% acetonitrile. After centrifuging at 12,000 g for 10 min, 10 μl of supernatant was applied to an Agilent 1200 Series liquid chromatography HPLC system (Agilent, Santa Clara, CA) equipped with a Tosoh TSK-Gel amide- 80 column (0.46 internal diam. × 25 cm) (Tosoh Bioscience, Tokyo, Japan). The liquid phase consisted of 70% (v/v) acetonitrile, and separation of the components at a flow rate of 0.7 ml/min, and eluted oligosaccharides were monitored by recording absorption at 210 nm (Koga et al. 1998). The sugar components of the reaction system were determined by comparison with the retention time of standard substance.
Results
Sequence analysis and 3D-modeling
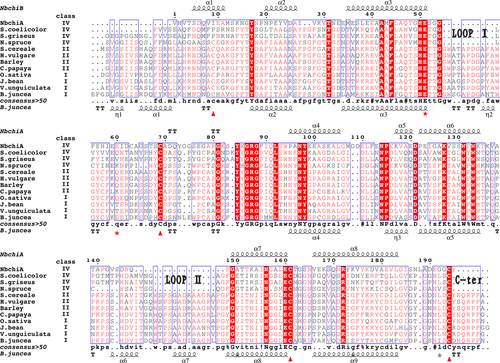
To compare NbchiA with GH19 chitinases for which X-ray structures have been determined, a structure-based sequence alignment was constructed. The multiple-sequence alignment of NbchiA and several GH19 chitinase homologs showed that NbchiA lacks three parts, Loop I, Loop II and C-ter (Fig. 1), when compared with class I and class II chitinases (Kezuka et al. 2006; Watanabe et al. 1999). Interestingly, the positions of the deletions observed in NbchiA are exactly the same as those observed in GH19 class IV chitinases (Fig. 1). In terms of the presence and positions of amino acid deletions, NbchiA can be classified as class IV.
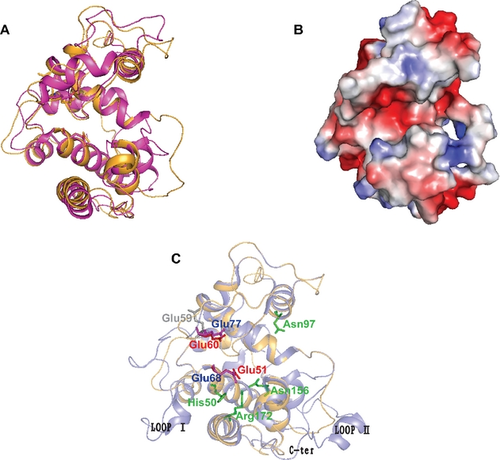
To determine the structural difference between the NbchiA and reported GH19 chitinases, we obtained a model of NbchiA using Swiss Model (Arnold et al. 2006). The overall predicted structure showed that NbchiA is complex, containing a total of nine α-helices (Fig. 2A). There is one deep cleft stretched across the molecular surface that is likely to function in substrate binding (Fig. 2B). Within this cleft, there are two putative catalytic residues, Glu51 and Glu60, that are conserved in all chitinases and are thought to constitute the active sites in other GH19 chitinases. Moreover, these two glutamine residues are also structurally conserved when compared with the S.coelicolor homolog (Hoell et al. 2006; Fig. 2C).
The optimal enzyme reaction pH and temperature of NbchiA
The effect of pH and temperature on enzyme activity was examined using glycolchitin as the substrate. The purified chitinase exhibited maximum activity at pH 7.0, and it showed a rapid decline from pH 8.0 to 9.0. pH values lower than 3.0 and higher than 9.0 strongly inhibited the activity of this chitinase (Fig. 3A). The enzyme was stable at broad pH value range from 3.0 to 10.0, reached a plateau at more than 60% hydrolytic activity from 3.0 to 6.0 and reached the maximum activity at pH 7.0. It was unstable above pH 10.0, and the relative activity of NbchiA was reduced to 5.5% at pH 11.0 compared to the maximum activity at pH 7.0 (Fig. 3B).
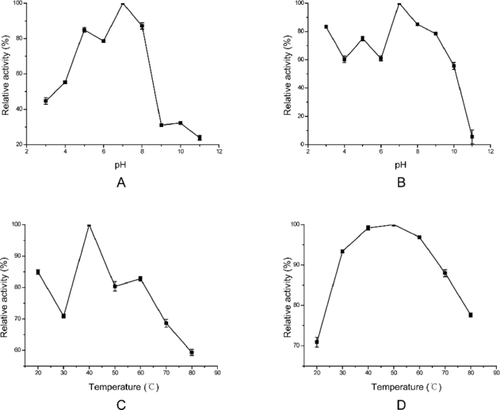
The effects of temperature on the hydrolytic activity of NbchiA were determined by incubation at a range of temperatures from 20 to 80 °C. NbchiA showed the highest catalytic activity around 40 °C and the activity was losing progressively as temperature increased (Fig. 3C). Moreover, the purified chitinase proved to be stable over a wide temperature range from 30 to 80 °C, and it still retained 77.6% activity of the maximum value at 80 °C (Fig. 3D).
HPLC analysis of NbchiA cleavage patterns
To identify the cleavage pattern of NbchiA, the enzyme activity toward (GlcNAc)2-5 was determined by high-performance liquid chromatography (HPLC; Koga et al. 1998). The substrate cleavage patterns were summarized as follows: (GlcNAc)5 was reduced to (GlcNAc)2 and (GlcNAc)3 after 1 h of hydrolysis (Fig. 4A). And after 24 h of hydrolysis, (GlcNAc)5 was completely reduced to (GlcNAc)2 and GlcNAc monomers (Fig. 4B). (GlcNAc)4 was turned to (GlcNAc)2 after no matter 1 or 24 h (Fig. 4A, B). There was almost no degradation of (GlcNAc)3 after 1 h. Nevertheless, the trisaccharide was completely cleaved into GlcNAc monomer and (GlcNAc)2 after 24 h of hydrolysis (Fig. 4B). The enzyme did not show any catalytic activity on the (GlcNAc)2 even after 24 h hydrolysis at a very high concentration (data not shown). These findings indicate that NbchiA hydrolyzes of chitin occurs mainly at the second glycosidic linkage from the reducing end of (GlcNAc)n (n ≥ 3).

Substrate specificity
Several substrates such as ethylene glycol chitin, colloidal chitin, soluble chitosan, powered chitin, chitin beads, and DCSCs were used to detect the substrate specificity of NbchiA. As shown in Table 1, NbchiA showed the greatest activity against soluble substrates such as ethylene glycol chitin and soluble chitosan, of which the activities were 58.63 U/μmol enzyme and 1.59 U/μmol enzyme, respectively. We also found that NbchiA showed relatively high activity toward chitin beads (3.13 U/μmol enzyme). By contrast, the hydrolytic activity was very low toward crystalline substrates such as powered chitin and colloidal chitin. We also could not detect the activity of NbchiA toward powered chitin and DCSCs, a insoluble forms of chitin substrate.
| Recombinant proteinsb | Degrading activity (μmol/min-per μmol enzyme)a | |||||
|---|---|---|---|---|---|---|
| Ethylene glycol chitin | Colloidal chitin | Soluble chitosan (DD 80%) | Powered chitin | Chitin beads | DCSCsc | |
| WT-NbchiA | 58.63 | 0.75 | 1.59 | < 0.01 | 3.13 | < 0.01 |
| NbchiA-E51A | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
| NbchiA-E60A | 24.47 | < 0.01 | 1.59 | < 0.01 | 2.13 | < 0.01 |
- DD = degree of deacetylation.
- a The amount of reducing sugar equivalent to GlcNAc liberated was measured for ethylene glycol chitin, collidal chitin, soluble chitosan, chitin power, chitin beads and spore coats.
- b The concentration of each enzyme in the reaction mixture was in the range of 100–350 nM.
- c The deproteinated chitin spore coats of Nosema bombycis (DCSCs).
Hydrolytic activity of the NbchiA mutants
According to the model structure of NbchiA (Fig. 2B), there is a deep cleft that functions in substrate binding on the surface of the enzyme. In this cleft, we found several sites that were conserved in both sequence and structural alignment, of which the Glu51 and Glu60 were reported to be the catalytic residues of the GH19 chitinase (Andersen et al. 1997; Hart et al. 1993; Hoell et al. 2006; Huet et al. 2008). Here, we produced two NbchiA mutants E51A and E60A by site-directed mutagenesis. Several substrates such as ethylene glycol chitin, colloidal chitin, soluble chitosan, powered chitin, chitin beads, and DCSCs were used to determine the substrate specificity and activities of the NbchiA and its mutants. As shown in Table 1, the mutant E51A lost activity against all of the tested substrates. By contrast, the activity for the E60A mutant toward soluble chitosan (DD 80%) was equal to that of the native protein, whereas the activity toward ethylene glycol chitin and chitin beads was decreased by about 40–60% compared with that of wild-type. These results suggest that Glu51 may act as the catalytic general acid, whereas Glu60 acts as the catalytic general base. Neither the wild-type nor mutated forms of the protein were effective in degrading crystalline chitin (colloidal chitin and powered chitin) or DCSCs. This may be due to the lack of chitin-binding modules (Heggset et al. 2009; Kawase et al. 2006).
Discussion
In this work, we cloned, purified and characterized the C-terminal domain of the N. bombycis chitinase, NbchiA, which belongs to glycoside hydrolase family 19 (GH19). According to the model structure of NbchiA (Fig. 2A), it is predominantly composed of α-helices, which is similar to other characterized GH19 chitinases (Robertus and Monzingo 1999). Chitinases are divided into two categories glycosyl hydrolase families, GH18 and GH19, based on the sequence similarity of the catalytic domain (Henrissat and Bairoch 1993). As microsporidia belong to the phylum Fungi (Gill and Fast 2006; Keeling 2003; Keeling et al. 2000; Weiss and Keohane 1999), NbchiA represents the first fungal GH19 chitinase to be characterized and reported.
GH19 chitinases are predominantly found in plants, where one of the physiological roles is to protect the plants from being infected by fungal pathogens (Kasprzewska 2003). However, NbchiA did not exhibit any antifungal activity (data not shown), perhaps because the catalytic domain of NbchiA which was reported in this paper lacked the chitin-binding domain.
In yeast and various fungi, chitinases participate in morphogenesis by taking part in remodeling cell wall structure and daughter cell separation, and also in some processes of pathogenesis (Cohen-Kupiec and Chet 1998; Patil et al. 2000; Shimono et al. 2002). The chitinase inhibitor allosamidin inhibits cyst formation in Entamoeba invadens, chitin is a component of Entamodeba cyst wall (de la Vega et al. 1997). Microsporidia are spore-forming parasites, Vavra and Chalupsky (1982) first described chitin as a component of microsporidia spore walls (Vavra and Chalupsky 1982), and the spore wall plays a major role in the protection against environmental stresses, allowing long-term survival of the parasite after its release from the host cell (Koudela et al. 1999; Shadduck and Polley 1978). The general life cycle pattern for the microsporidia can be divided into three phases: the infective or environmental phase, the proliferative phase identified by some merogony, and the sporogonic or spore-forming phase (Cali and Takvorian 1999). The latter ultimately transform into mature spores where the deposition of chitin in the cell wall is first observed. Our immunofluorescent assay revealed that NbchiA is regularly distributed at the spore wall of N. bombycis (Fig. S1). The time-course RT-PCR experiments indicated that 3 and 4 d postinoculation, NbchiA is up-regulated (Fig. S2). Coincidentally, depending on the cell culture model of Nosema ceranae and Nosema apis, microsporidia develops into primary spores from meronts (Gisder et al. 2011), and the formation of the chitin-containing spore wall also occurs from this time point. Therefore, it is attractive to hypothesize that NbchiA may be involved in the deposition and modeling of chitin within the endospore during sporogony. The chitinase in the microsporidia spores may also be one of the reasons for the germination and polar tube extrusion of spores, participating in pressure adjustment and the procession of polar tube through the chitinous spore wall.
Nosema bombycis is a silkworm pathogen that causes the epidemic disease pébrine in silkworms. Research into the infection and virulence mechanism of N. bombycis is necessary to understand host–parasite interactions, and hopefully will reduce the impact of pébrine disease on the silkworm host. Some pathogenic bacteria, including adherent invasive E. coli, Listeria monocytogenes, and Vibrio cholerae are likely to express some chitinases or chitin-binding proteins as accessory molecules, to initiate their adhesion to host cells (Bhowmick et al. 2007; Chaudhuri et al. 2010; Francetic et al. 2000). Some bacterial chitinases are thought to be virulence factors during infection. The peritrophic membrane (PM) of silkworms is a thin membrane which consists of protein, chitin and other carbohydrate compounds. Ultrastructural study has produced direct evidence that the PM is a barrier to infection in mosquitoes (Sieber et al. 1991). It has been reported that Plasmodium gallinaceum motile ookinetes can produce chitinase to penetrate the chitinous PM in the mosquito midgut (Huber et al. 1991). Under natural conditions, silkworms are infected by N. bombycis orally, and the PM is destroyed upon infection with N. bombycis. As there are several pieces evidence that the parasites chitinase can penetrate the PM during invasion, and we discovered that the recombinant NbchiA can degrade several kinds of chitin substrates, the role of NbchiA in the penetration of the chitin-containing PM and in the invasion of N. bombycis is an important subject and will be investigated in our future study.
Acknowledgments
We gratefully acknowledge Makoto Shimosaka from Shinshu University for assistance with the classification of NbchiA. We also appreciate the efforts of Michael Fishman and Dr. Pengchao Guo for their modifications of this paper. This study was supported by the grants from National High-tech R&D Program (863 Program, no. 2012AA101301 – 3, no. 2013AA102507), National Basic Research Program of China (no. 2012CB114604), Natural Science Foundation of China (no. 31072089, 31272504, 31270138), Chongqing Science & Technology Commission (no. CSTC2010AA1003), the Program of Introducing Talents of Discipline to Universities (no. B07045).




