The usefulness of a dermocosmetic containing Myrtus communis extract and azelaic acid for maintenance phase of adult female acne: Results from a randomized exploratory investigator-blinded comparative study
Abstract
Background
Acne is a very common condition. Currently, there are relatively few studies available to help guidance-based decisions for its long-term management, especially studies with cosmetic care products. We have developed a skin care product dedicated to adult female acne.
Objectives
Evaluate the efficacy and tolerance of the test product containing Myrtus communis extract and azelaic acid compared with a light moisturizing cream (LCM) in adult females in the acne maintenance phase.
Methods
A clinical study was conducted as a Brazilian, multicentre, randomized, investigator-blinded trial in adult females with clear or almost clear facial acne after anti-acne treatment. The test group (26 subjects) applied the test product and the comparative product group (27 subjects) applied LCM. Both groups applied the products twice daily on the whole face. Subjects were evaluated every 4 weeks over 16 weeks. Efficacy was evaluated according to acne relapse; Investigator's Global Assessment (IGA); acne lesions counting; AcneQoL questionnaire; Subject Global change Assessment (SGA) of acne severity; and the number of Post-Inflammatory Hyperpigmentation (PIH) and Post-Inflammatory Erythema (PIE) lesions. Tolerance was assessed according to a 5-point scale.
Results
Over 16 weeks, the number of acne relapse was more than double in the comparator compared to the test product group (eight subjects vs. three subjects respectively). There was no statistical difference in the evolution of the mean IGA from baseline between the two groups; however, 85% of subjects were assessed as clear or almost clear in the test product group and 67% in the comparative group.
Conclusions
This study demonstrated the effectiveness topical application of the test product compared to LCM on acne severity in the maintenance phase of adult female acne. Efficacy results after 16 weeks suggested a trend to limit acne relapses and a benefit of the test product in maintaining long-term remission.
Acne vulgaris is a very common disease in adolescents but adults, particularly women, are frequently affected, with at least half of women estimated to exhibit some form of acne (either persistent, the most common or late-onset or recurrent).1-4 As in adolescent patients, acne lesions in adults may result in post-inflammatory hyperpigmentation (PIH) and erythema (PIE) or true scars.1, 2 The severity of acne in women is mainly mild or moderate but it is a chronic disease which is prone to recurrence after discontinuing a successful treatment. These chronic and unpleasant visual manifestations may have significant and enduring emotional and psychological effects; therefore, long-term treatment is highly recommended.5 The objective of maintenance should be to maintain acne remission, achieved with an initial successful treatment, and to minimize the risk of relapse.6 Currently, there are relatively few studies available demonstrating the long-term management of this disease, especially studies with dermocosmetic care products. We have developed a dermocosmetic care product, which is already commercialized in several countries worldwide. This skin care product is adapted for acne-prone skin by the addition of Myrtus communis extract and the naturally occurring saturated dicarboxylic acid, azelaic acid. Myrtus communis has been shown in in vitro assays to exhibit antibacterial and anti-inflammatory activities7, 8 and to inhibit Cutibacterium acnes biofilm formation.9 The presence of biofilms in the pilosebaceous follicles of acne patients is now known to be linked to C. acnes virulence and resistance of the bacterial phylotype IA1 to antibiotics.9, 10 In a split-face clinical trial, a lotion containing Myrtus communis was shown to be effective and safe for the treatment of mild to moderate acne vulgaris.11 Azelaic acid has been shown to be effective in treating and maintenance treatment of acne by reducing acne lesions, decreasing the amount of secreted sebum, as well as alleviating PIH.12-16 The aim of our exploratory study was to evaluate the efficacy of the test product containing these two anti-acne ingredients compared with a light moisturizing cream in adult females in acne maintenance phase.
A clinical study was conducted as a Brazilian, multicentre, randomized, investigator-blinded trial in adult females (24–49 years old). The main inclusion criteria were as follows:1 Clear or almost clear facial acne, according to the Investigator's Global Assessment (IGA) of acne severity, with at least one acne-related lesion of PIE (describing residual erythema) or PIH (describing pigment change) on the face.2 Responder to a topical anti-acne treatment (alone or in combination with an oral anti-acne treatment), applied for mild to moderate facial acne, for at least 12 weeks and stopped within 7 days before inclusion. The test group (26 subjects) applied the test product and the comparative product group (27 subjects) applied light moisturizing cream. Both groups applied the products twice daily on the whole face for 16 weeks. The subjects were evaluated every 4 weeks, with a total of five planned visits and additional visit(s) in case there was suspicion of subject's acne relapse. The main efficacy assessments included: acne relapse (defined by the necessity to introduce an anti-acne treatment); IGA; acne lesions counting; Acne Quality of Life (QoL) questionnaire; Subject Global Change Assessment (SGA) of acne severity; and PIH and PIE lesions counting. Tolerance was assessed according to a 5-point scale for each subject.
An important consideration regarding the interpretation of the clinical data is that the population should be comparable between the control and test product groups. In this trial, both groups were comparable in terms of demography (number of subjects per group, age and phototype), the type of skin and history of acne. They were also comparable regarding topical anti-acne treatments applied in the previous 3 months (topical retinoids [adapalene, tretinoin, retinoic acid], benzoyl peroxide, azelaic acid and adapalene/benzoyl peroxide combination). None of the subjects used an oral anti-acne treatment in combination. The population was also globally comparable between both groups in terms of the acne severity at baseline. According to the IGA score, the majority of subjects were assessed as almost clear, with a higher percentage in the comparative product group (96%) compared to the test product group (81%). The number of acne lesions was slightly higher in the test product group (Table 1).
| Test product group | Comparative product group | |
|---|---|---|
| Number of subjects per group | 26 | 27 |
| Age | Mean = 32 (24–46) | Mean = 35.7 (25–49) |
| Phototype |
21 subjects (80.8%) with phototype III 5 subjects (19.2%) with phototype IV |
23 subjects (85.2%) with phototype III 4 subjects (14.8%) with phototype IV |
| History of acne |
|
|
| Skin nature |
|
|
| Acne severity (IGA score) |
|
|
| Acne lesions | ||
| Inflammatory lesions | Mean number of lesions: 5.69 | Mean number of lesions: 5.81 |
| Non inflammatory lesions | Mean number of lesions: 24.27 | Mean number of lesions: 21.81 |
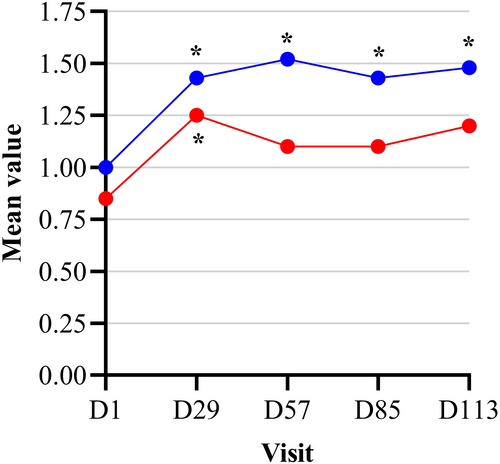
After 16 weeks of application, the number of acne relapses was more than double in the comparative group compared to the test product group (eight subjects [30%] vs. three subjects [12%] respectively). In addition, the probability of not having an acne relapse was 88% and 70% in the test and comparative group respectively. There was a significant worsening of the mean IGA from baseline at all the following visits in the comparative group, whereas in the test product group there was a significant worsening on D29 only (Figure 1). There was no statistical difference in the evolution of the mean IGA from baseline between the two study groups (p > 0.05); however, 85% of patients were assessed as clear or almost clear at the end of the study (D113) in the treated group compared to only 67% in the comparator group.
A defining feature of acne is the presence of non-inflammatory and inflammatory lesions, with the latter localized to the pilosebaceous unit.17, 18 This study showed a significant decrease from baseline of the mean number of total acne (Figure 2a) and non-inflammatory (Figure 2b) lesions at D29, D57 and D85 and globally over the 16 weeks of use the test product. By contrast, a significant decrease in the mean number of total acne and non-inflammatory lesions from baseline in the comparator group was only observed on D113. Moreover, the intergroup comparison showed a statistically significant difference in favour of the test product at D29, D57 and D85 and globally over the 16 weeks of use the test product. There was no significant evolution from baseline of the number of inflammatory acne lesions for test or comparative product groups or a significant difference between the two study groups throughout the study (Figure 2c). There was no statistical difference in the SGA score observed between the two study groups during the study.

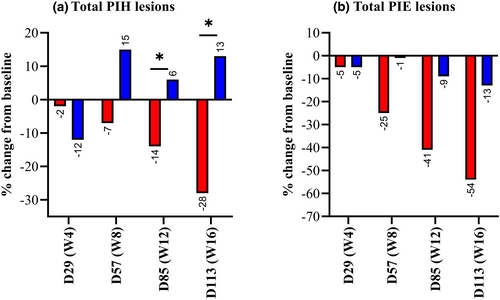
PIH is a reactive process resulting from increased melanin production, abnormal distribution and/or the presence of melanophages secondary to inflammation.19, 20 Since alterations in skin pigmentation can be long-lasting, difficult to manage, and even more distressing than the active acne lesions, significant efforts have been invested in developing treatments that alleviate PIH. In this study, the test product resulted in a significant decrease from baseline of the number of PIH lesions globally over the whole study and at D85 and D113. By contrast, there were no significant decreases from baseline of the number of PIH lesions in the comparative product group. There was also a significant difference in the evolution from baseline of the mean number of PIH lesions between the two study groups globally over the 16 weeks of use and at D85 and D113, in favour of test product (Figure 3a). In the test product group, there was a significant decrease from baseline of the number of PIE lesions globally over the whole study and at each visit on and after D57. A decrease in this value was only observed on D113 in the comparative product group. There was no significant difference in the evolution from baseline of the mean number of PIE lesions between the two study groups throughout the study (Figure 3b).
Acne is associated with psychosocial repercussions, especially in female patients, resulting in emotional distress and a poorer quality of life.4 Both the test product and the comparative product groups resulted in a significant improvement of the mean total score of Acne-QoL globally over the whole study and at each visit, with no significant difference between the two groups. Another aim of the study was to evaluate the tolerance of the test product in the adult female population by the investigators, as well as the patient cosmetic satisfaction. These are important attributes of acne treatment, since they increase the likelihood of good compliance which is essential for the management of this chronic disease. The test product exhibited a good to excellent tolerance in 88% of subjects, this result can be expected given the nature of the product ingredients and the target population. Most patients were also satisfied with cosmetic qualities and efficacy of the test product.
In conclusion, the results of this study demonstrated the efficacy of topical treatment with a dermocosmetic product containing Myrtus communis extract and azelaic acid for adult female acne in the maintenance phase. Efficacy results after 16 weeks suggest a trend to limit acne relapses and a benefit of the product in maintaining long-term remission.
ACKNOWLEDGEMENTS
The authors would like to thank the following people: Ana Coutinho for her support, Roberta Trefiglio (Ideal Clinops) for monitoring the study, Dominique NDeli (Axiodis) for the data management, and all the study investigators and their co-investigators for recruiting and evaluating the patients. Medical writing wad provided by Nicolas Hewitt and funded by Pierre Fabre Dermo-Cosmétique.
FUNDING INFORMATION
This work was funded by Pierre Fabre Dermo-Cosmétique.
CONFLICT OF INTEREST
M. D. Thouvenin, A. Bacquey, S. Baradat, C. Lauze, V. Mengeaud are employees of Pierre Fabre Dermo-Cosmétique. The other authors report no conflicts of interest in this work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




