No effect of topical digoxin and furosemide for patients with actinic keratosis
Bertine W. Huisman and Lauren E. H. Nené are shared first authors.
Dear Editor,
Actinic keratoses (AK) are common skin lesions that appear clinically as erythematous, scaly papules and plaques on sun-exposed skin. The pathogenesis of these lesions is associated with a mutation in the tumour suppressor gene p53, leading to defective apoptosis and hyperproliferation of cells. Besides AK being cosmetically bothersome for affected patients, AKs are also known as precursor lesions of squamous cell carcinoma (SCC). The annual burden of the treatment costs of AK has been estimated at around 900 million dollars in the USA.1 Current topical treatments for AK include, e.g. 5-fluorouracil and imiquimod, which can lead to redness, swelling and crusting influencing treatment compliance.2 Targeting the human papillomavirus (HPV) in AK could be a novel treatment paradigm, as it has been suggested that this DNA-based virus plays a role in the development of AK into SCC.3-5 Active HPV replication may contribute to carcinogenesis. For replication, DNA viruses rely on the cell membrane ion cotransporters accountable for K+ influx. We hypothesized that topical ionic contraviral therapy, composed of digoxin (0.125%) and furosemide (0.125%), could serve as a potential treatment for HPV-mediated and associated diseases. The ionic properties of digoxin and furosemide interact with the cell membrane ion cotransporters Na+/K+-ATPase and Na+-K+-2Cl co-transporter-1 and thereby inhibit the K+ influx on which DNA viruses rely for replication.6-8
To test whether treatment with topical digoxin and furosemide could improve AK, a randomized controlled trial (RCT) was performed from October 2018 until October 2019 to assess safety and tolerability, and to explore pharmacodynamics and clinical efficacy of this treatment in patients with AK. Thirty-two subjects with two facial fields of approximately 15 cm2 containing at least two AKs per field were enrolled. These patients were randomized to either topical gel with digoxin and furosemide (dual agent), digoxin (single agent), furosemide (single agent) or placebo (1:1:1:1 ratio). The gel was applied once daily for 42 consecutive days on one randomized facial field; the second facial field was used as an untreated comparator. Demographics and baseline characteristics were comparable across the four treatment groups. Therapy compliance was managed by daily contact via an electronic mobile application.9 To assess safety and tolerability, laboratory safety testing was performed, and adverse events, vital signs and ECGs were monitored. Clinical efficacy was primarily assessed by clinical scoring (Investigator Global Score [IGS], lesion count and clearance, field morphology scores), measurement of viral load, HPV expression and histology.
In the group treated with digoxin and furosemide, an average dose of 177 mg/day was applied. The topical treatments were found safe and generally well-tolerated. All treatment-emergent adverse events were of mild severity (n = 36); six of these were classified as treatment-related namely administration site irritation (n = 4) and skin exfoliation (n = 2). These adverse events were resolved without intervention. No between-group differences in clinical scores (Figure 1), viral load and HPV expression were observed after treatment with both the dual and single agents. Also, no difference was found for HPV viral load over time; they were found to be extremely low or absent in the swab and biopsy samples. For the explorative endpoints, a remarkable statistically significant finding was observed in field morphology, a lower score for pigmentation in the dual agent (digoxin and furosemide) group compared with placebo was observed. Investigation of blood samples did not show any measurable digoxin levels, indicating that no systemic effects were detectable.
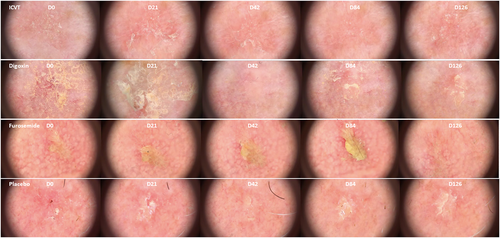
The results of this study are in line with a recent randomized controlled trial on the dual agent composed of digoxin and furosemide in anogenital warts showing it was safe to administer, but no effect on pharmacodynamic activity or clinical efficacy after 6 weeks of treatment was found.8 Overall, the results of this phase 2 study indicate that topical ionic contraviral therapy applied on facial AKs has a favourable safety profile but does not improve clinical scores or pharmacodynamic features of this disease. The subtle improvement in pigmentation is not considered clinically relevant. Either the concentration of the digoxin and furosemide in the gel or the duration of application was too low to eliminate HPV, or HPV plays a less important role in AK for topical ionic contraviral therapy to be an effective treatment.
ACKNOWLEDGEMENTS
The authors would like to thank Nicole Kukutsch for her expert advice on dermatoscopy of AK lesions.
FUNDING INFORMATION
Cutanea Life Science, Wayne, Pennsylvania, USA. Maruho Co., Ltd. Shimogyo-ku, Kyoto, Japan.
CONFLICT OF INTEREST
One of the co-authors (Gary Feiss) was employed at the co-funder (Cutanea Life Sciences) of the trial. The final draft was approved by this co-author on behalf of the co-founder. However, no major comments were made. All other authors declared no competing interests to this work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.