Association between particulate matter 2.5 and diabetes mellitus: A meta-analysis of cohort studies
Abstract
Aims/Introduction
The present meta-analysis was carried out to assess the association between exposure to the level of atmospheric particulate matter 2.5 (PM2.5; fine particulate matter with aerodynamic diameter less than 2.5 μm) and type 2 diabetes mellitus or gestational diabetes mellitus (GDM).
Materials and Methods
We searched the Medline, EMBASE, Cochrane and Web of Science databases to obtain articles according to the responding literature search strategies. Among a total of 279 identified articles, 55 were reviewed in depth, of which 10 articles (11 cohort studies) satisfied the inclusion criteria. Only cohort studies that disclosed the association between PM2.5 and type 2 diabetes mellitus or GDM were included in this article. A fixed-effects model was selected if P > 0.1 and I2 < 50%; otherwise, a random-effects model would be used to calculate the total effect value. Subgroup analysis was further carried out according to the types of diabetes mellitus (type 2 diabetes mellitus and GDM). The relative risk was used to estimate the association between PM2.5 and diabetes mellitus.
Results
The positive associations between PM2.5 and the incidence of type 2 diabetes mellitus were found in the long-term exposure period (relative risk 1.25, 95% confidence interval 1.10–1.43), which showed that with every 10-μg/m3 increase in PM2.5, the risk of type 2 diabetes mellitus would increase by 25% in the long-term exposure. Although the significant associations were not identified between maternal exposure to PM2.5 and GDM in the first trimester, the second trimester and the entire pregnancy periods, we could conclude that maternal exposure to PM2.5 in the entire pregnancy period would be more likely to lead to developing GDM (relative risk 1.162, 95% confidence interval 0.806–1.675) than the other two periods.
Conclusions
Long-term exposure to PM2.5 would be more likely to lead to developing type 2 diabetes mellitus, but more studies would be required to confirm the association between PM2.5 and GDM. It might be a wise to take effective measures to reduce PM2.5 exposure in vulnerable populations, especially for pregnant women.
Introduction
The prevalence of type 2 diabetes mellitus has rapidly increased in the past few decades worldwide, and it has contributed a large disease burden to the population's health1, 2. It was estimated that at least 366 million people suffered from type 2 diabetes mellitus in 2011, and it is estimated that this number will reach 566 million at the end of 20303. The increasing incidence of type 2 diabetes mellitus depends on the effects of genetic factors, obesity, social factors and other potential risk factors, such as air pollution4.
In recent years, air pollution has become a major public health issue, raising concerns around the world, especially for particulate matter 2.5 (PM2.5; fine particulate matter with aerodynamic diameter less than 2.5 μm). For its small size, PM2.5 can easily enter the depths of the respiratory tract and participate in blood circulation5, eliciting a wide range of biological responses. Hence, the association between exposure levels of PM2.5 and the effects on people's health has become a hot topic. Several major epidemiological studies showed that air pollution had adverse effects on cardiovascular disease, lung cancer and natural-cause mortality6-10. However, the effect of air pollution on type 2 diabetes mellitus risk has not been clearly described because of the inconsistent results. A great deal of experimental evidence has established possible pathways, including endothelial dysfunction4; immune response alterations in visceral adipose tissues; and endoplasmic reticulum stress resulting in alterations in insulin transduction11, insulin sensitivity and glucose metabolism4, 12. Meanwhile, a growing number of epidemiology studies have been trying to explore the associations between air pollution and type 2 diabetes mellitus13-20 or gestational diabetes mellitus (GDM)21-24. Some meta-analyses have also been published to show the associations between exposure to PM2.5 and type 2 diabetes mellitus25-27. However, a meta-analysis only including three articles showed an increased risk of type 2 diabetes mellitus by 10% per 10-μg/m3 increase in exposure to PM2.5 (odds ratio [OR] 1.10, 95% confidence interval [CI]: 1.02–1.18)25. A meta-analysis including four cohorts showed that the summary hazard ratio (HR) of the association between long-term exposure to PM2.5 and the incidence of type 2 diabetes mellitus was 1.11 (95% CI: 1.03–1.19) per 10-μg/m3 increase26. A Meta-analysis of five cohorts showed that the relative risks (RRs) of type 2 diabetes mellitus were significant for increments in concentrations of PM2.5 (1.39 per 10 mg/m3 increment, 95% CI: 1.14–1.68)27. However the last literature search in the aforementioned meta-analyses was only updated to 16 June 2014, and several new articles have been published since the last review. In addition, none of the meta-analyses described the association between exposure to PM2.5 and GDM until now.
Therefore, the purpose of the present meta-analysis of cohort studies was to estimate the long-term effects of exposure to PM2.5 on type 2 diabetes mellitus, and the effects of exposure to PM2.5 on GDM by different trimesters according to the standard guideline for carrying out and reporting meta-analyses of observational studies (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) in order to draw a coherent and consistent conclusion, and subsequently providing evidence for tackling the health effects of air pollution.
Methods
Databases
We searched the Medline, EMBASE, Cochrane and Web of Science databases with the following keywords: ‘particulate matter,’ ‘particulate matter 2.5,’ ‘PM2.5,’ ‘diabetes mellitus,’ ‘diabetes,’ ‘T2DM,’ ‘type 2 DM,’ ‘gestational diabetes’ and ‘gestational diabetes mellitus.’ The search strategy in the Medline database was as follows: (‘particulate matter’ OR ‘particulate matter 2.5′ OR ‘PM2.5’) AND (‘diabetes mellitus’ OR ‘diabetes’ OR ‘T2DM’ OR ‘type 2 DM’ OR ‘gestational diabetes’ OR ‘gestational diabetes mellitus’). Similar literature retrieval processes were carried out in the other databases according to different requirements. Bibliographic references and lists of relevant review articles were manually searched. Only peer-reviewed original articles published in English were considered. We limited our search to articles published from start to 10 August 2016.
Selection of articles and extraction of data
Inclusion criteria: (i) the definition of PM2.5 and diabetes mellitus had to be clearly stated; (ii) diabetes mellitus had to be diagnosed by physicians (or have clear medical records) or based on using related medications for controlling diabetes mellitus; (iii) we included two kinds of diabetes mellitus (type 2 diabetes mellitus and GDM), and excluded type 1 diabetes mellitus; (iv) original studies provided RRs or HRs, and their 95% CIs; and (v) only cohort studies were included. Exclusion criteria: (i) reviews, comments and lecture; (ii) repeat literature; (iii) the results in studies could not be transformed into RRs and their 95% CIs; (iv) animal studies; and (v) only exploring the mechanism of diabetes mellitus. Data were extracted by two independent investigators (Shaowen Wu and Haiping Zhao), and conflicts were adjudicated by a third investigator (Dian He). The study design, study population, adjustment and potential confounders ARE summarized in Table 1.
| First author, publication year | Locations | Study design | Sample size | Exposure | Outcomes | Data type | Adjustment | Time of follow up | Range of PM2.5 | Quality† |
|---|---|---|---|---|---|---|---|---|---|---|
| Brook RD (2013)15 | Canada | Prospective cohort | 2,145, 400 participants | PM2.5 | Type 2 diabetes mellitus | Per 10 mg/m3 for PM2.5 | High school diploma, adults with low income cut-off quintile, and adults unemployed determined at both the census divisions and census tracts levels | 1991–2001 | 8.7 ± 3.9 μg/m3 | 6 |
| Chen H (2013)16 | Ontario, Canada | Prospective cohort | 62,012 participants | PM2.5 | Type 2 diabetes mellitus | Per 10 mg/m3 for PM2.5 | Age, sex, BMI, education, race/ethnicity, household income adequacy, physical activity, smoking, and annual mean concentration of PM2.5 | 1996–2010 | Mean 10.6 μg/m3 (range 2.6–19.1) | 9 |
| Coogan PF (2012)13 | Los Angeles, USA | Prospective cohort | 3,992 participants | PM2.5, NOX | Type 2 diabetes mellitus | Per 10 mg/m3 for PM2.5 | Age, BMI, income, number of people in the household, history of diabetes mellitus, smoking, h/week of vigorous physical activity, and neighborhood socioeconomic status score in quintiles | 1995–2005 | 20.7 ± 2.10 μg/m3 | 8 |
| Puett RC (2011)NHS17 | Northeastern and Midwestern, USA | Prospective cohort | 74,412 participants | PM2.5, PM10 | Type 2 diabetes mellitus | Per 4 mg/m3 for PM2.5 | Age, season, calendar year, state of residence, time-varying cigarette smoking, time-varying hypertension, baseline BMI, time-varying alcohol intake, baseline physical activity, and time-varying diet | 1989–2002 | 17.5 ± 2.7 μg/m3 | 9 |
| Puett RC (2011) HPFS17 | Northeastern and Midwestern, USA | Prospective cohort | 15,048 participants | PM2.5, PM10 | Type 2 diabetes mellitus | Per 7 mg/m3 for PM2.5 | Age, season, calendar year, state of residence, time-varying cigarette smoking, time-varying hypertension, baseline BMI, time-varying alcohol intake, baseline physical activity, and time-varying diet | 1989–2002 | 18.3 ± 3.1 μg/m3 | 9 |
| To T (2015)20 | Ontario, Canadian | Prospective cohort | 29,549 participants | PM2.5 | Heart disease, Type 2 diabetes mellitus, asthma and COPD | Per 10 mg/m3 for PM2.5 | Potential confounders: baseline age, smoking, BMI, marital status, education and occupation | 1992–2013 | 12.47 ± 2.40 μg/m3 | 7 |
| Gudrun Weinmayr (2015)18 | Heinz Nixdorf, Germany | Prospective cohort | 3,607 participants | PM10 and PM2.5 | Type 2 diabetes mellitus | Per 2.29 mg/m3 for PM2.5 | Adjusted for age, sex, lifestyle variables, BMI, individual and neighborhood SES, and city | Mean follow-up 5.1 years | 16.7 μg/m3 (IQR 2.29 μg/m3) | 8 |
| Sung Kyun Park (2015)19 | Chicago, New York, and St. Paul, USA | Prospective cohort | 5,839 participants | PM2.5 and NOX | Type 2 diabetes mellitus | Per 2.43 mg/m3 for PM2.5 | Sex, age, race/ethnicity, family history of diabetes mellitus, educational level, smoking status, alcohol consumption, physical activity level, neighborhood socioeconomic status index, and body mass index and site | Median of 9 years of follow-up | 17.3 ± 3.1 μg/m3 | 7 |
| Hui Hu, (2015)22 | Florida, USA | Retrospective cohort | 410,267 women | PM2.5 and O3 | GDM | Per 5 mg/m3 for PM2.5 | Maternal age, race, education, marital status, season of conception, year of conception, prenatal care began, urbanization and median household income | 2004–2005 | 9.93 ± 1.67 μg/m3 | 6 |
| Abby F Fleisch, (2016)24 | Massachusetts, USA | Prospective cohort | 159,373 women | PM2.5 | GDM | Per 4.5 mg/m3 for PM2.5 | Maternal characteristics (age, race/ethnicity, education, prenatal insurance, smoking habits), census tract characteristics (median household income, percent open space, and median value of owner occupied housing), and timing of birth (season and date) | 2003–2008 | Range 1.3–19.3 μg/m3 | 7 |
| Abby F Fleisch, (2014)23 | Boston, USA | Prospective cohort | 2,093 women | PM2.5 | IGT, GDM | Per 2 mg/m3 for PM2.5 | Age, prepregnancy BMI, pregnancy weight gain, education, race/ethnicity, family history of diabetes, prior GDM and season of last menstrual period | 1999–2002, | range 8.5–15.9 μg/m3 | 8 |
| Candace A. Robledo, (2014)21 | 9 areas, USA‡ | Retrospective cohort | 219,952 women | PM2.5, NOX, SO2 and O3 | GDM | Per 5.28 mg/m3 for PM2.5 | Maternal age, race and study site | 2002–2008 | IQR 9.25 –14.53 μg/m3 | 7 |
- †The Newcastle–Ottawa Scale was used to assess the quality of the study. The higher the score mean the better quality the study. ‡Nine areas: Springfield, Massachusetts; Los Angeles, California; Newark, DE; Washington, DC; Indianapolis, Indiana; Salt Lake City, Utah; Brooklyn, New York; Cleveland, Ohio; Akron, Ohio. BMI, body mass index DM, diabetes mellitus; GDM, gestational diabetes mellitus; HPFS, Health Professionals Follow-up Study; IGT, impaired glucose tolerance; NHS, Nurses' Health Study; NOX, nitrogen oxides; PM2.5, particulate matter 2.5.
The quality of the included studies was assessed using the Newcastle Ottawa Scale for the cohort study28. It includes the following items: (i) representativeness of the exposed cohort; (ii) selection of the non-exposed cohort; (iii) ascertainment of exposure; (iv) demonstration that the outcome of interest was not present at the start of the study; (v) comparability of cohorts on the basis of the design or analysis (maximum of two scores); (vi) assessment of outcome; (vii) was follow up long enough for outcomes to occur; and (viii) adequacy of follow up of cohorts. Quality was categorized as three grades according the total score of items: 7–9 scores for excellent quality, 4–6 scores for good quality, and 0–3 scores for suboptimal quality. Only studies with excellent or good quality were finally included into the present meta-analysis.
Statistical analysis

All of the statistical analyses were carried out in Stata version 12.0 (StataCorp, College Station, Texas, USA), and a P-value <0.05 was considered statistically significant. Heterogeneity among studies was estimated using the Q-test. A fixed-effects model would be selected if P > 0.1 and I2 < 50%; otherwise, a random-effects model would be selected. Subgroup analysis was further carried out by types of diabetes mellitus (type 2 diabetes mellitus and GDM). Begg's funnel plot and Egger's test were used to assess publication bias. A sensitivity analysis was used to assess the stability of the results.
Results
In total, 279 articles were obtained from the literature search. Among them, 224 articles were excluded for being without eligible titles and abstracts. After reading the full texts of the remaining 55 articles, finally 11 cohort studies from 10 articles met the inclusion criteria. One article (Puett 2011)17 included two different study populations (Nurses' Health Study and Health Professionals Follow-up Study). There were 3,131,544 participants included from these 11 cohort studies.
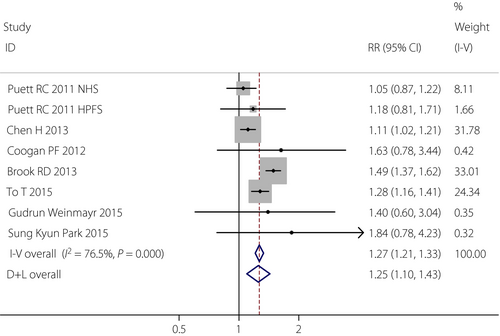
Seven studies explored the association between exposure to PM2.5 and type 2 diabetes mellitus13-20. The positive association between PM2.5 and the incidence of type 2 diabetes mellitus was found on the long-term exposure (RR 1.25, 95% CI: 1.10–1.43), which showed that with every 10-μg/m3 increase in PM2.5, the risk of type 2 diabetes mellitus would increase by 25% with long-term exposure to PM2.5. Four studies investigated the effects of maternal exposure to PM2.5 on GDM21-24. Although the significant associations were not identified between maternal exposure to PM2.5 and GDM in the first trimester, the second trimester and the entire pregnancy period, we could see that maternal exposure to PM2.5 in the entire pregnancy period would be more likely to lead to developing GDM (RR 1.162, 95% CI: 0.806–1.675) than the other two periods. It meant that longer-term exposure to PM2.5 would be more likely to lead to developing type 2 diabetes mellitus or GDM. The flow chart of selecting articles is shown in Figure 1. The details of these studies are given in Table 1.
PM2.5 and diabetes mellitus
In the present meta-analysis, seven cohort studies examined the risk of PM2.5 on type 2 diabetes mellitus based on the long-term exposure. Heterogeneity was observed for PM2.5 with type 2 diabetes mellitus, and the random-effects model was used to analyze the overall effect based on I2 of 76.5%, with the overall RR of 1.25 (95% CI: 1.10–1.43). This result showed that with every 10-μg/m3 increase in PM2.5, the risk of type 2 diabetes mellitus would increase by 25% (Figure 2). According to Egger's test, no publication bias was found in this analysis, with the value of P = 0.967 (Figure 3). After sequentially excluding each study, similar results were obtained. As all RR values of sensitivity analyses were significantly more than 1, it suggested that the results of the present meta-analysis were robust (Table 2).

| Study omitted | Estimate (RR) | Low of 95% CI | Up of 95% CI |
|---|---|---|---|
| Puett RC 2011 NHS | 1.288 | 1.225 | 1.355 |
| Puett RC 2011 HPFS | 1.269 | 1.209 | 1.332 |
| Chen H 2013 | 1.348 | 1.272 | 1.429 |
| Coogan PF 2012 | 1.266 | 1.206 | 1.328 |
| Brook RD 2013 | 1.170 | 1.103 | 1.241 |
| To T 2015 | 1.263 | 1.195 | 1.335 |
| Gudrun Weinmayr 2015 | 1.267 | 1.207 | 1.329 |
| Sung Kyun Park 2015 | 1.266 | 1.206 | 1.328 |
- 95% CI, 95% confidence interval; RR, relative risk.
PM2.5 and GDM
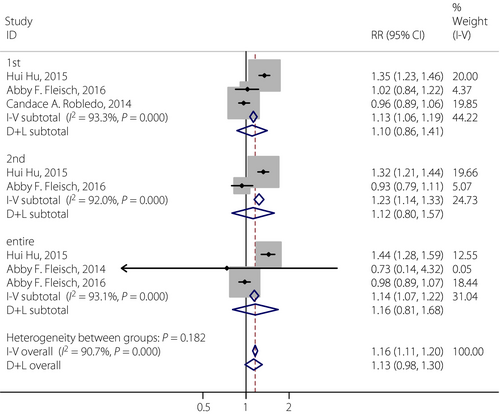
In the present meta-analysis, four cohort studies were reviewed to assess the effect of exposure to PM2.5 on GDM during the first trimester, second trimester and the entire pregnancy period. Heterogeneity for PM2.5 and GDM was observed, with I2 above 90%. Hence, the random-effects model was applied to analyze the overall effect. Although the significant associations were not identified between maternal exposure to PM2.5 and GDM in the first trimester (RR 1.102, 95% CI: 0.864–1.40), the second trimester (RR 1.121, 95% CI: 0.798–1.575) and the entire pregnancy period (RR 1.162, 95% CI: 0.806–1.675), we could see that maternal exposure to PM2.5 during the entire pregnancy period would be more likely to lead to developing GDM than the other two periods. The result showed that with every 10-μg/m3 increase in PM2.5, the risk of GDM would increase by 16% during the entire pregnancy (Figure 4). Just four studies were included in the present meta-analysis for PM2.5 and GDM, therefore Egger's test and sensitivity analyses were only carried out within all trimesters (Figure 5). Because the P-value was 0.689 in Egger's test, no publication bias was found for all trimesters. After removing each article sequentially, statistically steady results were obtained, which suggested the results of the present meta-analysis were robust.
Discussion
The present meta-analysis showed a deleterious effect of long-term exposure PM2.5 on the incidence of type 2 diabetes mellitus. Several previous studies explored the associations between PM2.5 with incident type 2 diabetes mellitus30, diabetes mortality15 or prevalent impaired glucose metabolism31. A large Canadian study, including more than 2 million participants, showed a strong positive association between PM2.5 and diabetes mortality15. Weinmayr et al.18 prospectively followed 3,607 individuals without diabetes at baseline (mean follow-up time 5.1 years). In that study, he found that adjusted RRs for PM2.5 and type 2 diabetes mellitus was 1.03 (95% CI: 0.95–1.12), and the result showed that long-term exposure to PM2.5 would increase the risk of type 2 diabetes mellitus in the general population. To et al.20 followed up women who enrolled in the Canadian National Breast Screening Study (n = 29,549), and the result showed a > 20% increase of diabetes mellitus per 10-μg/m3 increase in PM2.5 (prevalence ratio 1.28, 95% CI: 1.16–1.41). In addition, other cohort studies also reported relatively strong associations between exposure to PM2.5 and the incidence of diabetes mellitus (RRs from 1.25 to 1.42)17, 31. Previous experimental studies showed that PM2.5 could lead to insulin resistance through oxidative damage, endothelial dysfunction and inflammation32-34. In the present meta-analysis, only the long-term exposure to PM2.5 was considered, hence the total association effect value of PM2.5 and type 2 diabetes mellitus might be higher than those values in other studies; and we should investigate the short-term effects of PM2.5 exposure and the incidence type 2 diabetes mellitus in the future.
The present study did not show that increases in GDM were associated with high levels of PM2.5. Meanwhile, previous studies lack consistent results of the association between PM2.5 exposure and GDM, especially for human observational studies35. The study by van den Hooven et al.36 investigated the association between residential proximity to traffic and various pregnancy outcomes in 7,339 pregnant women. Their result showed that mothers exposed to residential traffic had no higher risk of GDM, but the authors believed that there was considerable variation in distance-weighted traffic density in that study. Hu et al.22 investigated the association between the risk of GDM and two kinds of air pollutants (PM2.5 and O3) during different trimesters among 410,267 women in Florida, USA. The result showed that the incidence of GDM increased with 5-μg/m3 increase in PM2.5 (OR first trimester 1.16, 95% CI: 1.11–1.21; OR second trimester 1.15, 95% CI: 1.10–1.20; OR pregnancy 1.20, 95% CI 1.13–1.26) in single-pollutant models. Fleisch et al.24 showed that the prevalence of impaired glucose tolerance in the second trimester was elevated in the highest (vs lowest) quartile of exposure to PM2.5 (OR 2.63, 95% CI: 1.15–6.01); however, exposure to PM2.5 was not positively associated with GDM. The study by Slama et al.37 showed that PM2.5 might affect pregnant women in their cardiovascular system and general health condition through hindering the input of glucose, oxygen, and insulin. Just four studies were included in the present meta-analysis for PM2.5 and GDM, hence the overall effect result between them was not strong, and more related studies will be required in the future.
The present meta-analysis from 11 cohort studies could confirm the association between exposure to PM2.5 and type 2 diabetes mellitus. Meanwhile, it is the first meta-analysis to describe the association between PM2.5 and GDM. However, several limitations to the present study should be considered. First, we found different degrees of heterogeneity across PM2.5 and diabetes mellitus, which could be partly explained by differences in population demography, sample size, exposure assessment, compounds of particulate matters and so on. Second, we only described the impact of single pollutants without taking the combined effects of multipollutants into account. Finally, relatively fewer numbers of studies focused on PM2.5 and GDM compared with type 2 diabetes mellitus. Thus, more studies related to the subject of PM2.5 and GDM are still required.
Disclosure
The authors declare no conflict of interest.




