Fine-tuning the composition of the cranberry weevil (Coleoptera: Curculionidae) aggregation pheromone
Abstract
The cranberry weevil Anthonomus musculus Say is a key pest of highbush blueberries (Vaccinium corymbosum L.) and cranberries (Vaccinium macrocarpon Aiton) in the northeastern United States. Previous studies have reported A. musculus adult attraction to traps baited with the aggregation pheromone of the pepper weevil Anthonomus eugenii Cano, likely because these two weevils share similar pheromone blends that differ only in two components. The A. musculus aggregation pheromone contains (Z)-2-(3,3-dimethylcyclohexylidene) ethanol (Z grandlure II), (Z)-(3,3-dimethylcyclohexylidene) acetaldehyde (grandlure III), (E)-(3,3-dimethylcyclohexylidene) acetaldehyde (grandlure IV) and (E)-3,7-dimethyl-2,6-octadien-1-ol (geraniol); whereas A. eugenii produces a pheromone blend that includes (E)-2-(3,3-dimethylcyclohexylidene) ethanol (E grandlure II) and (E)-3,7-dimethyl-2,6-octadienoic acid (geranic acid) in addition to the four A. musculus pheromone components. Here, we hypothesized that differences in pheromone composition between these two species influence A. musculus adult attraction to its aggregation pheromone. To test this, we studied the response of A. musculus to its pheromone blend with and without E grandlure II and geranic acid, a commercial A. eugenii pheromone lure and a no-lure control in highbush blueberry and cranberry fields in New Jersey and Massachusetts, respectively. Regardless of crop type, A. musculus adults were more attracted to their four-component pheromone blend and the blend plus geranic acid than the commercial A. eugenii pheromone and the no-lure controls. The A. musculus pheromone blend plus E grandlure II and the A. eugenii pheromone blend also captured more A. musculus adults than the no-lure control but not compared to the commercial A. eugenii pheromone. Further analysis showed that A. musculus adults are significantly (~27%) less attracted to their pheromone blend if it contains E grandlure II, although the addition of geranic acid did not affect their response. These findings may help guide future efforts towards the development of behaviour-based tools to monitor and manage A. musculus.
1 INTRODUCTION
The cranberry weevil Anthonomus musculus Say (Coleoptera: Curculionidae) is a key pest of highbush blueberries (Vaccinium corymbosum L.) and cranberries (Vaccinium macrocarpon Aiton) (Ericaceae) in the northeastern United States (Averill & Sylvia, 1998; Long & Averill, 2003; Marucci, 1966). In spring, overwintered adults move into fields from surrounding wooded areas and start feeding on flower and leaf buds, females lay eggs inside flower buds and larvae consume flowers; infested flower buds darken and fail to open, resulting in less fruit set (Szendrei & Rodriguez-Saona, 2009; Rodriquez-Saona & Polk, 2017).
Currently, overwintered A. musculus adults are monitored from bud break until bloom using beating tray and sweep-net sampling in blueberry and cranberry, respectively, which can be time consuming and labour intensive. A pheromone-based trapping system could provide an economic, easy-to-use alternative. The A. musculus aggregation pheromone includes (Z)-2-(3,3-dimethylcyclohexylidene) ethanol (Z grandlure II), (Z)-(3,3-dimethylcyclohexylidene) acetaldehyde (grandlure III), (E)-(3,3-dimethylcyclohexylidene) acetaldehyde (grandlure IV) and (E)-3,7-dimethyl-2,6-octadien-1-ol (geraniol) (Szendrei, Averill, Alborn, & Rodriguez-Saona, 2011). In contrast, the pepper weevil, Anthonomus eugenii Cano, aggregation pheromone contains (E)-2-(3,3-dimethylcyclohexylidene) ethanol (E grandlure II) and (E)-3,7-dimethyl-2,6-octadienoic acid (geranic acid) plus the above A. musculus pheromone components (Eller et al., 1994). Despite differences in pheromone blend composition between these species, field studies reported that A. musculus adults are captured as bycatch on traps baited with the A. eugenii aggregation pheromone (Ingerson-Mahar, Eichinger, & Holmstrom, 2015; Szendrei et al., 2011). Szendrei et al. (2011) caught A. musculus adults on yellow sticky cards baited with the A. eugenii pheromone in highbush blueberry fields in New Jersey. Yellow sticky cards captured more A. musculus adults than any other traps designed for weevils (Silva, Salamanca, Kyryczenko-Roth, Alborn, & Rodriguez-Saona, 2018).
The study by Szendrei et al. (2011) did not rule out the presence of other minor components in the A. musculus pheromone blend. In fact, further analysis of A. musculus adult emissions indicated that there are two previously undetected minor compounds (an acid similar to nerolic/geranic acid and an alcohol similar to nerol/geraniol, neither of which could be identified as known Anthonomus pheromone components) released by males, which might have behavioural significance (unpublished data). To determine the role of additional pheromone components, we conducted field studies in New Jersey highbush blueberries and Massachusetts cranberries to test A. musculus adult attraction to yellow sticky cards baited with its aggregation pheromone blend with or without the addition of E grandlure II and geranic acid.
2 MATERIALS AND METHODS
2.1 Study sites and experimental design
Field experiments were conducted in 2014 to test adult A. musculus attraction to blends containing A. musculus and A. eugenii aggregation pheromone components. The experiments were done in six commercial farms: three in highbush blueberries in New Jersey (one in Burlington County [39°51′20″N, 74°41′12″W] and two in Atlantic County [39°32′00″N, 74°41′12″W]) and three in cranberries in Massachusetts (Plymouth County [41°54′43″N, 70°43′00″W]). Yellow sticky cards (23 × 28 cm Pherocon AM No-Bait; Trécé Inc.) (Silva et al., 2018) were baited with one of six treatments. We used a double-split plot design with plot nested within site (farm) nested within crop, with three (blueberry) or four (cranberry) replications (plots) at each of the six farms (N = 21 plots). Treatments (lures + cards) were rotated weekly in each plot. In both crops, cards within plots were placed 10 m apart along the field edge facing wooded borders and hung from a metal pole, as described by Szendrei et al. (2011). In blueberries, cards were placed at mid-canopy level (~1 to 1.5 m aboveground) according to Silva et al. (2018). In cranberries, cards were placed just off the bog in the grassy ditch edge. Cards were checked weekly between early-April and mid-June (11 weeks) in blueberries and in late-May until mid-June (3 weeks) in cranberries. Lures were attached to the cards by using 22-gauge green florist wire and, on blueberry, were replaced with new lures every 3 weeks; lures were not replaced in cranberries. Captured weevils were removed and counted; sexes were not separated because Szendrei et al. (2011) showed no differences between A. musculus male and female responses to their aggregation pheromone.
2.2 Treatments
A list of treatments is presented in Table 1. These included: (a) a blend containing the four A. musculus aggregation pheromone components (treatment 1); (b) treatment 1 + geranic acid; (c) treatment 1 + E grandlure II; (d) treatment 1 + geranic acid and E grandlure II (= A. eugenii aggregation pheromone); (e) a commercial A. eugenii pheromone lure (PHEROCON® Pepper Weevil Dual Lure; Trécé Inc.); and (f) an unbaited (no-lure) control. All custom-made lures (treatments 1–4) were formulated by ChemTica Internacional S.A. at ratios that mimicked the A. eugenii pheromone (Table 1). Lures were loaded at 30 mg per lure and had a release rate of ca. 1 mg/day (Szendrei et al., 2011). Chemicals were used neat in a hermetically sealed sachet of proprietary semipermeable membrane. E grandlure II, Z grandlure II and grandlure III and IV mixture (1:1) were purchased from Bedoukian Research Inc., geraniol was purchased from Aldrich Chemical Co., and geranic acid was produced by ChemTica Internacional S.A.
| Treatment no. | Compound | Ratio | Amount (mg/lure) | Purity |
|---|---|---|---|---|
|
1a (A. musculus pheromone) |
Grandlure II Isomer Z | 22.6 | 26.59 | ≥95% |
| Grandlure II Isomer E | 0.0 | 0.0 | — | |
| Grandlure III & IVb | 1.9 | 2.24 | ≥95% | |
| Geraniol | 1.0 | 1.18 | ≥95% | |
| Geranic Acid | 0.0 | 0.0 | — | |
|
2 (A. musculus pheromone + geranic acid) |
Grandlure II Isomer Z | 24.1 | 21.08 | ≥95% |
| Grandlure II Isomer E | 0.0 | 0.0 | — | |
| Grandlure III & IV | 2.1 | 1.84 | ≥95% | |
| Geraniol | 1.0 | 0.87 | ≥95% | |
| Geranic Acid | 7.1 | 6.21 | ≥95% | |
|
3 (A. musculus pheromone + E grandlure II) |
Grandlure II Isomer Z | 24.0 | 16.82 | ≥95% |
| Grandlure II Isomer E | 15.8 | 11.07 | ≥95% | |
| Grandlure III & IV | 2.0 | 1.40 | ≥95% | |
| Geraniol | 1.0 | 0.70 | ≥95% | |
| Geranic Acid | 0.0 | 0.0 | — | |
|
4c (A. musculus pheromone + geranic acid + E grandlure II) |
Grandlure II Isomer Z | 23.3 | 14.41 | ≥95% |
| Grandlure II Isomer E | 15.4 | 9.53 | ≥95% | |
| Grandlure III & IV | 2.0 | 1.24 | ≥95% | |
| Geraniol | 1.0 | 0.62 | ≥95% | |
| Geranic Acid | 6.8 | 4.21 | ≥95% | |
| 5 | Commercial Anthonomus eugenii aggregation pheromone lured | N/A | N/A | N/A |
| 6 | Control (no-lure) | — | — | — |
- a Treatment 1 corresponds to the Anthonomus musculus aggregation pheromone.
- b 1:1 mixture.
- c Treatment 4 corresponds to the Anthonomus eugenii aggregation pheromone.
- d PHEROCON® Pepper Weevil Dual Lure; Trécé Inc. N/A = not available due to proprietary rights.
2.3 Statistical analyses
Anthonomus musculus numbers were summed for all four plots within each of the six farms and then averaged across weeks to obtain the number of adults/farm/week. Data were checked for normality (Shapiro–Wilk test) and equal variances (Levene's test, 'car' package in R); then, two analyses were conducted. First, we tested for treatment differences in A. musculus numbers using a generalized linear model (GLM) with a quasipoisson distribution and a logit-link function that included treatment, crop, treatment-by-crop interaction and site nested within a crop (site(crop)) as independent variables, with site as a random effect. If treatment was significant, treatment means were compared using Tukey's honest significant difference (HSD) test ('agricolae' package in R). We also tested the effects of adding E grandlure II and geranic acid to the A. musculus pheromone by GLM with a quasipoisson distribution and a logit-link function. Data from treatments 1–4 were used for this analysis. The model included E grandlure II (presence/absence), geranic acid (presence/absence), and their interaction; crop; and site(crop); with sites as a random effect. Data were analysed in R 3.3.1 (R Development Core Team, 2016).
3 RESULTS
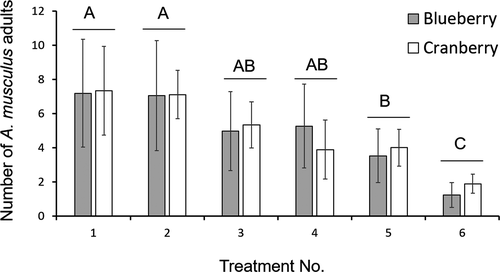
Treatment had an effect on A. musculus adult numbers (F5,39 = 15.03, p < .001). Anthonomus musculus adults were more attracted to the blend containing the four A. musculus aggregation pheromone components (treatment 1) and the A. musculus pheromone blend plus geranic acid (treatment 2) than the commercial A. eugenii pheromone and the unbaited control (treatments 5 and 6, respectively) (Figure 1). The A. musculus pheromone blend plus E grandlure II (treatment 3) and the A. eugenii pheromone blend (treatment 4) captured more A. musculus adults than the unbaited control but not compared to the commercial A. eugenii pheromone (Figure 1). There was an effect of crop (F1,29 = 7.82, p = .01) but not a treatment-by-crop interaction (F5,24 = 0.53, p = .74).
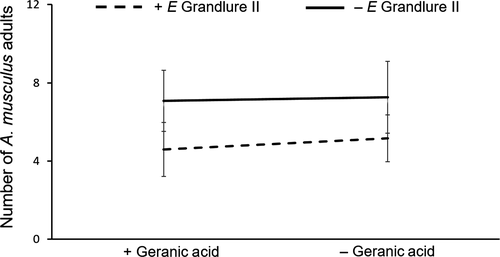
We found that A. musculus adult attraction to their aggregation pheromone was reduced by 27% due to the addition of E grandlure II (F1,22 = 10.39, p < .01), but geranic acid did not influence their response (F1,21 = 0.2, p = .65) (Figure 2). There was no interaction between E grandlure II and geranic acid (F1,19 = 0.08, p = .77) on A. musculus numbers; although the effect of crop was significant (F1,20 = 7.54, p = .01), indicating that weevil numbers differed by crop.
4 DISCUSSION
The aggregation pheromone blends of A. musculus and A. eugenii are similar in composition, except that the A. eugenii blend contains two additional components: E grandlure II and geranic acid. Here, we demonstrated that A. musculus adults are less attracted to their aggregation pheromone blend if it contains E grandlure II, but the response is not affected by the addition of geranic acid. E grandlure II is produced by A. eugenii but not by A. musculus (Szendrei et al., 2011) or the boll weevil (Anthonomus grandis Boheman) (Chang, Benedict, Payne, Camp, & Vinson, 1989; Tumlinson et al., 1969) and is produced only in trace amounts by the strawberry blossom weevil Anthonomus rubi Herbst. (Innocenzi, Hall, & Cross, 2001), suggesting that it may provide pheromone specificity for A. eugenii. The inhibitory response of E grandlure II on A. musculus was not influenced by host plant, as similar responses were observed in highbush blueberries and cranberries (no treatment-by-crop interaction). Szendrei et al. (2011) also found no effect of these cropping systems on the response of A. musculus to its aggregation pheromone.
In contrast, geranic acid had no effect on the A. musculus response to its aggregation pheromone. Geranic acid is produced by A. eugenii but not by A. musculus, A. grandis or A. rubi (Szendrei et al., 2011). Geranic acid also did not influence the response of A. musculus to E grandlure II. Although the A. eugenii pheromone components are produced at different ratios than those reported for A. musculus, particularly Z grandlure II, this did not seem to affect A. musculus attraction to its pheromone.
In conclusion, we showed that adding E grandlure II and geranic acid does not improve the attraction of A. musculus adults to their aggregation pheromone. Although our current 4-component pheromone blend is attractive to A. musculus, the response of overwintered adults (the damaging “spring generation”) to it is weaker than that of the “summer generation” (Szendrei et al., 2011). This indicates a need for improvement by identifying new pheromone components or testing the synergistic interaction between the aggregation pheromone with other cues, such as host plant volatiles (e.g., Szendrei, Malo, Stelinski, & Rodriguez-Saona, 2009).
ACKNOWLEDGEMENTS
We thank Kris Dahl, Sarah Ongaro and Gabrielle Pintauro for field assistance. Many thanks to the Massachusetts cranberry and New Jersey blueberry growers who provided the field sites, to three anonymous reviewers for their helpful comments on an earlier version of the manuscript and to Dr. Jordano Salamanca for help with statistics. Funding for this project was provided by the USDA Pest Management Alternatives Program (PMAP) (project no. 2012-34381-20108), and a Hatch project (no. NJ08192) to C.R.-S.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest with regard to this work.
AUTHORS' CONTRIBUTION
CR-S, HTA, CO and ALA conceived research and wrote the manuscript. ST, VK-R and MMS conducted experiments. CR-S analysed data. CR-S and ALA secured funding. All authors reviewed and approved the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Raw data are accessible as supplementary file: Raw data supplementary material.




