Role of vitamin D in energy and bone metabolism in postmenopausal women with type 2 diabetes mellitus: A 6-month follow-up evaluation
Abstract
Aims/Introduction
Resting energy expenditure was associated with a serum bone turnover marker in postmenopausal women with type 2 diabetes (T2DMPW) in the present cross-sectional study. To clarify the fundamental pathological factor for the correlation of bone metabolism and basal metabolism in type 2 diabetes, a 6-month prospective follow-up study was carried out with supplementation of vitamin D.
Materials and Methods
A total of 44 T2DMPW were enrolled. The following factors were evaluated at the beginning and the end of the summer: procollagen type 1 N-terminal propeptide, carboxy-terminal collagen crosslinks-1, intact parathyroid hormone and 25-hydroxyvitamin D (25[OH]D), as well as diabetic complications, body composition, respiratory quotient and resting energy expenditure. A total of 23 patients with low 25(OH)D levels (˂20 ng/mL) were instructed to increase vitamin D levels by lifestyle change. Among them, 15 patients with osteoporosis were also administered alfacalcidol.
Results
Serum 25(OH)D increased in 25 patients and decreased in 19 patients. Patients who did not receive the study intervention at the start tended to have a decreased 2525(OH)D level; therefore, the average 25(OH)D level of all patients was not changed. Changes in resting energy expenditure were positively correlated with those of procollagen type 1 N-terminal propeptide/carboxy-terminal collagen crosslinks-1. Changes in the respiratory quotient correlated with the mean glycated hemoglobin levels; procollagen type 1 N-terminal propeptide levels positively correlated with serum 25(OH)D after the intervention. These correlations were prominent in patients with increased 25(OH)D and those with alfacalcidol supplementation.
Conclusions
Restoration of vitamin D level might be a prerequisite for a normal correlation between bone and basal metabolism in T2DMPW. Lifestyle intervention for retention of vitamin D level is important even in summer, in T2DMPW.
Introduction
Diabetes mellitus is a risk factor for osteoporosis, and the physiological manifestations of osteoporosis are different between patients with type 1 and type 2 diabetes mellitus1. Bone mineral density (BMD) is not a suitable marker for the diagnosis of osteoporosis in patients with type 2 diabetes mellitus2-4. Candidate markers of osteoporosis in type 2 diabetes mellitus include those of bone metabolism, such as serum or urine levels of products resulting from bone tusrnover5. Among many biochemical markers of bone turnover, carboxy-terminal collagen crosslinks-1 (CTX-1), a marker of bone resorption, and procollagen type 1 N-terminal propeptide (P1NP), a marker of bone formation, are collagen markers that are expected to reflect early changes in bone turnover6. In our previous study, we reported a positive association between basal metabolism and bone turnover using these bone metabolic markers, and we also observed a close correlation between serum 25-hydroxyvitamin D (25[OH]D) levels and the respiratory quotient (RQ), an energy source index, in postmenopausal women with type 2 diabetes mellitus7. Bone and blood glucose metabolism are thought to be closely related8, and this hypothesis is supported by physiological studies that reveal decreased bone turnover with high blood glucose levels in patients with diabetes mellitus7, 9. These findings suggest that low basal metabolism might be a risk factor for osteoporosis.
Vitamin D is an important factor involved in both bone and basal metabolism10-12. A study by Suzuki et al. showed that more than half of patients with type 2 diabetes mellitus had low 25(OH)D levels (≤20 ng/mL)13, accompanied by a relatively high insulin requirement. However, vitamin D activation was shown to be reduced in a diabetic rat model14, 15. Furthermore, vitamin D supplementation was shown to ameliorate hypoinsulinemia and hyperglycemia in a diabetic rat model16. In contrast, low vitamin D levels have been shown to be associated with an increased incidence of metabolic disease and type 2 diabetes mellitus17-19. However, the effects of vitamin D supplementation with regard to type 2 diabetes mellitus prevention and improved blood glucose control have been inconsistent20-24. In our previous study, patients with low vitamin D levels also showed a low C-peptide immunoreactivity (CPR) level7. Vitamin D level and insulin sensitivity are novel predictors of the resting energy expenditure (REE) in healthy controls12; therefore, vitamin D level might affect basal metabolism as well as bone metabolism.
In the present prospective study of postmenopausal women with type 2 diabetes mellitus, which follows our previous cross-sectional study7, we show that restoration of normal vitamin D levels is important for maintaining the normal correlation between energy and bone metabolism.
Methods
Participants
The study design is shown in Figure 1. A total of 56 postmenopausal Japanese women (aged >50 years) with type 2 diabetes mellitus were initially enrolled in the study7. Of these, 10 patients were excluded based on a history of dietary supplement intake or a positive glutamic acid decarboxylase antibody test result. The remaining 46 patients did not show signs of complications, overt proteinuria or symptoms of major diseases other than hypertension, dyslipidemia and mild obesity (body mass index ≤30 kg/m2). No patient had received hormonal drugs, including for thyroid diseases. They underwent an examination, the results of which were set as the baseline (0 months). The first examination was carried out in spring (March–May), and the second examination followed in autumn (September–November; 6 months). None of the patients changed their oral hypoglycemic agents during the observational period.

Half of the patients had serum 25(OH)D levels of ≤20 ng/dL, and one-third of the patients had a comparatively lower bone mineral density (BMD). A total of 23 patients with a low vitamin D level (25[OH]D ≤ 20 ng/mL) were instructed to take vitamin D from their diets and not to avoid sunlight, in order to boost their vitamin D levels. Vitamin D activation is supposed to be reduced in diabetes patients; therefore, among patients with low vitamin D levels, 15 patients with low BMD (T-score ≤−2.5) were prescribed alfacalcidol at a dose of 0.25–0.50 μg/day between the first examination and the 6-month re-examination. Out of 23 patients who had serum 25(OH)D levels >20 ng/mL, two patients were withdrawn during the course of the study: one changed her physician in charge owing to social circumstances, and the other as a result of an incidental detection of thyroid carcinoma. Ultimately, 44 patients completed the 6-month examination.
Written informed consent was obtained from all patients before their participation in the study. This study was approved by the ethics committee of the Tokyo Women's Medical University (IRB number: 2396).
Measurements
To evaluate orthostatic hypotension, clinical blood pressure was measured within a 3-min interval, with the patient in both the supine and standing positions. Blood samples were collected after a 10-h overnight fast and were used for all the tests carried out in the present study. Serum CPR was calculated by a chemiluminescent enzyme immunoassay (Fujirebio Inc., Tokyo, Japan), and serum 25(OH)D was calculated by a double antibody radioimmunoassay (DiaSorin Inc., Stillwater, Minnesota, USA). Serum levels of P1NP and CTX-1 were measured at Roche Diagnostics (Tokyo, Japan) in a blinded manner. Serum levels of intact parathyroid hormone, 25(OH)D, calcium and phosphate were evaluated at the Diabetes Center, Tokyo Women's Medical University, Tokyo, Japan, using aliquots of the same serum samples. General serum tests were also carried out to measure levels of aspartate transaminase, alanine transaminase, cholesterol, triglycerides and creatinine. Microalbumin content in the patients' first morning urine samples was quantified to evaluate complications. Motor and sensory nerve conduction velocities were calculated using the Neuropack X1 system (Nihon Kohden, Tokyo, Japan). The R-R interval was calculated as the maximum difference in pulses/min under deep breathing conditions. A questionnaire to determine lifestyle momentum was provided to the participants.
Body composition was calculated by the impedance method using a body composition analyzer (Tanita, Tokyo, Japan). REE and RQ were calculated over a 20-min period by respiratory gas analysis in a thermoneutral environment (25°C) using Vmax Spectra indirect calorimetry (Cardinal Health, Dublin, Ohio, USA). A diagnosis of retinopathy was recorded within the 6 months before the patient's participation in the present study. BMD was evaluated using dual-energy X-ray absorptiometry (Hologic, Bedford, Massachusetts, USA) at the non-dominant distal radius, and the BMD T-score was calculated.
Statistical analysis
The results are expressed as means ± SD. All analyses were carried out using SPSS software (version 21.0; SPSS, Chicago, Illinois, USA). Intergroup comparisons were carried out using Student's t-test (participants with 25[OH]D ≤ 20 ng/mL vs participants with 25[OH]D > 20 ng/mL, or participants who increased 25[OH]D levels vs who decreased 25[OH]D levels) and Pearson's χ2-test (with or without habit of exercise). To evaluate the relationship between basal metabolism and bone metabolism, various factors were subjected to a correlation analysis. Each pair of variables that showed a significant correlation was subjected to a regression analysis, and all coefficients of determination were adjusted (r2). The standardized partial regression coefficient was denoted as β. Differences were considered statistically significant at a two-side P-value of <0.05.
Results
Vitamin D levels
The baseline characteristics of the participants were reported previously7. The characteristics at the end of the study (at 6 months) are listed in Table 1. The mean serum 25(OH)D levels were 21.4 ± 7.6 ng/mL at 0 months, and 20.4 ± 6.3 ng/mL at 6 months. A total of 21 participants had a serum 25(OH)D level of <20 ng/mL at the start of the observation period. During the study period, the 25(OH)D level increased in 25 patients and decreased in 19 patients, with high variability (average 0.09 ± 6.3 ng/mL) in 25(OH)D levels. In addition, 13 of the patients whose 25(OH)D levels increased had taken alfacalcidol. The characteristics of the participants whose 25(OH)D levels had either increased or decreased at 6 months are summarized in Table 2. Participants with low levels of 25(OH)D had significantly lower CPR levels than did those with normal levels of 25(OH)D at 0 months, as described previously7. These patients were advised to increase their vitamin D levels by diet, ultraviolet (UV) exposure and/or alfacalcidol administration; as a result, the serum CPR levels were lower (1.43 ± 0.6 and 1.86 ± 0.8 ng/mL with increasing or decreasing 25(OH)D levels, respectively; P < 0.01), and serum phosphate levels were slightly elevated in patients with increasing 25(OH)D levels (3.62 ± 0.1 and 3.58 ± 0.1 mg/dL with increasing and decreasing 25(OH)D, respectively; P < 0.05). After the observation period, serum 25(OH)D levels were low (<20 ng/mL) in 21 participants, and >20 ng/mL in 23 participants. Serum CPR levels were generally lower in participants with low 25(OH)D levels (1.59 ± 0.9 ng/mL) than they were in participants with serum 25(OH)D > 20 ng/mL (1.64 ± 0.8 ng/mL); however, the difference was not significant.
| Age (years) | 65.0 ± 5.9 | Ca (mg/dL) | 9.4 ± 0.3 |
| BMI (kg/m2) | 24.4 ± 3.5 | P (mg/dL) | 3.5 ± 0.5 |
| Tobacco use (+/−) | 4/40 | 25(OH)D (ng/mL) | 20.4 ± 6.2 |
| Alcohol use (+/−) | 11/33 | iPTH (pg/mL) | 54.6 ± 17.3 |
| Serum fasting blood sugar (mg/dL) | 149.1 ± 49.7 | Serum CTX-1 (ng/mL) [normal] | 0.30 ± 0.13 [0.56 ± 0.23]† |
| HbA1c (%) | 7.7 ± 1.2 | Serum P1NP (ng/mL) [normal] | 35.9 ± 10.0 [45.1 (20.3–76.3)]‡ |
| Mean HbA1c for prior 6 months (%) | 7.6 ± 1.2 | ||
| Family history of bone fracture (+/−) | 15/31 | Fat mass (kg) | 20.3 ± 5.8 |
| Past history of bone fracture (atraumatic/traumatic/none) | 9/1/36 | Respiratory quotient | 0.84 ± 0.10 |
| Duration of diabetes (years) | 15.8 ± 8.7 | REE/estimated by Harris–Benedict (Cal) | 957.3 ± 122.4/1,144.7 ± 201.0 |
| Insulin/OHA/diet | 13/30/3 | ||
| SU/thiazolidine/DPPIV-I/biguanide | 21/1/14/16 | ||
| Antihypertensive drug (+/−) | 24/20 | ||
| Lipid-lowering agent (statins) | 28 (22)/16 | ||
| Retinopathy (P/S/N) | 5/7/31 | MCV ulnar/peroneal | 55.7 ± 5.0/50.2 ± 4.5 |
| Urine albumin (+/−) | 6/38 | SCV ulnar/sural | 50.2 ± 4.5/47.9 ± 7.5 |
| Creatinine (mg/dL) | 0.67 ± 0.13 | R-R interval (s) | 115 ± 5.9 |
| HDL (mg/dL) | 66.0 ± 16.7 | Resting (upright) clinical systolic BP (mmHg) | 131.9 ± 17.0 (131.4 ± 17.4) |
| Triglyceride (mg/dL) | 117.0 ± 56.0 | ||
| LDL (mg/dL) | 123.2 ± 30.2 | Resting (upright) clinical diastolic BP (mmHg) | 72.0 ± 7.6 (75.8 ± 8.8) |
| CPR (ng/mL) | 1.6 ± 0.9 |
- Total n = 44. Blood pressure values in parentheses indicate the pressure in the upright position. Data for non-diabetic subjects are shown in brackets. †Mean ± standard deviation in normal postmenopausal women. &Mean (5–95th percentile) in normal postmenopausal women without hormone replacement therapy. 25(OH)D, 25-hydroxyvitamin D; BMD, bone mass density; BMI, body mass index; BP, blood pressure; Ca, calcium; CPR, C-peptide immunoreactivity; CTX-1, carboxy-terminal collagen crosslinks-1; DPPIV-I; dipeptidyl peptidase-IV inhibitor; Hb1Ac, glycated hemoglobin; HDL, high-density lipoprotein; iPTH, intact parathyroid hormone; LDL, low-density lipoprotein; MCV, motor nerve velocity; OHA, oral hypoglycemic agent; P, phosphate; P1NP, procollagen type 1 N-terminal propeptide; REE, resting energy expenditure; SCV, sensory nerve velocity; SU, sulfonylurea.
| Characteristics | 25(OH)D increase (n = 25) | 25(OH)D decrease (n = 19) | P |
|---|---|---|---|
| Age (years) | 63.8 ± 6.5 | 63.7 ± 6.7 | NS |
| BMI (kg/m2) | 24.7 ± 3.9 | 24.8 ± 7.3 | NS |
| Percent body fat | 34.3 ± 6.3 | 35.4 ± 5.0 | NS |
| Lean body mass (kg) | 37.3 ± 3.0 | 37.4 ± 3.3 | NS |
| REE (Cal) | 937.0 ± 129.5 | 955.4 ± 189.3 | NS |
| Alfacalcidol (+/−) | 13/12 | 2/17 | |
| Diabetic duration (year) | 15.8 ± 8.1 | 15.0 ± 9.7 | NS |
| Fasting blood glucose (mg/dL) | 143.6 ± 4.1 | 158.9 ± 57.2 | NS |
| HbA1c (%) | 7.7 ± 1.4 | 7.5 ± 0.9 | NS |
| Average HbA1c over the previous 6 months (%) | 7.7 ± 1.3 | 7.6 ± 0.7 | NS |
| Serum creatinine (mg/dL) | 0.65 ± 0.1 | 0.67 ± 0.17 | NS |
| HDL (mg/dL) | 65.4 ± 17.4 | 68.3 ± 16.5 | NS |
| Triglyceride (mg/dL) | 144.6 ± 130.5 | 122.7 ± 62.1 | NS |
| LDL (mg/dL) | 122.8 ± 29.8 | 125.6 ± 30.5 | NS |
| Ca (mg/dL) | 9.2 ± 0.3 | 9.2 ± 0.4 | NS |
| P (mg/dL) | 3.62 ± 0.1 | 3.58 ± 0.1 | P < 0.05 |
| CPR (ng/mL) at start after 6 months |
1.43 ± 0.6 1.48 ± 0.6 |
1.86 ± 0.8 1.98 ± 1.0 |
P < 0.001 |
| CTX-1 (ng/mL) | 0.33 ± 0.11 | 0.39 ± 0.14 | NS |
| PINP | 35.4 ± 8.7 | 40.4 ± 14.8 | NS |
| BMD total | 0.500 ± 0.08 | 0.462 ± 0.06 | NS |
| 25(OH)D at start | 19.0 ± 6.8 | 25 ± 7.3 | P < 0.01 |
| 25(OH)D ≤ 20 ng/mL/alfacalcidol taken | (19/13) | (4/2) | |
| 25(OH)D after 6 months | 21.0 ± 7.0 | 19.2 ± 4.9 | NS |
| Habit of exercising >30 min twice per week | 14 (56%) | 15 (78%) | NS |
| Average daily walking time (min) | 21.3 ± 31.1 | 20 ± 27.9 | NS |
- 25(OH)D, 25-hydroxyvitamin D; BMD, bone mass density; BMI, body mass index; BP, blood pressure; Ca, calcium; CPR, C-peptide immunoreactivity; CTX-1, carboxy-terminal collagen crosslinks-1; Hb1Ac, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NS, not significant; P, phosphate; P1NP, procollagen type 1 N-terminal propeptide; REE, resting energy expenditure.
Basal metabolism and Bone metabolism
The results of a regression analysis of basal metabolism and bone metabolism that showed a significant correlation are summarized in Table 3. P1NP/CTX-1 levels at 6 months positively correlated with REE. β-Values were high in participants taking alfacalcidol and those with increased serum 25[OH]D levels (β = 0.327, adjusted R2 = 0.086, P = 0.03; β = 0.471, adjusted R2 = 0.188, P = 0.017; and β = 0.720, adjusted R2 = 0.482, P = 0.002 for all participants, those with increased 25[OH]D and those taking alfacalcidol, respectively; Figure 2a). In contrast, no correlation was observed between P1NP/CTX-1, REE values calculated using the Harris–Benedict equation and fat mass. The change in P1NP/CTX-1 levels at 6 months (ΔP1NP/CTX-1) positively correlated with the change in REE (ΔREE; β = 0.365, adjusted r2 = 0.134, P = 0.015; β = 0.439, adjusted r2 = 0.158, P = 0.028; and β = 0.687, adjusted r2 = 0.431, P = 0.005; for all participants, those with increased 25[OH]D and those taking alfacalcidol, respectively; Figure 2b). The relationship between the ΔP1NP/CTX-1 and ΔREE in participants with increased levels of 25(OH)D had a high β-value (β = 0.439, R2 = 0.158, P = 0.028); this was particularly prominent for participants taking alfacalcidol (β = 0.687, R2 = 0.431, P = 0.005; Figure 2c).
| P1NP | CTX-1 | P1NP/CTX-1 | ΔP1NP/CTX-1 | 25(OH)D | Mean HbA1c | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | P | R 2 | β | P | R 2 | β | P | R 2 | β | P | R 2 | β | P | R 2 | β | P | R 2 | |
| All (n = 44) | ||||||||||||||||||
| REE | −0.117 | 0.449 | −0.010 | −0.212 | 0.167 | 0.022 | 0.327 | 0.030a | 0.086 | 0.015 | 0.496 | −0.012 | −0.024 | 0.878 | −0.023 | 0.324 | 0.032a | 0.084 |
| ΔREE | 0.092 | 0.553 | −0.015 | 0.062 | 0.690 | −0.020 | 0.163 | 0.291 | 0.003 | 0.365 | 0.015a | 0.113 | 0.009 | 0.953 | −0.024 | −0.006 | 0.967 | −0.024 |
| RQ | 0.107 | 0.441 | −0.012 | 0.055 | 0.721 | −0.021 | 0.006 | 0.971 | −0.024 | −0.171 | 0.267 | 0.006 | 0.325 | 0.032a | 0.084 | −0.046 | 0.767 | −0.022 |
| ΔRQ | −0.120 | 0.437 | −0.009 | 0.003 | 0.982 | −0.024 | −0.101 | 0.516 | −0.013 | −0.001 | 0.997 | −0.024 | 0.086 | 0.577 | −0.016 | −0.269 | 0.078 | 0.05 |
| HbA1c | −0.213 | 0.165 | 0.023 | −0.302 | 0.046a | 0.070 | 0.342 | 0.023a | 0.096 | 0.270 | 0.076 | 0.051 | −0.156 | 0.312 | 0.001 | 0.963 | 0.000 | 0.925 |
| Mean HbA1c | −0.299 | 0.103 | 0.040 | −0.289 | 0.057 | 0.062 | 0.254 | 0.097 | 0.042 | 0.180 | 0.243 | 0.009 | −0.232 | 0.130 | 0.031 | – | – | – |
| 25(OH)D | 0.374 | 0.012a | 0.119 | 0.201 | 0.192 | 0.017 | 0.128 | 0.409 | −0.007 | 0.044 | 0.775 | −0.022 | – | – | – | −0.232 | 0.130 | 0.031 |
| Taking alfacalcidol (n = 15) | ||||||||||||||||||
| REE | 0.023 | 0.936 | −0.076 | −0.355 | 0.194 | 0.059 | 0.720 | 0.002a | 0.482 | 0.606 | 0.017a | 0.318 | 0.455 | 0.088 | 0.146 | 0.437 | 0.103 | 0.129 |
| ΔREE | −0.083 | 0.770 | −0.070 | −0.334 | 0.223 | 0.043 | 0.669 | 0.006a | 0.404 | 0.687 | 0.005a | 0.431 | 0.348 | 0.203 | 0.054 | 0.366 | 0.180 | 0.067 |
| RQ | −0.186 | 0.597 | −0.040 | −0.105 | 0.710 | −0.065 | 0.022 | 0.939 | −0.076 | −0.105 | 0.710 | −0.065 | 0.102 | 0.717 | −0.066 | −0.466 | 0.080 | 0.157 |
| ΔRQ | −0.298 | 0.280 | 0.019 | −0.102 | 0.716 | −0.066 | −0.132 | 0.638 | −0.058 | −0.063 | 0.823 | −0.073 | −0.054 | 0.848 | −0.074 | −0.630 | 0.012a | 0.351 |
| HbA1c | −0.059 | 0.834 | −0.073 | −0.295 | 0.286 | 0.017 | 0.633 | 0.011a | 0.354 | 0.599 | 0.018a | 0.309 | 0.298 | 0.281 | 0.018 | 0.966 | 0.000 | 0.929 |
| Mean HbA1c | −0.072 | 0.800 | −0.071 | −0.249 | 0.371 | −0.010 | 0.502 | 0.057 | 0.194 | 0.432 | 0.108 | 0.124 | 0.153 | 0.587 | −0.052 | – | – | – |
| 25(OH)D | 0.653 | 0.008a | 0.382 | 0.346 | 0.207 | 0.052 | 0.334 | 0.223 | 0.043 | 0.373 | 0.171 | 0.073 | – | – | – | 0.153 | 0.587 | −0.052 |
| 25[OH]D increase(n = 25) | ||||||||||||||||||
| REE | −0.233 | 0.263 | 0.013 | −0.386 | 0.057 | 0.112 | 0.471 | 0.017a | 0.188 | 0.243 | 0.242 | 0.018 | −0.113 | 0.591 | −0.030 | 0.426 | 0.034a | 0.146 |
| ΔREE | −0.050 | 0.812 | −0.041 | −0.151 | 0.470 | −0.020 | 0.337 | 0.100 | 0.075 | 0.439 | 0.028a | 0.158 | 0.154 | 0.462 | −0.019 | −0.005 | 0.982 | −0.043 |
| RQ | 0.135 | 0.520 | −0.024 | −0.013 | 0.950 | −0.043 | 0.104 | 0.622 | −0.032 | −0.009 | 0.967 | −0.054 | 0.342 | 0.094 | 0.078 | −0.056 | 0.789 | −0.040 |
| ΔRQ | −0.630 | 0.764 | −0.039 | 0.022 | 0.918 | −0.043 | −0.077 | 0.713 | −0.037 | 0.052 | 0.803 | −0.041 | 0.111 | 0.598 | −0.031 | −0.530 | 0.006a | 0.250 |
| HbA1c | −0.179 | 0.391 | −0.010 | 0.377 | 0.063 | 0.105 | 0.450 | 0.024a | 0.168 | 0.204 | 0.329 | 0.000 | −0.172 | 0.412 | −0.013 | 0.973 | 0.000 | 0.945 |
| Mean HbA1c | −0.186 | 0.374 | −0.007 | −0.338 | 0.099 | 0.076 | 0.358 | 0.079 | 0.090 | 0.097 | 0.646 | −0.034 | −0.258 | 0.214 | 0.026 | – | – | – |
| 25(OH)D | 0.492 | 0.013a | 0.209 | 0.345 | 0.092 | 0.081 | 0.066 | 0.754 | −0.039 | 0.075 | 0.723 | −0.038 | – | – | – | −0.258 | 0.214 | 0.026 |
| 25(OH)D decrease (n = 19) | ||||||||||||||||||
| REE | −0.065 | 0.791 | −0.054 | −0.098 | 0.69 | −0.049 | 0.156 | 0.523 | −0.033 | −0.021 | 0.931 | −0.058 | 0.288 | 0.232 | 0.029 | 0.128 | 0.603 | −0.042 |
| ΔREE | 0.2 | 0.411 | −0.016 | 0.246 | 0.309 | 0.005 | −0.06 | 0.808 | −0.055 | 0.32 | 0.182 | 0.049 | −0.17 | 0.487 | −0.028 | −0.023 | 0.925 | −0.058 |
| RQ | 0.078 | 0.752 | −0.052 | 0.131 | 0.593 | −0.041 | −0.153 | 0.531 | −0.034 | −0.34 | 0.155 | 0.063 | 0.331 | 0.167 | 0.057 | −0.032 | 0.898 | −0.058 |
| ΔRQ | −0.176 | 0.47 | −0.026 | 0.007 | 0.976 | −0.059 | −0.175 | 0.473 | −0.026 | −0.079 | 0.749 | −0.052 | 0.001 | 0.996 | −0.059 | 0.258 | 0.286 | 0.012 |
| HbA1c | −0.279 | 0.247 | 0.024 | −0.207 | 0.396 | −0.014 | 0.099 | 0.687 | −0.048 | 0.383 | 0.105 | 0.097 | −0.128 | 0.601 | −0.041 | 0.942 | 0.000 | 0.881 |
| Mean HbA1c | −0.385 | 0.104 | 0.098 | −0.254 | 0.294 | 0.009 | 0.027 | 0.914 | −0.058 | 0.323 | 0.178 | 0.052 | −0.157 | 0.521 | −0.033 | – | – | – |
| 25(OH)D | 0.309 | 0.198 | 0.042 | 0.067 | 0.786 | −0.054 | 0.224 | 0.356 | −0.006 | −0.032 | 0.897 | −0.058 | – | – | – | −0.157 | 0.521 | −0.033 |
- a P < 0.05. All coefficients for determination data were adjusted (r2).
- 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; Hb1Ac, glycated hemoglobin; REE, resting energy expenditure; RQ, respiratory quotient.
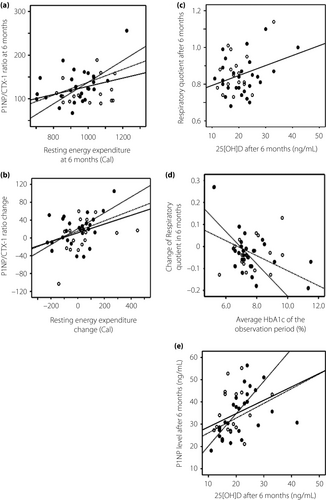
At the 0-month examination, the RQ was also found to be positively correlated with the 25(OH)D level at 6 months (β = 0.325, R2 = 0.084, P = 0.046). In contrast, a negative correlation was observed between the change in RQ (ΔRQ) and the average glycated hemoglobin level after the vitamin D intervention (β = −0.530, adjusted r2 = 0.250, P = 0.006 and β = −0.630, adjusted r2 = 0.351, P = 0.012 for those with increased 25[OH]D and those taking alfacalcidol, respectively; Figure 2d). For patients who took alfacalcidol, a striking correlation was observed between the ΔRQ and average glycated hemoglobin level during the observation period. Similarly, the 25(OH)D and P1NP levels were also positively correlated at 6 months (β = 0.374, R2 = 0.119, P = 0.012; Figure 2e). These correlations were also significant among patients with increased levels of 25(OH)D (β = 0.492, R2 = 0.209, P = 0.013) and those taking alfacalcidol (β = 0.653, R2 = 0.382, P = 0.008).
Discussion
Serum biochemical markers of bone turnover (for both formation and resorption) are accurate predictors of osteoporosis or osteopenia in women25. In the present study, we observed correlations between the P1NP (bone formation marker) and CTX-1 levels (bone resorption marker) ratio (P1NP/CTX-1), which was expected to reflect an early change in bone turnover with REE and P1NP/CTX-1, as well as a further change in REE during the observational period (Figure 2a,b). Accordingly, an association of bone metabolism with basal metabolism was consistently observed at the 6-month follow up. In contrast, no such association was observed when REE was estimated using the Harris–Benedict equation. Therefore, a correct evaluation of basal metabolism through indirect calorimetry is important.
We further considered that a factor other than fat mass or bodyweight (which will influence the Harris–Benedict equation) might underlie the relationship between basal and bone metabolism. One candidate factor is vitamin D, which plays an important role in both bone and basal metabolism10, 12. Patients with type 2 diabetes mellitus and metabolic syndrome were reported to have low vitamin D levels26-28. The frequency of vitamin D deficiency in our subjects at the beginning of the trial was similar (50%) to that reported in a previous Japanese study13. The low vitamin D level possibly affected the present results; therefore, according to our hypothesis, guidance on diet to increase the intake of vitamin D and UV exposure or supplementation with alfacalcidol for patients with low vitamin D levels (serum 25[OH]D < 20 ng/mL) was implemented. The participation of all patients began in spring (March–May), and the observation period ended in autumn (September–November). The summer season was selected as the observation period in this study, as in the northern hemisphere vitamin D is easily activated in summer because of UV exposure29. We set the observation period for summer in anticipation of increased vitamin D levels; unfortunately, patients with 25(OH)D levels >20 ng/mL at baseline, who did not receive instructions for vitamin D supplementation, showed decreased 25(OH)D levels after the observation period (Table 2). Therefore, the average 25(OH)D levels were almost identical before and after the partial interventions. One reason is the tendency of Japanese women to believe that UV exposure can cause skin problems; therefore, they avoid UV exposure by using UV-blocking cosmetics or a sunshade. A second possible reason is that the summer in Tokyo has become very hot and humid. Heat stroke has recently been noted as a public problem in Japan, especially in older people. People have generally been instructed to avoid outdoor exercise during the day in the summer, and to instead exercise indoors or outdoors in the early morning or late evening. Thus, UV exposure has consequently been low. Participants with low serum 25(OH)D levels were instructed to take vitamin D from natural food sources along with UV exposure. The participants who were given those instructions increased their 25(OH)D levels. Although the amount of time spent outdoors was not examined, we set the observation period for almost the same time to exclude seasonal effects. Neither participants with nor without increasing serum 25(OH)D levels showed any differences in other measurements, except for the serum CPR level (0.43 ng/mL) and a minimal change in the phosphate level (−0.04 mg/dL; Table 2), which was considered to be as a result of the vitamin D deficiency at baseline. A total of 57% of the participants in the increased vitamin D group had low 25(OH)D levels (<20 ng/mL), and also low serum CPR levels at the start of the observation period (CPR levels were 1.29 ± 0.59 and 1.98 ± 0.6 ng/mL in participants whose 25[OH]D level were <20 ng/mL [n = 22] and >20 ng/mL [n = 22], respectively). It was consistent with other reports that vitamin D deficiency was associated with a deficiency of insulin secretion30, 31. Consequently, participants with serum 25(OH)D levels ≤20 ng/mL showed lower serum CPR levels than did participants with serum 25(OH)D levels >20 ng/mL after the observation period, although not to a significant degree. This finding suggests that a continuous intervention to maintain vitamin D levels is important for all postmenopausal women with type 2 diabetes, regardless of the season or their 25(OH)D level. In addition, this result also suggests that insulin secretion is not directly activated by 25(OH)D in patients with type 2 diabetes mellitus, at least during a period of 6 months. The amount of exercise the patients carried out, which was evaluated using the lifestyle questionnaire, did not considerably change during the observation period; however, 60% of patients showed an increase in REE over the baseline level (Table 1). All patients had been regularly instructed on how to manage their diet and exercise for diabetes therapy. It was particularly effective to educate them at the beginning of the trial that exercise with UV exposure ensured an increase in vitamin D production and activation, because Japanese women tend to excessively avoid sun exposure. This result also shows that education for blood glucose control with retention of vitamin D is important for patients with type 2 diabetes mellitus. Although an increase in vitamin D appears to have directed this increase in REE, a direct correlation between the serum 25(OH)D level and REE was not found. Compared with vitamin D3, calcifediol was reported to improve gait speed in early postmenopausal women32; it is therefore possible that alfacalcidol treatment led to an increase in activity, although this change was not significant. Vitamin D supplementation was shown to ameliorate deoxyribonucleic acid damage in the liver and pancreas in a diabetic rat model by restoring a glucose utilization pathway33; however, other physiological reports of patients with type 2 diabetes mellitus who received vitamin D interventions also failed to reproduce the results from animal models11, 34-37. The long-term maintenance of adequate vitamin D levels38 or supplementation during an early stage of β-cell damage might be necessary to improve their ability to utilize glucose.
Considering the change in vitamin D level as an important factor, the association between the changes in bone and basal metabolism (ΔP1NP/CTX-1 and ΔREE) was strong (high β-value), and therefore significant in patients receiving alfacalcidol and those with increased 25(OH)D levels (Figure 2b; dotted lines). In contrast, no such correlation was observed in patients with decreased 25(OH)D levels during this period. Participants who took alfacalcidol were chosen because they had both low 25(OH)D levels and low BMD; therefore, the low serum 25(OH)D level in these participants had likely been ongoing for a long time before this intervention. As vitamin D activation was reduced in patients with diabetes mellitus14, 15, 39, taking activated vitamin D, even low-dose alfacalcidol, might be effective with taking natural vitamin D. The present data show that alfacalcidol affected the relationship with the effect of naturally increased vitamin D additively. Alfacalcidol does not affect serum 25(OH)D levels. Therefore, this unexpected result led us to hypothesize that vitamin D supplementation might be necessary for a normal association between basal and bone metabolism in postmenopausal diabetes patients with low vitamin D levels. Although it is possible that lifestyle changes could have affected the result, these data suggest that vitamin D itself affected the relationship between basal and bone metabolism. These data also show that vitamin D could be a key regulator of the correlation between basal and bone metabolism.
An increase in the vitamin D level is thought to influence REE; however, a direct correlation between serum 25(OH)D level and REE was not found in the present study. Consistent with our previous report of the baseline data7, RQ was correlated with the vitamin D level after the study intervention (Figure 2c). RQ reflects the inner respiratory function, decreases with lipid utilization and increases with glucose utilization40-42. Therefore, vitamin D might have enhanced glucose utilization in the present participants. Vitamin D is thought to improve insulin sensitivity, insulin secretion and lipid metabolism16, 43-45; however, the present data from the serum analyses did not show such improvements. In contrast, the ΔRQ correlated with the average glycated hemoglobin level during the observation period in patients receiving alfacalcidol and those with increased 25[OH]D levels (Figure 2d). The association of blood glucose control with RQ after an intervention with vitamin D supplementation (Figure 2d; fine dotted line) provided further support for the effect of vitamin D on metabolism in patients with type 2 diabetes mellitus.
Although vitamin D is thought to affect bone resorption during bone turnover46-48, the present data showed a positive relationship between 25(OH)D and P1NP, a marker of bone formation, in postmenopausal women with type 2 diabetes mellitus (Figure 2e). This correlation was prominent in patients taking alfacalcidol, similar to the relationship between P1NP/CTX-1 and REE. In ovariectomized mouse model studies, eldecalcitol, a vitamin D analog, was found to use a different mode of action that depends on the original phase of bone turnover. Eldecalcitol mainly affected the suppression of bone resorption during the high bone turnover phase, but it also increased bone formation in the low bone turnover phase49, 50. In addition, alfacalcidol has been shown to enhance the collagen quality in an ovariectomized rat model51. P1NP is a protein that reflects bone formation in collagen52. Therefore, the effectiveness of vitamin D with regard to increasing P1NP in postmenopausal women with type 2 diabetes mellitus and low bone turnover is consistent with data from previous animal studies.
Although the present study had a small number of participants, the inclusion criteria and observation season were strict when compared with those of other studies. Again, we confirmed an association between basal metabolism and bone metabolism. Vitamin D level was also closely related to bone mass53, and has been reported to be a determinant of basal metabolism12. Findings from our prospective partial intervention study suggested that vitamin D might play an important role, directly or indirectly, in a positive relationship between basal metabolism and bone metabolism. A low vitamin D level is a risk factor for fracture in postmenopausal women54. Therefore, the importance of vitamin D should be emphasized for postmenopausal women, especially those with type 2 diabetes mellitus. The present data also show that continuous interventions of vitamin D are necessary to restore vitamin D levels in patients at a high risk of fracture.
In conclusion, vitamin D is an important component in the control of RQ through glucose utilization, and therefore plays a critical role in the positive relationship between basal and bone metabolism. Vitamin D levels decrease easily, and so it is important for clinicians to provide continuous instruction regarding vitamin D restoration and supplementation to postmenopausal women with diabetes mellitus.
Acknowledgments
We thank Professor T Matsumoto for his advice, Ms M Tomioka for her assistance with the data analyses and Mr M Tomoda for de-identifying the patients' data. This study was partially supported by Grants-in-Aid (22510214) for Scientific Research from MEXT, the National Center for Global Health and Medicine (21A114) from the MHLW of Japan (NI), and the 8th research grant in the area of bone from Lilly (MO). The sponsors had no control over the interpretation, writing or publication of this work.
Disclosure
YU has received research funding and consultancy fees from Novartis Pharma K. K., Astellas Pharma Inc., Pfizer Inc., Chugai Pharmaceutical Co., Ltd., Boehringer Ingelheim Japan Inc., AstraZeneca K.K., Kyowa Hakko Kirin Co., Ltd., Alcon Japan Ltd., Otsuka Pharmaceutical Co., Ltd., Nipro Co., Eli Lilly Japan K. K., Kowa Co. Ltd., Eisai Co. Ltd., Takeda Pharmaceutical Co., Ltd., Sanofi K.K., Mitsubishi Tanabe Pharma Corporation., MSD K. K., Ono Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Terumo Corporation, Sumitomo Dainippon Pharma Co., Ltd. and Daiichi Sankyo Co., Ltd.; and honoraria for clinical trials: Novo Nordisk Pharma Ltd., Sanofi K. K., Eli Lilly Japan K. K. and Chugai Pharmaceutical Co., Ltd. MO received research funding from Eli Lilly Japan K.K. The other authors declare no conflict of interest.




