Wasp Pollination: Mechanisms, Evolution and Ecological Significance in Neglected Pollinator Groups
Funding: This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (303986/2023-9), Fundação de Amparo à Pesquisa do Estado de São Paulo (2022/11911-3) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
ABSTRACT
Wasp-mediated pollination encompasses diverse mechanisms, presenting a relevant yet underexplored facet of plant–insect interactions. This review synthesises current knowledge on wasp pollination's ecological and evolutionary dynamics. For clarity, I classified wasp pollinators into three categories: (1) generalist pollinators associated with resource-rich plants, (2) specialised pollinators of resource-rich plants and (3) specialised pollinators of resource-less plants (deceptive pollination). The latter category includes sexual and food deception. These pollination types will be described in detail throughout the text. Generalist wasps pollinate flowers with less morphological specialisation, which are also visited by other insect groups. In contrast, certain groups, such as the subfamilies Masarinae and Thynninae, the genus Hemipepsis (Pompilidae) and the family Agaonidae (fig wasps), have specialised in certain taxa or plant guilds. Deceptive pollination strategies, primarily in orchids, exploit wasps through sexual and food mimicry, reflecting intricate evolutionary adaptations. Fig wasps exhibit a long history of co-diversification with their host plants, presenting a key innovation that combines wind-mediated and chemotactic pollen dispersal. This review highlights the ecological implications and evolutionary aspects of wasp pollination by examining these diverse mechanisms. It concludes that wasps should not be neglected as pollinators, as they play an important role in the reproduction of many plant species.
1 Introduction
Insects constitute the primary agents of pollination in many ecosystems (Schowalter 2000; Torezan-Silingardi, Silberbauer-Gottsberger, and Gottsberger 2021), with hymenopterans, particularly bees, standing out as one of the main groups of pollinators of wild and cultivated angiosperms (Danforth et al. 2006; Khalifa et al. 2021; Junqueira et al. 2022). Wasps (i.e., all Hymenoptera of the suborder Apocrita, excluding bees and ants), on the other hand, are generally considered less efficient pollinators and are often overlooked in classical pollination studies (Fægri and van der Pijl 1979; Proctor 1991). This generalisation is partly due to social wasps, which exhibit less specialised interactions with the plant species they visit (Santos, Aguiar, and Mello 2010; Mello et al. 2011). Moreover, more generalist wasps use flowers with less specialised morphology, which are also accessible to other groups of insects. In addition, cultural biases heavily influence the focus of research efforts, as bees are universally admired. At the same time, wasps are universally disliked, leading to a disparity in both public perception and scientific attention towards these taxa (Sumner, Law, and Cini 2018).
For pollination to occur, the insect must exhibit behaviour and morphology that enable pollen transport from the anther to the stigma of a flower of the same species. Simply put, an efficient pollinator must have a size and flower-accessing behaviour that allows its body to touch the floral structures involved in reproduction. Additionally, for cross-pollination, the insect must visit flowers from different individuals of the same species within a short time interval (Willmer 2011; Torezan-Silingardi, Silberbauer-Gottsberger, and Gottsberger 2021). Many species of wasps that visit flowers do not meet these basic requirements and do not function as pollinators. For instance, Bembix u-scripta W. Fox, 1895, and Myzinum navajo (Krombein, 1938) forage for nectar in Kallstroemia grandiflora Torr. ex A. Grey (Zygophyllaceae) flowers in the southwestern U.S. deserts, but they do not act as pollinators. Bembix u-scripta perches beneath the blossoms, inserting its tongue between the sepals to sip nectar, not contacting the flower's reproductive structures. Similarly, M. navajo accesses the nectaries from within the blossom, but its small size prevents contact with the pollen-bearing anthers (O'Neill 2001).
However, some groups of wasps are specialised pollinators of fig trees and certain species of orchids, Apocynaceae and Asparagaceae (Weiblen 2002; Gaskett 2011; Shuttleworth and Johnson 2012; Wiemer et al. 2012). Despite including fewer pollinating species, wasps are notable for participating in various pollination mechanisms. In fact, some social wasp species can rival or even exceed bees in pollen transport and deposition, particularly in late-blooming native plants, indicating that wasps may occupy unique ecological niches and play a more relevant role in pollination networks than previously recognised (Borchardt et al. 2024).
The larvae of most wasp species are carnivorous, feeding on other arthropods or parasitising insects (i.e., parasitoid wasps) (Willmer 2011). In contrast, adult wasps typically visit flowers to forage for nectar, which they use to meet their energy needs or sustain the colony (in social species). During these visits, they may inadvertently transport pollen on their bodies. Indeed, wasps are floral visitors to many angiosperm families (Tooker and Hanks 2000; Robertson and Klemash 2003; Antonini et al. 2005; Hermes and Köhler 2006; Wiesenborn, Heydon, and Lorenzen 2008; Clemente et al. 2012; Somavilla and Köhler 2012). In some cases, such as in the pink pepper Schinus terebinthifolius Raddi (Anacardiaceae), which is a native species in Brazil subjected to extractivism (Jesus and Gomes 2012), more than 50% of the sampled floral visitors had a substantial amount of pollen grains adhered to their bodies (Sühs et al. 2009).
The amount of pollen transported depends on the density of bristles that wasps have on their bodies. Generally, they have relatively few bristles; however, some groups, such as pollen wasps (Masarinae), have more bristles adapted for pollen collection and transport (O'Neill 2001). Other wasps do not transport any pollen at all, as they rob nectar by cutting the base of the flower without coming into contact with the floral structures involved in reproduction (Antonini et al. 2005). Some non-pollinating wasps exhibit defensive mutualism with the plants they visit. For example, Pachodynerus brevithorax (de Saussure, 1852) (Vespidae: Eumeninae) and Brachygastra lecheguana (Latreille, 1824) (Vespidae: Polistinae) patrol Banisteriopsis malifolia (Nees & Mart.) B. Gates (Malpighiaceae) plants and prey on endophytic larvae of beetles from the genus Anthonomus (Curculionidae) that develop inside the flower buds (Torezan-Silingardi 2011; Alves-Silva et al. 2013).
The generalist habit of social wasps appears to be associated with the high carbohydrate demand for colony development, prompting them to exploit any concentrated sugar source. Besides floral nectar, social wasps collect plant sap, fruit exudates, honeydew excreted by plant-sucking insects and artificial sources like sugary liquid foods (Richter 2000). In addition to groups that forage for floral nectar, some wasps visit flowers seeking food for their offspring in the form of pollen (Gess 1996), animal prey (Alves-Silva et al. 2013), sites for oviposition (Weiblen 2002), or are deceived by false cues suggesting the presence of food or mating opportunities, that is, pollination by deceit (Renner 2006).
Classifying the pollination mechanisms mediated by wasps is not a simple task due to the vast diversity of life histories in this group of insects and their various interactions with pollinated plants. However, some general patterns can be identified. Based on this literature review, plant and pollinating wasp associations can be categorised into generalist pollinators, specialised pollinators of resource-less plants (deceptive pollination) and specialised pollinators of resource-rich plants (Figure 1). These categories serve a didactic purpose, as establishing natural groups is not always possible. Wasps belonging to a single phylogenetic lineage can interact with plants through different pollination mechanisms, and independent wasp lineages can engage in the same pollination mode. For example, the family Vespidae includes poorly adapted generalist species and highly specialised pollinator groups (O'Neill 2001; Brodmann et al. 2008). Additionally, various families of wasps are attracted to orchids by false sexual cues and pollinate their flowers without receiving rewards (Jersáková, Johnson, and Kindlmann 2006). On the other hand, fig-pollinating wasps (Agaonidae) exemplify a natural group that has radiated exclusively in association with the genus Ficus (Cruaud et al. 2010, 2012).
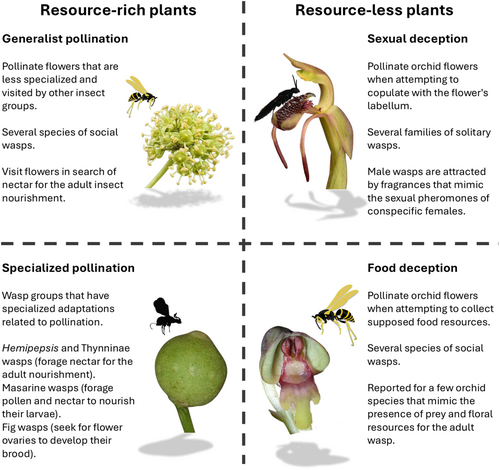
Subsequently, each of the three classes of wasp-mediated pollination will be discussed. Generalist pollinators will be treated cautiously, as detailed studies may reveal more specialised interactions. The deceptive pollination group includes cases of sexual and food deception. Finally, specialised resource-based pollination will encompass all interactions where the plant and its primary pollinator exhibit adaptations suggesting a shared evolutionary history. This group is highly diverse both taxonomically and biologically, mainly encompassing species of Apocynaceae and orchids pollinated by wasps from the families Pompilidae and Vespidae, as well as plants associated with pollen wasps and fig wasps. The interaction between fig trees and their pollinating wasps will be addressed in greater depth due to the extensive ecological, biological and evolutionary information available in the literature.
2 Generalist Pollinators
Several species of social wasps from the genera Brachygastra, Belonogaster, Polistes, Polybia (Polistinae), Dolichovespula, Vespa and Vespula (Vespinae) act as (co-)pollinators for plants in the families Apocynaceae (Asclepiadoideae), Araliaceae, Asteraceae, Erythroxylaceae, Iridaceae, Mitrastemonaceae and Polygonaceae when they visit their flowers in search of nectar (Momose and Inoue 1993; Barros 1998; Vieira and Shepherd 1999; Coombs, Peter, and Johnson 2009; Jacobs et al. 2010; Horsburgh, Semple, and Kevan 2011; Suetsugu 2019). More comprehensive studies on pollination biology within communities of flower-visiting wasps will likely expand the list of plants effectively pollinated by these insects (Clemente et al. 2013). These wasps are considered generalists because they pollinate flowers that are less specialised and visited by other insect groups. Examples include Polygonum thunbergii Sieb. et Zucc. (Polygonaceae) and Hedera helix L. (Araliaceae). In Japan, P. thunbergii was visited by 64 species from 30 insect families. Among these visitors, Vespidae was the second most abundant group, with 42% of the collected individuals carrying pollen grains (Momose and Inoue 1993). In England, Hedera helix L. is visited by at least twenty insect species, with 55% of these visitors being wasps from the genus Vespula. These wasps are likely pollinators, as they exhibit a high frequency of visits, relatively high foraging rates and many pollen grains on their bodies (Jacobs et al. 2010).
Species of Asclepiadoideae (Apocynaceae) are frequently associated with wasps. Social wasps of the subfamily Polistinae forage on flowers visited by other insect groups. However, their morphology and foraging behaviour make them more effective pollinators (Vieira and Shepherd 1999; Coombs, Peter, and Johnson 2009). Polybia ignobilis (Haliday, 1836) is the principal pollinating wasp species for four sympatric species of Oxypetalum in Brazil (Vieira and Shepherd 1999). Insects from various orders were observed visiting the flowers of these Oxypetalum species. Still, only P. ignobilis transported pollinaria, which adhered to their mouthparts when inserted into the floral tube to access the nectar. In South Africa, another species of Asclepiadoideae, Gomphocarpus physocarpus E. Mey., exhibited an ecologically generalised wasp pollination system composed of several species from the genera Belonogaster and Polistes (Coombs, Peter, and Johnson 2009). Unlike Oxypetalum, the pollinaria of G. physocarpus adhere to the arolium (a small pad located between the tarsal claws) of the insect when the wasps grasp the flowers to lap up nectar from the relatively exposed nectaries of this plant species. Similarly, the apocynacean Morrenia odorata (Hook. & Arn.) Lindl. is pollinated by various wasp groups, including pompilid and sphecid wasps. The flowers of M. odorata appear to have evolved a specialised mechanical fit with the wasps' body parts. When the wasps insert their mouthparts to access nectar, they engage with the floral guide rails, facilitating the efficient removal and transport of pollinaria (Wiemer et al. 2012).
Velvet ants (Hymenoptera: Mutillidae and Myrmosidae) have recently been recognised as potential generalist pollinators, visiting flowers from several plant families, including Apiaceae, Asteraceae, Euphorbiaceae and Fabaceae (Parejo-Pulido, Díaz-Calafat, and Robla 2024). These solitary ectoparasitoid wasps, which primarily parasitise the immature stages of other insects, exhibit strong sexual dimorphism, with wingless females resembling ants and winged males (Brothers 1989; Brothers, Tschuch, and Burger 2000). A global review of their flower visitation patterns revealed that velvet ants are generalist flower visitors, interacting with a broad range of plant species (Parejo-Pulido, Díaz-Calafat, and Robla 2024). In 42.7% of recorded interactions, pollen was observed attached to the bodies of these wasps, suggesting their potential role in pollination. Additionally, the review highlighted differences in flower visitation behaviours between males and females, with males being more generalist flower visitors. While their effectiveness as pollinators is not yet fully established, the presence of pollen on their bodies indicates that velvet ants may play a role in pollination networks, particularly in ecosystems where they are abundant.
However, caution is advisable when considering social wasps as generalist pollinators. A more in-depth investigation into the role of floral fragrances in attracting specific pollinators and the efficiency of other floral visitors as pollinators may reveal specialisations in these plant–insect interactions (Burger et al. 2017; Phillips, Bohman, and Peakall 2021). Detailed observations are essential to validate the pollination hypothesis, as some studies use indirect data based on visitation behaviour and insect morphology to infer the efficiency of wasps as pollinators (Barros 1998; Horsburgh, Semple, and Kevan 2011; Hordzi 2024).
3 Specialised Pollinators on Resource-Less Plants
Plants pollinated by deception signal the presence of a resource without providing it to the pollinator. It is estimated that approximately 7500 species across 32 families of angiosperms are pollinated by deception. About 6500 (87%) species belong to the family Orchidaceae (Renner 2006). If these estimates are accurate, at least one-third of the remaining 1000 species belong to the genus Ficus. Despite the large number of species pollinated by deception in these two groups (approximately 30% of orchids and 50% of fig trees), resource-less plants represent only 3.7% of all angiosperm species (Renner 2006).
Most resource-less orchids pollinated by wasps emit attractants that signal mating opportunities (i.e., sexual deception). In contrast, orchids pollinated by wasps that signal the presence of food (i.e., food deception) are rarely reported, despite being more common in orchids pollinated by bees (Jersáková, Johnson, and Kindlmann 2006). In fig trees, the resource offered to wasps is oviposition sites. In monoecious species, the wasp lays eggs in some pistillate flowers, and seeds are produced in the remaining non-oviposited flowers of the inflorescence. In gynodioecious species, female plants are pollinated by deceit, as they signal the presence of a resource, but the flowers are inaccessible for the fig wasp oviposition (Kjellberg et al. 1987). The following section will address deceit pollination in orchids. Examples involving fig trees will be discussed later in the section on fig tree pollination.
3.1 Sexual Deception
Orchids pollinated by sexual deception mimic female insects, attracting males who act as pollinators by attempting to copulate with the flower's labellum. Males attracted by these false cues expend time, real mating opportunities and sometimes sperm without receiving any reward. The attraction process occurs in two stages. Initially, males are attracted by fragrances that mimic the sexual pheromones of conspecific females. Subsequently, at close range, the flower's shape and colours, which may resemble the females, guide the insects (Gaskett 2012; Bohman et al. 2016, 2017; Cuervo et al. 2017; Francisco and Ascensão 2024).
The analysis of semiochemicals reveals these compounds' crucial importance in pollination by sexual deception in various orchid species (Bohman et al. 2016). Different groups of chemical compounds are associated with distinct plant groups and their pollinators. For example, in bee-pollinated Ophrys species, alkenes and alkanes have been identified as the main attractants. In O. speculum, pollinated by scoliid wasps, the primary compounds are keto-acid and hydroxy-acids. In Chiloglottis and Drakaea orchids, pollinated by thynnine wasps, cyclohexanediones and pyrazines are involved, respectively (Bohman et al. 2016). Additionally, Caladenia (spider orchids) attract their pollinator, the thynnine wasp Campylothynnus flavopictus (Smith, 1859), with a unique system of (methylthio) phenols, being the first occurrence of sulphurous sex pheromones in Hymenoptera (Bohman et al. 2017).
Wasps from the families Sphecidae and Pompilidae pollinate orchids of the genus Disa in South Africa by deception. In Australia, most orchid species pollinated by sexual deception exploit wasps from the families Ichneumonidae, Scoliidae, Thynnidae and Tiphiidae (Gaskett 2011; Menz et al. 2013; Weinstein et al. 2016; Bohman et al. 2017). In South America, there is a report of the genus Geoblasta being pollinated by wasps of the family Scoliidae (Ciotek et al. 2006). Representatives of the genus Ophrys in Europe, primarily associated with bees from the families Andrenidae, Colletidae, Megachilidae and Apidae, are also pollinated by wasps from the families Scoliidae and Sphecidae (Gaskett 2011) (Table 1).
| Wasp family | Occurrence | Orchid genera |
|---|---|---|
| Sphecidae | South Africa | Disa |
| Pompilidae | South Africa | Disa |
| Ichneumonidae | Australia | Cryptostylis |
| Scoliidae | Australia | Calochilus |
| Tiphiidae | Australia | Arthrochilus, Caladenia, Caleana, Chiloglottis, Drakaea, Leporella, Paracaleana, Spiculaea |
| Ichneumonidae | New Zealand | Cryptostylis |
| Scoliidae | South America | Geoblasta |
| Scoliidae | Europe | Ophrys |
| Sphecidae | Europe | Ophrys |
Likely due to the chemical mimicry of sexual pheromones, orchid species pollinated by sexual deception exploit one or a few species of pollinating wasps, which may vary in different regions. However, in contact zones between allopatric species, orchids may attract pollinators of phylogenetically related species due to the similarity of the semiochemicals used. This occurs in Australia with the orchids Chiloglottis trapeziformis Fitzg. and C. valida D.L. Jones, which are pollinated by the wasps Neozeleboria cryptoides (Smith, 1959) and N. monticola (Turner, 1909), respectively (Schiestl and Peakall 2005).
Solitary and parasitoid wasps are the most reported pollinators in orchids pollinated by sexual deception. This appears to be related to various characteristics of the mating systems of these wasps, which facilitate or act as pre-adaptations for exploitation by orchids (Menz et al. 2013; Whitehead and Peakall 2013). Males of solitary wasp species are generally easily attracted by airborne sexual pheromones and are highly vigilant, responding promptly to female pheromones. Since female wasps are generally monogamous or have a higher chance of being fertilised in their first mating, there is intense selective pressure on males to locate and mate with virgin females quickly. Consequently, orchids benefit from these characteristics of male wasps, which result in a predisposition to indiscriminate mating attempts (Gaskett 2011).
The cost of sexual deception to insects and the evolution of males' ability to discriminate false signals are current topics of debate (Schiestl 2004; Renner 2006). Addressing this issue requires specifying which biological scale is considered, whether at the individual or species level (Gaskett 2011). At the species level, orchids pollinated by deception likely have little influence on the evolution of their pollinators, as they do not provide benefits to the insects and usually do not impose apparent costs on their pollinators (Gaskett 2011; Brunton-Martin, O'Hanlon, and Gaskett 2022). However, an experimental study on Cryptostylis orchids and the ichneumonid wasp Lissopimpla excelsa (Costa, 1864) demonstrates that pseudocopulation imposes costs on the pollinator (Brunton Martin, O'Hanlon, and Gaskett 2020). Male wasps that engage in pseudocopulation with the orchid experience significant sperm depletion. This depletion occurs because the orchids trigger the release of sperm during false mating attempts. The study also found evidence of population-level adaptation in these wasps, where males from populations regularly exposed to Cryptostylis orchids exhibited reduced sperm release during pseudocopulation. This adaptive response suggests that wasp populations have evolved mechanisms to mitigate sperm depletion, allowing them to conserve reproductive resources despite frequent deceptive interactions with orchids (Brunton Martin, O'Hanlon, and Gaskett 2020).
The evolutionary persistence of wasp exploitation may be explained by the hypothesis of low encounter rates and frequency-dependent selection (Gaskett 2011; Brunton-Martin, O'Hanlon, and Gaskett 2022). Under this hypothesis, the cost to the pollinator species is minimal because orchids are typically rare or bloom sporadically. Additionally, not all insects that encounter an orchid are deceived by its false signals. As a result, many individuals within the species either never encounter an orchid or do so infrequently. In this context, the benefit of responding quickly to sexual attractants and securing multiple matings with real females outweighs the occasional cost of copulating with an orchid flower. Alternatively, Brunton-Martin, O'Hanlon, and Gaskett (2022) proposed the robustness hypothesis to explain the persistence of exploitative interactions, such as sexual deception. This hypothesis suggests that certain species possess intrinsic characteristics that make them resilient to the costs of exploitation. In systems where pollinators are deceived into wasting sperm on non-reproductive interactions, such as the relationship between Cryptostylis orchids and L. excelsa wasps, the hymenopteran's haplodiploid reproductive system may provide robustness. Haplodiploidy allows populations to reproduce despite temporary sperm depletion, as non-inseminated females produce haploid males. This biological advantage may enable such species to persist in deceptive relationships over evolutionary timescales, even when exploitation imposes significant fitness costs on individuals.
3.2 Food Deception
While orchids pollinated by sexual deception primarily exploit male insects of solitary species, orchids that signal false food cues are pollinated by female social wasps. Food deception wasp pollination has been reported for a few orchid species Coelogyne fimbriata Lindl., Dendrobium sinense Tang & F.T. Wang, and Steveniella satyrioides (Steven) Schltr. (Nazarov 1995; Brodmann et al. 2009; Cheng et al. 2009; Fateryga, Ivanov, and Fateryga 2013). Despite this mechanism being the most common form of deceptive pollination in orchids that exploit bees (Jersáková, Johnson, and Kindlmann 2006; Gaskett 2011).
The difference in the number of orchid species that exploit these two groups of insects may be related to the type of resources sought by bees and wasps. Bees generally visit orchid flowers for floral resources such as nectar, oil and pollen. Thus, they are likely under strong selective pressure to respond to general floral resource signals, such as inflorescence shape, flower colour, fragrance and nectar guides (Jersáková, Johnson, and Kindlmann 2006). On the other hand, wasps forage for nectar for adult insect nourishment and arthropods to provision their larvae. The interest in such contrasting resources would require more complex signalling by the plant, which would only be selected for situations where the benefits outweighed signalling costs. Indeed, orchids that exploit wasps through food deception emit signals that mimic the presence of prey (Nazarov 1995; Brodmann et al. 2009) and floral resources for the adult individual (Cheng et al. 2009). Thus, not all cases of food deception pollination in wasps fit the definition of generalised food deception (sensu Jersáková, Johnson, and Kindlmann 2006), which is based on signalling general floral resources. Nazarov (1995) defines the mechanism of food deception in wasps as the ‘false prey syndrome’; however, this term has not been mentioned in more recent reviews (Jersáková, Johnson, and Kindlmann 2006).
The first report of food deception pollination involving wasps was in Steveniella satyrioides (Steven) Schltr. in Crimea (Nazarov 1995). This resource-less orchid is pollinated by two wasp species, Paravespula vulgaris (Linnaeus, 1758) and Dolichovespula sylvestris (Scopoli, 1763). The base of the floral labellum has a reddish-brown colouration that likely mimics a piece of animal prey. The wasps of these two species bite the base of the labellum, apparently trying to remove tissue fragments. In this process, they press against the flower's column, causing the pollinia to adhere to the face of the insect. Additionally, they seem to search for nectar by inserting their heads into the flower's spur. Another example is Coelogyne fimbriata Lindl., which has flowers with a green-yellow colouration typical of wasp-pollinated orchids, but they do not produce nectar or any other resource. This species appears to exploit the high carbohydrate demand of social wasps by emitting floral fragrances that mimic the scent of carbohydrate sources such as fruits and flowers of other plants (Cheng et al. 2009). An example of the complexity of food deception pollination is the pollinator attraction mechanism of Dendrobium sinense Tang & F.T. Wang, an orchid species endemic to Hainan Island, China. The flowers of D. sinense emit floral fragrances and compounds typically present in the alarm pheromones of Asian honeybees (Apis cerana Fabricius, 1793) and European honeybees (A. mellifera Linnaeus, 1758). These compounds are attractive to the social wasp Vespa bicolor Fabricius, 1787 females, which act as pollinators. Since V. bicolor females frequently hunt bees to feed their larvae, it is suggested that the flowers of D. sinense mimic the alarm pheromones of honeybees to attract predatory wasps that would act as pollinators (Brodmann et al. 2009). Indeed, Brodmann et al. (2009) observed that these wasps do not land on the flowers as other pollinating insects do; instead, they make swift strikes at the red centre of the floral labellum, resembling the behaviour of attacking prey. During these attacks on the flowers, the pollinia are deposited on the pronotum of the insect.
4 Specialised Pollinators on Resource-Rich Plants
Although relatively underrepresented in species number, some groups of wasps that pollinate resource-rich flowers exhibit extremely specialised adaptations related to pollination. Although most species of solitary wasps use animal prey to feed their larvae, pollinating wasps of the subfamily Masarinae (Vespidae) exhibit morphological and behavioural adaptations for collecting nectar and pollen to provision their nests, resembling the feeding regime of solitary bees in several aspects (Gess 1996). The fig wasps (Agaonidae) exemplify highly specialised pollinators, exclusively pollinating plants of the genus Ficus. The primary resource offered is the site for offspring development, positioning them as the most specialised group among pollinating wasps (Weiblen 2002). The following sections will discuss these three groups of pollinating wasps in greater detail.
4.1 Flower Wasps
Flower wasp is a common name for the subfamily Thynninae (Hymenoptera: Thynnidae). These wasps are relevant yet underappreciated in plant pollination, particularly in Australian ecosystems. In Australia, approximately 600 flower wasp species are described across 48 genera, with an estimated 2000 species currently represented in collections, including many new genera (Brown and Phillips 2014).
The biology of the flower wasps remains largely underinvestigated and poorly understood. Courtship is initiated by the female releasing pheromones that rapidly attract males. Copulation occurs in flight, with the female curled underneath the male's abdomen. Since female flower wasps are flightless, they rely on males for food during these courtship flights. Coupling can last up to 2 days in captivity. Food provision may occur via regurgitation or by the male flying the female to a food source while mating continues. After mating, the female is dropped to the ground, where she parasitises subterranean scarab larvae (Alcock and Gwynne 1987; Peakall 1990; Brown and Phillips 2014).
These wasps exhibit diverse feeding behaviours, primarily visiting nectar-rich flowers of the Myrtaceae family, such as Eucalyptus, Leptospermum and Chamelaucium, which facilitate pollination (Phillips et al. 2009; Menz et al. 2013; Brown and Phillips 2014). Although there was an apparent preference for myrtacean plants, they were also observed feeding on Xanthorrheaceae and Proteaceae species (Phillips et al. 2009). Their pollination activities extend beyond nectar foraging. Thynnine wasps are pollinators of some sexually deceptive orchids (Peakall 1990; Reiter 2002; Reiter et al. 2018, 2019; Phillips et al. 2020). These specialised interactions highlight the ecological importance of thynnine wasps in plant pollination.
4.2 Hemipepsis Wasps
Species of the genus Hemipepsis (Pompilidae) pollinate flowers with relatively unspecialised morphology, obtaining nectar as a resource. However, the mechanism of attracting these insects through volatile substances is highly specialised, making them exclusive pollinators of certain species. Some species in the families Apocynaceae (Asclepiadoideae), Asparagaceae and Orchidaceae in South Africa form a guild with a high level of functional specialisation. Most of its members are exclusively pollinated by Hemipepsis wasps (Table 2). The species in this guild have flowers with relatively unspecialised morphology, featuring pale greenish or white-brownish colouration, always with reddish spots, a sweet-acrid scent and exposed nectar (Shuttleworth and Johnson 2012).
| Family | Genus | No of species |
|---|---|---|
| Apocynaceae Asclepiadoideae | Asclepias | 1 |
| Aspidoglossum | 1 | |
| Miraglossum | 3 | |
| Pachycarpus | 6 | |
| Periglossum | 1 | |
| Xysmalobium | 3 | |
| Woodia | 2 | |
| Asparagaceae | Eucomis | 3 |
| Orchidaceae | Disa | 2 |
| Satyrium | 1 |
As the nectar is exposed in Hemipepsis-pollinated flowers, it is potentially accessible to other groups of floral visitors. However, the pollinating wasps are selectively attracted by the floral fragrances. Field and Y-tube choice experiments have demonstrated that the pollinators of Pachycarpus grandiflorus (L. f.) E. Mey. and Eucomis spp. are attracted by floral scent rather than visual cues (Shuttleworth and Johnson 2009a, 2009b). The palatability of the nectar, at least in some species of Apocynaceae, plays a role in the selection of pollinator species. In experiments conducted by Shuttleworth & Johnson (2006, 2009c c), nectar from Pachycarpus and a sugar solution of the same concentration were offered to bees (A. mellifera). The bees consumed the sugar solution but rejected the nectar, suggesting that the nectar composition acts as a filter for floral visitors. However, other Apocynaceae species in the genus Xysmalobium, also pollinated by Hemipepsis wasps, appear to have nectar that is more palatable to other insects (Shuttleworth and Johnson 2009b). Indeed, Xysmalobium is visited by a broader range of non-pollinating insects, reinforcing the hypothesis that nectar's unpalatability is important in limiting visits from nectar robbers (Shuttleworth and Johnson 2009c).
Hemipepsis wasps constitute an excellent model for studying the role of non-morphological traits in the specialisation of pollination systems, contrasting with cases where specialisation occurs through floral morphological traits. The latter cases are exemplified by the flies Moegistorhynchus longirostris (Wiedemann, 1819), which have the longest proboscides among dipterans (60 to 100 mm in length) and exclusively pollinate plants with tubular flowers of corresponding size (Johnson and Steiner 2000). Therefore, investigating the role of chemical substances in pollinator selection in wasp-pollinated plants is a promising field and may reveal unnoticed specialisations in systems considered generalist based solely on floral morphology and visitation patterns.
4.3 Pollen Wasps
Within Vespidae, the subfamily Masarinae (‘masarine’) is the group most closely associated with flowering plants and is likely the principal pollinator for several species. The morphology and behaviour of pollen wasps are generally compatible with the shape of the flowers they visit and are potentially suitable for effective pollination (Gess 1996). However, this group of insects has been largely neglected in formal studies of pollination biology. Their role as potential pollinators is inferred from indirect information on floral visitation and the presence of pollen on their bodies. Thus, the pollination mechanism by these wasps remains an open field for investigation.
Masarinae is distributed globally between latitudes 50° N and S, except in the eastern parts of North America and Asia's eastern and southern regions. The records are concentrated in the Mediterranean, temperate, hot semi-arid and arid areas outside the tropical zone. These wasps are thus found in warmer climates with relatively low rainfall, dominated by open and tufted vegetation. The subfamily is divided into two tribes, Gayellini, restricted to the Neotropical region, and Masarini, with a broader distribution in the Nearctic, Neotropical, Palearctic, Afrotropical and Australian regions (Gess 1992). About 300 species of Masarinae are described, with half being endemic to southern Africa. Twenty-three species are known in the Neotropics, including four genera (Masarini: Ceramiopsis and Trimeria; Gayellini: Gayella and Paramasaris) (Hermes and Garcete-Barrett 2009).
Masarinae are solitary wasps with complete metamorphosis. The stages from egg to pupa are confined in cells within multicellular nests, either excavated in the soil or constructed externally on rocks, plant branches, or pre-existing holes. However, some species of the genus Quartinia build nests inside snail shells (Mollusca: Gastropoda). The wasps use water, nectar, or silk as cement for nest construction, making many species water collectors. Once the nest is constructed, the female deposits one egg per cell and then provisions it with a macerated mixture of nectar and pollen to serve as food for the larva. The prepupal stage enters diapause, and the transition to pupal and adult stages occurs the following spring or summer, potentially extending over several years in some cases (Gess 1996; Gess and Gess 2010). These wasps represent the only group within the family Vespidae that collects pollen and nectar to feed their larvae. In this aspect, they are considered functionally equivalent to solitary bees, as the habit of provisioning larvae with pollen and nectar represents a convergent evolution in both groups. The pollen used to feed the offspring is transported in the crop of the insect and, therefore, is not available for pollination. Consequently, the pollen adhered to the wasp's body is responsible for the pollination.
Pollen wasps are highly adapted for foraging floral nectar. While wasps generally have relatively short tongues, most masarine wasps have relatively long tongues, some as long as the length of their bodies. Consequently, they can access nectar from a wide range of flower forms (Gess 1996). Regarding the plants they utilise, pollen wasps are generally more oligophagous (i.e., they use a restricted number of plant groups) than bees and other wasp groups (Gess and Gess 2004). An extreme case occurs with a species of Crassulaceae endemic to South Africa, Tylecodon hallii (Tolken) Tolken. This species appears to be visited exclusively by Masarina tylecodoni Gess, Gess, and Gess 1997, which exhibits morphology and behaviour compatible with pollination (Gess, Gess, and Gess 1997).
Pollination by masarine wasps falls under the melittophily syndrome. However, flowers frequently visited by pollen wasps are not equally associated with bees (Gess and Gess 2004), suggesting that these flowers possess specific traits related to pollen wasp visitation. The spectrum of flower forms preferentially utilised by masarine wasps is relatively broad due to the diversity of species visited. However, some general characteristics can be recognised: diurnal anthesis; light colouration; sweet odour (no visits were recorded to flowers with fruity or putrid odours); generally tubular shape, with flowers either solitary or aggregated in capitula (e.g., Asteraceae) or highly differentiated (e.g., Leguminosae); and dilute nectar that is hidden (protected from evaporation) within the flower (Gess 1996).
The plant families preferentially associated with pollen wasps vary according to biogeographic regions (Gess 1992) – Australasia: Myrtaceae and Goodeniaceae; Nearctic: Scrophulariaceae and Hydrophyllaceae; and Afrotropic: Aizoaceae, Asteraceae, Campanulaceae, Scrophulariaceae and Leguminosae (tribe Crotalarieae). The greater diversity in the Afrotropic region is likely due to the higher concentration of studies in this area. Data from the Neotropical and Palearctic regions are scarce, but there are records in Verbenaceae, Asteraceae and Leguminosae in the Neotropics and Asteraceae in the Palearctic (Gess 1992; Mechi 1999).
4.4 Fig Wasps
In some plant groups, the reward to pollinators is providing a site for oviposition and offspring development. This interaction is called brood-site mutualism, sometimes referred to as nursery pollination (Willmer 2011). Brood-site pollination is known in 12 angiosperm families and one gymnosperm family (Dufaÿ and Anstett 2003). Although widely distributed among plant groups, the oviposition site as a resource is best known in Ficus (Moraceae) and Yucca (Agavaceae) (Baker 1986). Insects of the orders Coleoptera and Lepidoptera are the most frequent pollinators in this type of mutualism, associating with eight plant families (Dufaÿ and Anstett 2003). In the order Hymenoptera, the family Agaonidae is the only reported group involved in this pollination mechanism, comprising the exclusive pollinators of fig trees.
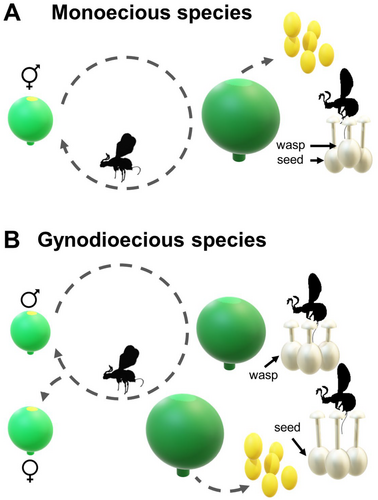
Ficus (Moraceae) encompasses approximately 800 described species with a pantropical distribution. One or a few wasp species pollinate each Ficus species (Ramírez 1970; Kjellberg et al. 2005). The genus is characterised by a globose inflorescence called a syconium (or fig), where the flowers are embedded. Approximately half of the Ficus species are monoecious, with pistillate and staminate flowers occurring in the same fig. The other species are structurally gynodioecious but functionally dioecious (Kjellberg et al. 2005). In monoecious species, pistillate flowers are arranged more compactly within the syconium, with ovaries layered. Since the stigmas of the flowers are at the same level in the syconium cavity, the flowers with ovaries closer to the fig wall have long styles, while those near the cavity have shorter styles. The floral arrangement is less complex in functionally dioecious species, forming a single layer of flowers (Verkerke 1988, 1989; Basso-Alves et al. 2014).
The pollination mechanism varies slightly between monoecious and dioecious species but shares common basic characteristics. In monoecious species (Figure 2A), fertilised female pollinators loaded with pollen are attracted by volatile substances released by receptive syconia (Cornille et al. 2012; Proffit et al. 2018, 2020). The wasps enter the inflorescence through an opening called the ostiole, pollinate the pistillate flowers and preferentially lay eggs in the ovaries of the short-styled flowers, inserting the ovipositor through the floral style. The flowers that receive eggs form galls, where the wasp offspring develop instead of fruit. The fruits are generally produced by the long-styled flowers. A few weeks later, shortly before the syconium matures, the wasp offspring complete their development. The first to emerge are the wingless males, whose activities are confined to the inside of the syconium. The males locate and copulate with the females, still inside their galls. The fertilised females then emerge, collect pollen (in some species, females do not collect pollen but transport it passively on their bodies) and leave the syconium searching for another tree with receptive syconia (Galil and Eisikowitch 1968). Subsequently, the syconia complete their maturation, becoming attractive to various species of frugivorous vertebrates that act as dispersers (Shanahan et al. 2001). The figs not consumed by these vertebrates fall to the ground and serve as a food resource for several insect groups to develop or complete their development (Palmieri and Pereira 2018).
Agaonid wasps regularly disperse pollen over surprisingly longer distances than typical insect-pollinated plants. Studies have shown that the average pollination distances within a fig population can reach several tens of kilometres (Compton et al. 1988; Nason, Herre, and Hamrick 1996; Ahmed et al. 2009; Nazareno and Carvalho 2009). In monoecious Ficus species, long-distance pollen dispersal is facilitated by wind. Collections of aerial plankton indicate that wasps pollinating these species are carried by the wind above the forest canopy over extensive distances (Compton et al. 2000; Harrison and Rasplus 2006). Upon detecting the scent plume from a receptive fig tree, a wasp descends from the main wind current and flies upwind to the tree (Ware and Compton 1994). The fig–fig wasp mutualism exemplifies a unique innovation in pollen dispersal by combining initial wind dispersal of the wasps with subsequent chemotaxis, enabling cross-pollination at remarkably low population densities (Ware and Compton 1994).
Functionally dioecious species (Figure 2B) have plants with figs that contain only pistillate flowers (functionally female plants). When a wasp enters such a fig, it deposits pollen (actively or passively, depending on the species). Still, it cannot lay its eggs because these flowers have long styles that prevent the ovipositor from reaching the appropriate location in the ovary. Thus, the flowers in the syconia of female plants are pollinated by deception, as the wasps are attracted by false resource signals. On the other hand, pollen production occurs in functionally male plants, which have figs containing pistillate and staminate flowers. These plants, although hermaphroditic, function solely in pollen production as their flowers do not produce seeds. The styles are short, allowing the deposition of eggs in the ovaries of all the flowers. The completion of wasp offspring development coincides with the maturation of the staminate flowers (as described for monoecious species), ensuring that the newly emerged wasps carry pollen to other plants. Notably, some of these wasps will find male trees and successfully reproduce, while others will encounter female plants, where they will only perform pollination (Weiblen, Yu, and West 2001).
Mimicry between the scents emitted by male and female plants makes it difficult for pollinating fig wasps to distinguish between resource-rich and resource-less figs. Studies have shown that male and female figs emit a similar blend of volatile organic compounds during the receptive phase (Hossaert-McKey et al. 2016; Chen et al. 2022). This intersexual mimicry in odour profiles is crucial for maintaining the mutualistic relationship between figs and their pollinating wasps. This strategy minimises the risk of wasps selectively visiting only male figs, which could lead to reproductive failures in female figs. In some gynodioecious Ficus species, there is likely minimal selective pressure for wasps to evolve traits that enable them to avoid female figs. This is because male and female trees do not flower simultaneously throughout the year (Kjellberg et al. 1987). As a result, wasps emerging from figs on male trees often encounter no receptive male figs when they emerge, leaving them no choice but to pollinate the female figs. In species where male and female trees flower simultaneously, the competitive pressure to quickly enter receptive figs (Conchou et al. 2014) selects against time-consuming behaviours, such as distinguishing between highly similar male and female fig scents. Wasps that hesitate to differentiate between the scents may miss the chance to enter a fig. This dynamic likely reduces the selective pressure for wasps to develop the ability to avoid female figs, maintaining the scent mimicry between male and female figs (Pereira and Kjellberg 2021).
Most species of fig wasps actively pollinate flowers due to morphological and behavioural adaptations. Active pollinating wasps possess pocket-like structures on their thorax for storing pollen actively collected from the anthers (Galil and Eisikowitch 1969; Ramírez 1969). Additionally, these wasps have comb-like setae on their hind coxae used to manipulate pollen during collection (Kjellberg et al. 2001). When entering a fig with receptive flowers, the pollinators use their hind legs to remove pollen from the thoracic pockets and deposit it onto the floral stigmas. Subsequently, the wasp inserts its ovipositor through the style and lays an egg in the flower's ovary (Kjellberg et al. 2001). The pollinator usually lays eggs in the flowers it has pollinated, leading to the development of galls (Jousselin and Kjellberg 2001). Seed production in monoecious fig trees occurs in the flowers with longer styles, where the wasp avoids laying eggs (Anstett 2001), or in nearby flowers due to the behaviour of pollen tubes that, during their growth, reach adjacent flowers (Jousselin and Kjellberg 2001). This behaviour is facilitated by the spatial arrangement of stigmas in actively pollinated syconia, which form a synstigma, a more or less cohesive platform (Teixeira et al. 2018, 2021).
Approximately one-third of fig wasp species pollinate flowers passively, lacking the morphological and behavioural adaptations seen in active pollinators. In passively pollinated species, the number of staminate flowers is greater, causing pollen to be distributed throughout the syconium's interior and covering the bodies of the wasps as they emerge from their galls. This difference is reflected in the anther-to-ovule ratio of syconia, which averages 0.6 in passively pollinated species and 0.08 in actively pollinated species (Kjellberg et al. 2001). The production of wasps and seeds in syconia of monoecious species pollinated passively is determined by the morphological specialisation of the styles and stigmas of the flowers, as observed in species of the section Pharmacosycea (Jousselin, Kjellberg, and Herre 2004; Teixeira et al. 2018). The short-styled flowers, whose ovaries are closer to the fig lumen, have a flat stigma and are preferentially used by wasps for egg deposition. In contrast, the long-styled flowers (ovaries closer to the fig wall) are preferentially pollinated and are more specialised for seed production. These flowers have a bifurcated stigma, with elongated branches that project like a brush into the interior of the fig, facilitating the passive transfer of pollen from the wasp's body to the stigmatic surface.
In addition to the agaonid pollinating wasps, many species from other chalcid families (e.g., Eurytomidae, Pteromalidae and Torymidae) that are gall-inducers, kleptoparasites, or parasitoids (Pereira, Teixeira, and Kjellberg 2007; Borges 2015; Elias et al. 2018; Silva and Pereira 2018) also utilise figs as a resource. These wasps mainly oviposit from outside the figs, but a few species, like the pollinators, enter the figs to oviposit. Internal-ovipositing species of the genera Diaziella and Lipothymus (Pteromalidae), when associated with passively pollinated Ficus species, act as supplementary pollinators as they transport substantial amounts of pollen on their body surface (Jousselin, Rasplus, and Kjellberg 2001; Zhang and Yang 2017).
In many cases, oviposition sites are a relatively high-cost resource for plants, especially in Ficus and Yucca, where plants offer ovaries to develop the pollinator's offspring. The cost to the plant in these systems directly affects its reproductive success, as the development of the pollinator's progeny occurs at the expense of ovaries that could produce seeds. The interaction cost may be high for the pollinator if it involves investment in specialised behaviours, such as active pollination. It is questioned how the active pollination behaviour evolved and is maintained in most species of fig wasps, considering that passive pollination would not incur costs related to pollen collection and deposition. The answer likely lies in the quality of the resource for offspring development. Experimental studies in actively pollinated fig trees have shown that larval mortality of wasps is higher in unpollinated syconia (Jousselin et al. 2003; Tarachai, Compton, and Trisonthi 2008). For the fig tree, the evolution of active pollination was a key innovation that likely conferred evolutionary advantages. Actively pollinated Ficus species diversified more rapidly and exhibited lower extinction rates than those pollinated passively (Bruun-Lund et al. 2018).
A study on the larval development and gall formation of an actively pollinated species, Ficus citrifolia Mill., revealed the mechanism that favours the larval development of the pollinator Pegoscapus aerumnosus (Grandi, 1938) in pollinated flowers. During the gall development, P. aerumnosus larvae exhibit two contrasting feeding strategies. In the first two developmental stages, the larvae behave as parasites of the flower ovary, staying close to the site of egg deposition (near the entrance of the stylar canal) and feeding on the nucellus. During the transition from the second to the third instar, the larva migrates to the micropylar region, where the plant embryo is located, and begins feeding on the hypertrophied endosperm. At this stage, the plant embryo disappears, likely consumed by the larva. Thus, the larvae of actively pollinating wasps appear to depend on the plant's embryogenesis, particularly the endosperm resulting from fertilisation (Jansen-Gonzalez, Teixeira, and Pereira 2012). This dependency explains why pollination may result in the production of galls with higher nutritional quality. This hypothesis is also supported by observations of similar coordination between larval development and plant embryogenesis in other groups of seed-feeding chalcid wasps (Jansen-González, Teixeira, and Pereira 2020).
The evolution of the Ficus–wasp mutualism remains a subject of debate. The development of phylogenetic reconstruction techniques based on molecular data at the end of the 20th century enabled the formal investigation of evolutionary processes within this mutualism, particularly in attempts to determine whether the Ficus–wasp pollinator interaction arose through coevolutionary processes (Lopez-Vaamonde et al. 2001; Machado et al. 2001; Jousselin et al. 2003; Weiblen 2004). Comparisons of the phylogenies of Ficus species and their pollinating wasps revealed some incongruities in terminal branches and divergence times between the two groups, thus not fully supporting the hypothesis of strict coevolution (Weiblen 2004; Cruaud et al. 2012). However, on a broader scale, there is a strong correspondence in the divergence of genera of pollinating wasps and sections or subsections of Ficus, suggesting that plants and insects codiversified (Cruaud et al. 2012). Indeed, each section of Ficus is generally pollinated by a particular genus of Agaonidae.
Multiple lines of evidence, including biogeography, diversification patterns and fossil records, indicate that fig trees and fig wasps originated in Eurasia approximately 75 million years ago and subsequently dispersed and diversified on other continents (Cruaud et al. 2012). The diversification of hemiepiphytic figs (subgenus Spherosuke) exemplifies the codiversification scenario within the genus. The ancestor of hemiepiphytic figs likely emerged around 50 million years ago. One clade, probably occurring in Eurasia, dispersed to India and Southeast Asia, giving rise to the section Conosycea, and Australasia, forming the section Malvanthera approximately 50–43 million years ago. Another clade dispersed to Africa, originating the section Platyphyllae (former Galoglychia), and to South America, forming the section Americanae around 38–32 million years ago (Cruaud et al. 2012). The codiversification in this plant–insect mutualism opened new evolutionary opportunities, influencing other groups of organisms. This highlights the significant role of fig trees in tropical ecosystems. They provide food and shelter to nearly all terrestrial animals, including birds, mammals, reptiles, insects, mites and nematodes (Greeff and Whiting 1999; Pereira, Semir, and Menezes Jr 2000; Walter 2000; Shanahan et al. 2001; Palmieri and Pereira 2018; Jauharlina et al. 2022).
5 Ecological and Economic Implications of Wasp Pollination
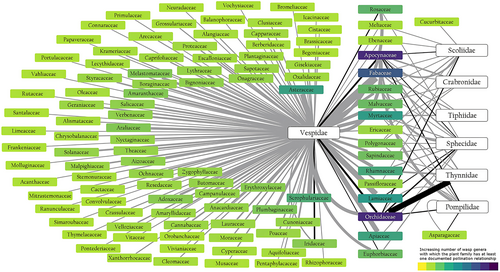
Wasps play a crucial ecological role in both wild and cultivated plants, not only by facilitating pollination but also by providing pest control services through predating phytophagous insects (Holm 2021). A comprehensive literature review on ecosystem services provided by aculeate wasps (Brock, Cini, and Sumner 2021) identified 960 plant species associated with pollinating or potentially pollinating aculeate wasps (Figure 3). These interactions include records of plants that depend exclusively on wasps for pollination (obligate pollination) and cases where wasps act as potentially facultative pollinators. These records do not include non-aculeate wasps, such as Agaonidae and Ichneumonidae, which are obligate pollinators of Ficus and Orchidaceae, respectively (Weinstein et al. 2016; Pereira and Kjellberg 2021). According to the review, obligate pollination by aculeate wasps has been documented in six plant families: Apocynaceae, Asparagaceae, Euphorbiaceae, Iridaceae, Orchidaceae and Rosaceae, covering 164 plant species. Five aculeate wasp families (i.e., Pompilidae, Scoliidae, Sphecidae, Thynnidae and Vespidae) are reported as obligate pollinators, representing 122 wasp species (Table 3).
| Plant family | Wasp family | N° of wasp species |
|---|---|---|
| Apocynaceae | Pompilidae | 7 |
| Scoliidae | 1 | |
| Sphecidae | 2 | |
| Vespidae | 8 | |
| Asparagaceae | Pompilidae | 1 |
| Euphorbiaceae | Vespidae | 1 |
| Iridaceae | Vespidae | 4 |
| Orchidaceae | Pompilidae | 3 |
| Scoliidae | 5 | |
| Sphecidae | 4 | |
| Thynnidae | 72 | |
| Vespidae | 12 | |
| Rosaceae | Vespidae | 2 |
Regarding the potentially facultative pollinators, Brock, Cini, and Sumner (2021) identified 602 species from nine aculeate wasp families – Chrysididae, Crabronidae, Pompilidae, Sapygidae, Scoliidae, Sphecidae, Thynnidae, Tiphiidae and Vespidae – that are associated with 798 plant species across 110 families (Figure 3). While not specialised pollinators, these wasps contribute to pollination by visiting flowers for nectar and occasionally facilitating pollen transfer. The presence of wasps in such a wide diversity of plant families highlights their ecological importance and potential contribution to pollination networks, particularly in environments where specialised pollinators are less prevalent.
From an economic perspective, wasps are reported as secondary pollinators for several commercial crops. Although they are not the primary pollinators, they have been recorded as potential pollinators of crops such as apples, cucumbers, mustard, onions, peaches, pears, pumpkins, Spanish broom, sunflowers and tomatoes (Akhter, Khanday, and Ahmad 2016). Additionally, agaonid wasps are the sole pollinators of the cultivated fig (Ficus carica L.) in many regions of the world, including the Mediterranean, parts of North Africa and California in the United States. The edible fig, commonly known as Smyrna fig, depends on wasp pollination for fruit development (Condit 1947; Eisikowitch et al. 2022). However, many parthenocarpic varieties have also been artificially selected, which do not require pollination to produce figs. These parthenocarpic varieties develop drupelets without fertilisation, and since they do not produce seeds, their propagation relies on stem cuttings (Achtak et al. 2010).
6 Conclusions
Wasps stand out for their involvement in various pollination mechanisms, playing a crucial role in the reproduction of many wild and cultivated plant species. For didactic purposes, pollinating wasps can be classified into generalist pollinators, pollinators specialised in resource-less plants (deceptive pollination), and pollinators specialised in resource-rich plants. When provided, the rewards offered to pollinators include nectar for the adult insect's nourishment (social wasps and solitary thynnine wasps), nectar + pollen for the offspring's nourishment (pollen wasps), or a site for offspring development (fig wasps). In more specialised pollination systems, plants seem to exploit the well-developed olfactory capabilities of wasps through long-distance attractive floral fragrances. The pollination of fig trees by Agaonidae wasps represents an extreme case of plant-pollinator codiversification. This interaction exhibits a unique innovation in pollen dispersal (wind transport + wasp chemotaxis), which opened evolutionary opportunities, enabling fig trees to disperse exceptionally across all continents.
Author Contributions
Rodrigo Augusto Santinelo Pereira: conceptualization, methodology, writing – original draft, writing – review and editing, data curation, funding acquisition.
Acknowledgements
I thank Simone P. Teixeira (FCFRP-USP) for her constructive comments on the manuscript.
Conflicts of Interest
The author declares no conflicts of interest.
Open Research
Data Availability Statement
The author has nothing to report.




