Prevention and treatment of peri-implant diseases—The EFP S3 level clinical practice guideline
Abstract
Background
The recently published Clinical Practice Guidelines (CPGs) for the treatment of stages I–IV periodontitis provided evidence-based recommendations for treating periodontitis patients, defined according to the 2018 classification. Peri-implant diseases were also re-defined in the 2018 classification. It is well established that both peri-implant mucositis and peri-implantitis are highly prevalent. In addition, peri-implantitis is particularly challenging to manage and is accompanied by significant morbidity.
Aim
To develop an S3 level CPG for the prevention and treatment of peri-implant diseases, focusing on the implementation of interdisciplinary approaches required to prevent the development of peri-implant diseases or their recurrence, and to treat/rehabilitate patients with dental implants following the development of peri-implant diseases.
Materials and Methods
This S3 level CPG was developed by the European Federation of Periodontology, following methodological guidance from the Association of Scientific Medical Societies in Germany and the Grading of Recommendations Assessment, Development and Evaluation process. A rigorous and transparent process included synthesis of relevant research in 13 specifically commissioned systematic reviews, evaluation of the quality and strength of evidence, formulation of specific recommendations, and a structured consensus process involving leading experts and a broad base of stakeholders.
Results
The S3 level CPG for the prevention and treatment of peri-implant diseases culminated in the recommendation for implementation of various different interventions before, during and after implant placement/loading. Prevention of peri-implant diseases should commence when dental implants are planned, surgically placed and prosthetically loaded. Once the implants are loaded and in function, a supportive peri-implant care programme should be structured, including periodical assessment of peri-implant tissue health. If peri-implant mucositis or peri-implantitis are detected, appropriate treatments for their management must be rendered.
Conclusion
The present S3 level CPG informs clinical practice, health systems, policymakers and, indirectly, the public on the available and most effective modalities to maintain healthy peri-implant tissues, and to manage peri-implant diseases, according to the available evidence at the time of publication.
Clinical Relevance
Scientific rationale for study: Peri-implant diseases, specifically peri-implant mucositis and peri-implantitis, are highly prevalent and their management is challenging, and are associated with significant morbidity. This clinical practice guideline (CPG) provides guidance on the management of peri-implant diseases. The recommendations described in this CPG have been formulated following a rigorous evidence-based and patient-centred decision-making process.
Principal findings: This guideline covers preventive and treatment interventions for peri-implant diseases to be implemented during the planning, execution and long-term follow-up of tooth replacement with dental implants. It identifies specific interventions demonstrated to be effective and structures them in needs-based care pathways. It also examines the current level of scientific support for a variety of widely employed approaches and techniques.
Practical implications: The application of this S3 level CPG will facilitate a consistent, interdisciplinary and evidence-based approach to the prevention and treatment of peri-implant diseases.
1 INTRODUCTION
1.1 The health problem
1.1.1 Definition
Peri-implant diseases are inflammatory conditions that affect the peri-implant tissues and are induced by peri-implant biofilms. There are two distinct conditions: peri-implant mucositis and peri-implantitis.
Peri-implant mucositis is ‘an inflammatory lesion of the peri-implant mucosa, in the absence of continuing marginal bone loss’ (Heitz-Mayfield & Salvi, 2018). It is characterized clinically by bleeding on gentle probing. Other clinical signs of inflammation may be present, such as erythema, swelling and/or suppuration, and an increase in probing depth (PD) is frequently observed in the presence of peri-implant mucositis due to oedema or a decrease in probing resistance (Berglundh et al., 2018). Peri-implant mucositis is primarily caused by a disruption of host–microbial homeostasis at the implant–mucosa interface and is a reversible condition when assessed indirectly at the host biomarker level (Heitz-Mayfield & Salvi, 2018). Additional factors associated with the onset and progression of peri-implant mucositis include biofilm accumulation, smoking and radiation therapy (Berglundh et al., 2018).
Peri-implantitis has been defined as a ‘peri-implant biofilm-associated pathological condition, occurring in tissues around dental implants, and characterized by inflammation in the peri-implant mucosa and subsequent progressive loss of supporting bone’ (Berglundh et al., 2018). Clinically, peri-implantitis sites exhibit inflammation, bleeding on probing (BOP) and/or suppuration, increased PDs and/or recession of the mucosal margin, in addition to radiographic bone loss compared with previous examinations (Berglundh et al., 2018). The primary etiological factor for peri-implantitis onset and progression is the accumulation of a peri-implant plaque biofilm. Important risk factors/indicators have been identified, including a history of severe periodontitis, poor plaque control and no regular supportive peri-implant care (SPIC) following implant therapy. Less conclusive evidence was found for smoking and diabetes, or local factors such as the presence of submucosal cement following prosthetic restoration of the implant, or positioning of implants limiting access to oral hygiene (OH) and maintenance. Other factors such as the absence of peri-implant keratinized mucosa (PIKM), occlusal overload, presence of titanium particles within peri-implant tissues, bone compression necrosis, overheating, micromotion or biocorrosion have been proposed as risk factors for peri-implant diseases onset and/or progression, but further research is required to clarify their true roles (Schwarz et al., 2018).
Peri-implant diseases, especially peri-implantitis, represent a growing public health problem due to their high prevalence and the associated consequences (implant and implant-supported prosthesis loss), including dental care costs, which are substantial.
1.1.2 Pathophysiology
To better understand the pathophysiology of peri-implant diseases, knowledge of the pathophysiology of periodontal diseases has been extensively used, and findings on peri-implant mucositis have been likened to those of biofilm-induced gingivitis. The same applies to peri-implantitis and periodontitis. However, when compared with periodontal tissues, peri-implant tissues lack cementum and periodontal ligament; thus, there are only two peri-implant tissue layers, alveolar bone and peri-implant mucosa. Additional differences are found in the peri-implant mucosa: the peri-implant epithelial attachment is usually longer; the connective tissue exhibits no fibres inserting into the supra-crestal area; and vascularization is lower.
Peri-implant biofilms are considered to be the primary aetiological factor for peri-implant mucositis, based on strong evidence derived from animal and human studies (Berglundh et al., 2018). Such biofilms form on the hard, non-shedding surfaces of the implant and implant-supported restorations, similar to the formation of dental plaque biofilms on teeth (Bermejo et al., 2019; Sanchez et al., 2014). Histologically, peri-implant mucositis is similar to gingivitis: a well-defined inflammatory lesion, adjacent to the junctional/pocket epithelium, richly infiltrated by vascular structures, plasma cells and lymphocytes, but not extending apically to the junctional/pocket epithelium, or into the supra-crestal area (Berglundh et al., 2018; Heitz-Mayfield & Salvi, 2018).
Evidence exists to support the contention that peri-implant mucositis is treatable, and can be successfully managed by careful control of the peri-implant biofilm. However, if allowed to persist, peri-implantitis develop, as it is believed that peri-implant mucositis always precedes peri-implantitis (Berglundh et al., 2018; Heitz-Mayfield & Salvi, 2018).
The primary aetiological agent for peri-implantitis is also the accumulation of the peri-implant biofilm, with human observational studies demonstrating a higher risk of incident peri-implantitis in patients with poor biofilm control and/or non-adherence to maintenance care, and based on intervention studies using anti-infective approaches (Berglundh et al., 2018).
Peri-implantitis lesions are larger than those associated with peri-implant mucositis or with periodontitis and are characterized by greater number of neutrophils and larger proportions of B cells when compared with peri-implant mucositis. Consistent with periodontitis lesions, plasma cells and lymphocytes predominate within the immune-inflammatory infiltrate (Schwarz et al., 2018). However, these characteristic histological features have not been associated with specific bacteria (Sahrmann et al., 2020) or proinflammatory cytokine profiles (Berglundh et al., 2018).
1.1.3 Prevalence
During the XI European Workshop in Periodontology (2014), entitled ‘Effective Prevention of Periodontal and Peri-implant Diseases’, a systematic review (SR) was specifically commissioned to address the prevalence of peri-implant diseases. Eleven studies were selected and the meta-analyses demonstrated a patient-level prevalence estimate of 43% (95% confidence interval—CI [32; 54]) for peri-implant mucositis and 22% (95% CI [14; 30]) for peri-implantitis (Derks & Tomasi, 2015). Another SR, comprising 47 studies, reported a prevalence of 46.83% (95% CI [38.30; 55.36]) for peri-implant mucositis and of 19.83% (95% CI [15.38; 24.27]) for peri-implantitis (Lee et al., 2017).
1.1.4 Consequences of failure to treat peri-implant diseases
As described above, peri-implant mucositis can be treated and resolved, but if left untreated, can progress to peri-implantitis; peri-implant mucositis is widely believed to precede peri-implantitis. Peri-implantitis can be initiated rapidly following prosthetic restoration and loading of the fixture during function, and if no treatment is provided, it is likely to progress in a non-linear accelerating pattern (Berglundh et al., 2018), and at a faster rate than is typically seen in periodontitis lesions (Schwarz et al., 2018).
Progression of peri-implantitis will most likely lead to the loss of the affected implant and the implant-supported prosthesis.
Limited information is available on the impact of peri-implant diseases on the quality of life. One study concluded that neither peri-implantitis nor surgical treatment of the same had any impact on Oral Health Related Quality of Life (Rustand et al., 2022), while another study assessing morbidity after non-surgical and surgical treatment of peri-implantitis concluded that pain levels were low to moderate and most pronounced in the first 2 days (Norum et al., 2019).
1.1.5 Financial aspects
According to a market analysis report (Grand View Research, 2022), the global market size of dental implants is estimated at US $4.6 billion in 2022 and is expected to grow at an annual rate of around 10%, up to 2030. The increase is based upon the demand for treatment with dental implants by the population and on the widening range of clinicians providing implant therapy. It is also associated with the growing need for longer term supportive care to avoid/control biological and mechanical complications, including managing complications with implant-supported restorations and maintaining peri-implant tissue health (Alani et al., 2014). There is increasing awareness of the need to plan long-term supportive care programmes during the treatment planning phase, and of the financial, biological and legal consequences of not doing so. For example, patients may be able to cover the initial cost of dental implants and their associated restorations at the time of implant placement, when they are employed and earning a living, but the long-term cost of supportive care may not be explained clearly to patients and may impact when they are no longer economically active (Alani et al., 2014). A Swedish study of 514 subjects recently calculated such costs (Karlsson et al., 2022), including the costs of preventive measures and of procedures to treat implant complications, over a period of 8.2 years. The mean cost ranged from €878 (single-tooth restoration) to €1210 (full-arch restoration), the larger proportion of the cost being for prevention (€741), while implant loss was the most expensive complication (€1508), followed by peri-implantitis (€1244).
A cost-effectiveness analysis was undertaken to assess preventive, non-surgical and surgical interventions (Schwendicke et al., 2015), with the model assuming that each implant was followed for 20 years. The annual provision of SPIC was dichotomized and the risk profile of patients was also considered, with implant loss and cost as primary outcomes. For management of peri-implantitis, 11 approaches (non-surgical and surgical instrumentation alone or with adjuncts) were compared. The authors concluded that, within the limitations of their study methodology, not providing annual SPIC increased the risk of peri-implant diseases. Conversely, providing SPIC could prevent or delay the onset of disease and was cost-effective, especially in high-risk groups.
Cost-effectiveness has also been evaluated for non-surgical treatment approaches of peri-implantitis (Listl et al., 2015). Change in PD was the primary outcome when comparing eight interventions. Instrumentation alone, use of an air-polishing device, or combining instrumentation with local antiseptics/antibiotics provided better value for money than Er:YAG laser, a specific ultrasonic device (Vector®), photodynamic therapy (PDT) or instrumentation combined with chlorhexidine.
Of relevance is the cost comparison of SPIC with that of the supportive care of teeth. This was assessed in a private practice in Norway (Fardal & Grytten, 2013) in 43 patients with 847 teeth and 119 implants. The mean number of ‘disease-free years’ was 8.66 for implants, 9.08 for neighbouring teeth, and 9.93 for teeth on the contra-lateral side of the mouth, with no statistically significant differences. However, due to the high prevalence of peri-implantitis, the extra cost of maintaining implants was five times higher than for teeth.
Finally, financial considerations should include the economic impact of edentulism. While not yet clearly established, at least two factors may support its importance: firstly, the need for rehabilitation and the associated costs; secondly, and in case of lack of rehabilitation, the negative consequences for quality of life, nutrition, systemic health and well-being. In addition, it is also widely contended that individual- and community-level social inequalities strongly impact on levels of edentulism (Ito et al., 2015).
2 AIM OF THE GUIDELINE
This guideline aims to identify best practice interventions for preserving the health of peri-implant tissues and, thereby, extending the longevity of complication-free survival of dental implants when used to replace missing teeth. The main objective, therefore, is to summarize the evidence-based recommendations for individual interventions used in the management (both prevention and treatment) of peri-implant diseases, based on the best available evidence and/or expert consensus. In so doing, this guideline aims to: (i) inform sound preventive/therapeutic approaches to the management of peri-implant diseases, and thereby improve the overall quality of peri-implant interventions undertaken in Europe and worldwide; (ii) reduce dental implant loss arising due to peri-implantitis; and (iii) ultimately reduce medical and dental costs and improve the quality of life of patients.
2.1 Target users of the guideline
Oral health professionals, together with stakeholders, related to oral health care. In addition, this CPG aims to inform medical professions, health systems, policymakers, patients and the public.
2.2 Target environments
Academic/hospital environments, community-based dental clinics and practices.
2.3 Target patient population
- People awaiting dental implant rehabilitation.
- People receiving dental implant rehabilitation.
- People with dental implants and, therefore, at risk of developing peri-implant diseases.
- People with peri-implant mucositis.
- People with peri-implantitis.
- People with peri-implant mucositis, following successful peri-implant treatment.
- People with peri-implantitis, following successful peri-implant treatment.
2.4 Exceptions from the guideline
This guideline does not consider in detail the health/economic cost–benefit ratio of the proposed therapies, since (i) the target users and patient populations include people in different countries with diverse, not readily comparable healthcare systems, and (ii) there is a paucity of sound scientific data available addressing this issue.
This guideline does not consider the management of other peri-implant tissue conditions, such as hard- and soft tissue deficiencies around dental implants (Hammerle & Tarnow, 2018), unusual peri-implant problems (such as peri-implant peripheral giant-cell granuloma, pyogenic granuloma, squamous cell carcinoma, metastatic carcinomas and malignant melanoma) or implant fractures, that may mimic or share certain clinical features with biofilm-associated peri-implant conditions (Renvert et al., 2018).
3 METHODOLOGY
3.1 General framework
This guideline was developed following methodological guidance published by the Standing Guideline Commission of the Association of Scientific Medical Societies in Germany (AWMF) (https://www.awmf.org/leitlinien/awmf-regelwerk/awmf-guidance.html) and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group (WG) (https://www.gradeworkinggroup.org/).
The guideline was developed under the auspices of the European Federation of Periodontology (EFP) and overseen by the EFP Workshop Committee. This guideline development process was steered by an Organizing Committee and a methodology consultant designated by the EFP. All members of the Organizing Committee participated in the EFP Workshop Committee.
To ensure adequate stakeholder involvement, the EFP established a guideline panel involving dental professionals representing national periodontal societies within the EFP, together with experts in Prosthodontics, Implant Dentistry and Oral Surgery (Table 1). These delegates were nominated and selected by the Organizing Committee and participated in the guideline development process with voting rights in the consensus conference. For the guideline development process, delegates were assigned to four WGs that were chaired by selected members of the Organizing Committee and guided by the methodology consultant. This panel was supported by key stakeholders from European scientific societies with a strong professional interest in periodontal care and from European organizations representing key groups within the dental profession (Table 2), and key experts from non-EFP member regions, such as North America and Australia.
| Scientific society/organization | Delegate(s) |
|---|---|
| European Federation of Periodontology (EFP) | Organizing Committee, Working Group Chairs (in alphabetic order): Tord Berglundh, Iain Chapple, David Herrera, Søren Jepsen, Moritz Kebschull, Panos Papapanou, Mariano Sanz, Frank Schwarz, Anton Sculean, Maurizio Tonetti |
| Methodologist: Ina Kopp | |
Clinical Experts (in alphabetic order): Mario Aimetti Juan Blanco Nagihan Bostanci Philippe Bouchard Nurcan Buduneli Elena Calciolari María Clotilde Carra Raluca Cosgarea Jan Cosyn Bettina Dannewitz Beatriz de Tapia Yvonne de Waal Jan Derks Henrik Dommisch Nikos Donos Peter Eickholz Bahar Eren Kuru Elena Figuero Moshe Goldstein Filippo Graziani Jasmin Grischke Fernando Guerra Lisa Heitz-Mayfield Karin Jepsen Odd Carsten Koldsland France Lambert Antonio Liñares Bruno Loos Phoebus Madianos Paula Matesanz Ana Molina Virginie Monnet Corti Eduardo Montero Frauke Müller Luigi Nibali Andrés Pascual Ioannis Polyzois Marc Quirynen Ausra Ramanauskaite Stefan Renvert Mario Roccuzzo Philipp Sahrmann Giovanni Salvi Nerea Sánchez Ignacio Sanz Lior Shapira Andreas Stavropoulos Meike Stiesch Wim Teughels Cristiano Tomasi Leonardo Trombelli Anders Verket Asaf Wilensky |
|
| Scientific societies | |
| European Dental Hygienists Federation | Gitana Rederiene |
| EFP—Executive Committee | Darko Božić |
| EFP—Executive Committee | Monique Danser |
| EFP—Executive Committee | Spyros Vassilopoulos |
| EFP—Executive Committee | Nicola West |
| European Society of Endodontology | Lise-Lotte Kirkevang |
| Other organizations | |
| Council of European Dentists | Paulo Melo |
| European Dental Students' Association | Ieva Tamošiūnaitė |
| Platform for Better Oral Health in Europe | Kenneth Eaton |
| Institution/society | Acronym | Answera | Representative |
|---|---|---|---|
| Association for Dental Education in Europe | ADEE | No proposal | None |
| Continental European Division of IADR | CED-IADR | No proposal | None |
| Council of European Chief Dental Officers | CECDO | No answer | None |
| Council of European Dentists | CED | Participant | Paulo Melo |
| European Association for Osseointegration | EAO | Participant | Cancelled |
| European Association of Dental Public Health | EADPH | No answer | None |
| European Dental Hygienists Federation | EDHF | Participant | Gitana Rederiene |
| European Dental Students' Association | EDSA | Participant | Ieva Tamošiūnaitė |
| European Federation of Conservative Dentistry | EFCD | No answer | None |
| European Orthodontic Society | EOS | No answer | None |
| European Prosthodontic Association | EPA | No answer | None |
| European Society of Endodontology | ESE | Participant | Lise Lotte Kirkevang |
| Platform for Better Oral Health in Europe | PBOHE | Participant | Kenneth Eaton |
- a Messages sent on April 4, 2022.
In addition, the EFP engaged an independent guideline methodologist to advise the panel and facilitate the consensus process (Prof. Dr. med. Ina Kopp [I.K.]). The guideline methodologist had no voting rights.
The EFP and the guideline panel attempted to involve patient forums/organizations but were unable to identify any groups focused on periodontal diseases at a pan-European level. In future updates, efforts will be undertaken to include the perspectives of citizens/patients (Brocklehurst et al., 2018). National societies will be encouraged to involve patient groups within individual countries as key stakeholders for the Adaptation, Adoption, De Novo Development—‘ADOLOPMENT’ of this CPG (Schunemann et al., 2017).
3.2 Evidence synthesis
3.2.1 Systematic search and critical appraisal of guidelines
- Guideline International Network (GIN)
- Guidelinecentral.com
- The National Institute for Health and Clinical Excellence (NICE)
- Canadian Health Technology Assessment (CADTH)
- European Federation of Periodontology (EFP)
- American Academy of Periodontology (AAP)
- American Dental Association (ADA)
- BIGG International database of GRADE guidelines
- ECRI Guidelines trust
- DynaMed database
- US Preventive Services Task Force
- Scottish Intercollegiate Guidelines Network, Healthcare Improvement Scotland (SIGN-HIS)
The last search was performed on 13 January 2023. Search terms used were
‘implant’, ‘dental implant’, ‘peri-implant*’, ‘guidelines’ and ‘clinical practice guidelines (CPG)’. In addition, content was screened by hand searches, see Table 3.
| Database | Identified, potentially relevant guidelines | Critical appraisal |
|---|---|---|
| Guideline International Network (GIN) International Guidelines Librarya | No thematically relevant hits | Not applicable |
| The National Institute for Health and Clinical Excellence (NICE)b | Insertion of customized exposed titanium implants, without soft tissue cover, for complex orofacial reconstruction (Jul 2013) | Focus on orofacial implants, therefore potentially relevant But: Data more than a decade old, does not directly address biological complications Not applicable |
| Insertion of customized titanium implants, with soft tissue cover, for orofacial reconstruction (Jul 2013) | Focus on orofacial implants, therefore potentially relevant But: Data more than a decade old, does not directly address biological complications Not applicable |
|
| Soft-palate implants for simple snoring (Nov 2007) | Focus on oral implants, therefore potentially relevant But: Data more than 15 years old, focus on palatal implants, does not directly address biological complications Not applicable |
|
| Soft-palate implants for obstructive sleep apnoea (Nov 2007) | Focus on oral implants, therefore potentially relevant But: Data more than 15 years old, focus on palatal implants, does not directly address biological complications Not applicable |
|
| Guidelinecentral.com ‘Dentistry’ category | Antibiotic prophylaxis for prevention of prosthetic joint infection (Jan 2015) | Does not readily address per-implant diseases Not applicable |
| Prevention of orthopaedic implant infection in patients undergoing dental procedures (Dec 2012) | Does not readily address per-implant diseases Not applicable |
|
| Agency for Healthcare Research and Qualityc | No thematically relevant hits | Not applicable |
| Canadian Health Technology Assessment (CADTH)d | Biological mesh: A review of clinical effectiveness, cost-effectiveness and guidelines—an update (Aug 2015) | Focus on implants in other areas, no direct relation to oral diseases Not applicable |
| Osseointegrated Prosthetic Implants for Lower Limb Amputation: A Review of Clinical Effectiveness, Cost-Effectiveness and Guidelines (Feb 2017) | Focus on implants in other areas, no direct relation to oral diseases Not applicable |
|
| Immediate osseointegrated implants for cancer patients: A review of clinical and cost-effectiveness (Jan 2015) | Focus on dental implants in very specific, selected patient group, peri-implantitis not directly addressed, 7-year-old data Not applicable |
|
| European Federation of Periodontology (EFP)e | EFP S3-level clinical practice guideline for stages I–III periodontitis | Indirectly applicable, high quality |
| EFP S3-level clinical practice guideline for stage IV periodontitis | Indirectly applicable, high quality | |
| American Academy of Periodontology (AAP)f | AAP best evidence consensus: Biologics in clinical practice (Oct 2022) |
Focus on periodontal defects only—peri-implantitis not addressed Not applicable |
AAP best evidence consensus: Periodontal phenotype (Jan 2020) |
Focus on tissues around teeth, rather than dental implants Not applicable |
|
AAP best evidence consensus: Laser therapy (Apr 2018) |
Potentially relevant: Two SRs address adjunctive laser use and photodynamic therapy, respectively, for peri-implant mucositis and peri-implantitis But: More than 4 years old, superseded by new SRs in current guideline Not directly applicable |
|
AAP best evidence consensus: Cone-beam computed tomography (Oct 2017) |
Does not readily address per-implant diseases Not applicable |
|
| American Dental Association (ADA)g | No thematically relevant hits | Not applicable |
| BIGG International database of GRADE guidelinesh | Antibiotic prophylaxis is not indicated prior to dental procedures for prevention of periprosthetic joint infections (2017) | Does not readily address per-implant diseases Not applicable |
| ECRI Guidelines Trusti | No thematically relevant hits | Not applicable |
| DynaMedj | Anaerobic bacterial infections | Does not readily address per-implant diseases Not applicable |
| Gingivitis and periodontitis in adults | Does not readily address per-implant diseases Not applicable |
|
| Oral healthcare in persons with diabetes | Potentially applicable, as it addresses an important risk factor But: No specific recommendations, no standardized methodology, no guideline Not applicable |
|
| US Preventive Services Task Forcek | Dental and periodontal disease: Counselling (1996) | More than two decades old, does not readily address peri-implant conditions Not applicable |
| Scottish Intercollegiate Guidelines Network, Healthcare Improvement Scotland (SIGN-HIS)l | No thematically relevant hits | Not applicable |
- a https://guidelines.ebmportal.com/.
- b https://www.nice.org.uk/guidance/published?type=csg,cg,mpg,ph,sg,sc.
- c https://www.ahrq.gov/gam/index.html.
- d https://www.cadth.ca/.
- e http://www.efp.org/publications/index.html.
- f https://www.perio.org/research-science/best-evidence-consensus-bec/.
- g https://ebd.ada.org/en/evidence/guidelines.
- h https://sites.bvsalud.org/bigg/en/biblio/.
- i https://www.ecri.org/solutions/ecri-guidelines-trust.
- j https://www.dynamed.com/.
- k https://www.uspreventiveservicestaskforce.org/uspstf/topic_search_results.
- l https://www.sign.ac.uk/our-guidelines/.
Only guidelines published in English and with full texts available were included. The methodological quality of these guideline texts was critically appraised using the AGREE II framework (https://www.agreetrust.org/agree-ii/).
We did not identify guidelines/documents directly relevant to the current guideline development process due to: (i) their publication time, (ii) their methodological approach or (iii) their stated inclusion criteria. We have referenced the EFP S3-level CPGs (Herrera et al., 2022; Sanz et al., 2020), where applicable.
3.2.2 Systematic search and critical appraisal of the literature
For this guideline, a total of 13 SRs were conducted to support the guideline development process (Carra et al., 2023; Cosgarea et al., 2023; de Waal et al., 2023; Dommisch et al., 2023; Donos et al., 2023; Gennai et al., 2023; Karlsson et al., 2023; Liñares et al., 2023; Ramanauskaite et al., 2023; Stiesch et al., 2023; Teughels et al., 2023; Verket et al., 2023; Wilensky et al., 2023). The corresponding manuscripts are published within this special issue of the Journal of Clinical Periodontology.
All SRs were conducted following the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA) framework (Moher et al., 2009), and were prospectively registered in PROSPERO.
3.2.3 Focused questions
In all 13 SRs, focused questions in population-intervention-comparison-outcome-study design (PICOS) format (Centre for reviews and dissemination, 2008; Guyatt et al., 2011) were proposed by the authors in February–March 2022 to a panel comprising the WG chairs and the methodological consultant in order to review and approve them (Table 4a–d). The panel took great care to avoid overlaps between the SRs or significant thematic omissions in order to ensure that they encompass the main interventions currently undertaken in the management of peri-implant diseases.
| Reference | Systematic review title | PICOS question (as written in the original article) |
|---|---|---|
| (a) | ||
| Carra et al. (2023) | Primary prevention of peri-implant diseases: A systematic review and meta-analysis. | What is the efficacy of preventive interventions, involving risk factor control, in patients (i) awaiting dental implant rehabilitation (primordial prevention), or (ii) already having dental implant(s) with healthy peri-implant tissues (primary prevention)? |
| Stiesch et al. (2023) | Supportive care for the prevention of disease recurrence/progression following peri-implantitis treatment: A systematic review. | #1. In patients treated for peri-implantitis (P) what is the efficacy of supportive care (I) in comparison with no supportive care (C), in terms of peri-implant tissue stability (O), as reported in prospective and retrospective studies of at least 3 years duration (S). |
| #2. In patients treated for peri-implantitis (P) what is the efficacy of supportive care with adjunctive local antiseptic agents (I) in comparison with supportive care without local antiseptic agents (C), in terms of peri-implant tissue stability (O), as reported in prospective and retrospective studies of at least 3 years duration (S)? | ||
| #3. In patients treated for peri-implantitis (P) what is the efficacy of supportive care with a frequency of more than once a year (I) in comparison with supportive care with a frequency of once a year or less (C) in terms of peri-implant tissue stability (O), as reported in prospective and retrospective studies of at least 3 years duration (S)? | ||
| (b) | ||
| Verket et al. (2023) | Non-surgical therapy of peri-implant mucositis—mechanical/physical approaches: A systematic review. | #1. In human subjects suffering peri-implant mucositis (P), has professionally administered non-surgical mechanical/physical therapy (I) any effect over no treatment (C), in terms of clinical/radiographic parameters and invasiveness (O), as shown in randomized clinical trials (RCTs) (S)? |
| #2. In human subjects suffering peri-implant mucositis (P), is any single mode of professionally administered non-surgical mechanical/physical therapy (I) superior to other single modes of professionally administered non-surgical mechanical/physical therapy (C), in terms of clinical/radiographic parameters and invasiveness (O), as shown in (RCTs) (S)? | ||
| #3. In human subjects suffering peri-implant mucositis (P), are combinations of treatment modes of professionally administered non-surgical mechanical/physical therapy (I) superior to single modes of professionally administered non-surgical mechanical/physical therapy (C), in terms of clinical/radiographic parameters and invasiveness (O), as shown in (RCTs) (S)? | ||
| #4. In human subjects suffering peri-implant mucositis (P), does repetition of professionally administered non-surgical mechanical/physical therapy (I) provide added benefits over single administration (C), in terms of clinical/radiographic parameters and invasiveness (O), as shown in (RCTs) (S)? | ||
| Dommisch et al. (2023) | Efficacy of chemical approaches during non-surgical sub-marginal instrumentation in the management of peri-implant mucositis: A systematic review. | In patients with peri-implant mucositis (P), what is the efficacy of (I) professionally administered topical antibiotics (with unsustained drug release), topical antiseptics (hydrogen peroxide, chlorhexidine, delmopinol hydrochloride, sodium hypochlorite, chitosan, acids) or photodynamic therapy during non-surgical sub-marginal peri-implant instrumentation compared to (C) non-surgical sub-marginal peri-implant instrumentation with or without additional control/placebo treatment in terms of (O) reduction of bleeding on probing (BOP) in (S) RCTs controlled clinical trials, prospective and retrospective case–control-studies, and case series with a follow-up of ≥3 month? |
| Gennai et al. (2023) | Efficacy of adjunctive measures in peri-implant mucositis. A systematic review and meta-analysis. | In systemically healthy humans with PiM, what is the efficacy of patient-performed or administered (by prescription) measures used adjunctively to sub-marginal instrumentation, as compared to sub-marginal instrumentation alone or combined with a negative control, in terms of reducing BOP, in randomized controlled clinical trials (RCTs) with at least 3-month follow-up? |
| (c) | ||
| Cosgarea et al. (2023) | Efficacy of mechanical/physical approaches for implant surface decontamination in non-surgical sub-marginal instrumentation of peri-implantitis. A systematic review. | #1. In patients with peri-implantitis, what is the efficacy of non-surgical sub-marginal peri- implant instrumentation with mechanical/physical decontamination methods (e.g., air- polishing, sonic/ultrasonic devices, lasers) alone or combinations thereof, compared to non-surgical sub-marginal instrumentation with placebo decontamination (non-aiming at mechanical/physical decontamination, e.g., scalers to remove hard deposits with adjunctive saline irrigation), in terms of change in peri-implant PD and/or change in BOP, in parallel-arm and split-mouth RCTs with ≥10 recruited/randomized subjects per treatment arm, in controlled clinical trials and prospective cohort studies with ≥30 recruited subjects with ≥6 months duration? |
| #2. In patients with peri-implantitis, what is the efficacy of non-surgical sub-marginal peri-implant instrumentation with mechanical/physical decontamination methods (e.g., air- polishing, sonic/ultrasonic devices, lasers) alone or combinations thereof and additional measures/interventions (e.g., irrigation with antiseptics), compared to non-surgical sub-marginal instrumentation with placebo decontamination (non-aiming at mechanical/physical decontamination, e.g., scalers to remove hard deposits with adjunctive saline irrigation) and additional measures/interventions (e.g., irrigation with antiseptics), in terms of change in peri-implant PD and/or change in BOP, in parallel- arm and split-mouth RCTs with ≥10 recruited/randomized subjects per treatment arm, in controlled clinical trials and prospective cohort studies with ≥30 recruited subjects with ≥6 months duration? | ||
| #3. In patients with peri-implantitis, what is the efficacy of non-surgical sub-marginal instrumentation with placebo decontamination (non-aiming at mechanical/physical decontamination, e.g., scalers to remove hard deposits with adjunctive saline irrigation) compared to no treatment or supramarginal mechanical cleaning in terms of change in peri-implant probing depth (PD) and/or change in bleeding on probing (BOP), in parallel-arm and split-mouth RCTs with ≥10 recruited/randomized subjects per treatment arm, in controlled clinical trials and prospective cohort studies with ≥30 recruited subjects with ≥6 months duration? | ||
| de Waal et al. (2023) | Efficacy of chemical approaches for implant surface decontamination in conjunction with sub-marginal instrumentation, in the non-surgical treatment of peri-implantitis. A systematic review and meta-analysis. | In adult patients with peri-implantitis (P), what is the efficacy of sub-marginal instrumentation combined with chemical surface decontamination (I) in comparison with sub- marginal instrumentation with or without placebo (C), in terms of changes in PDs and/or BOP (O), as reported in RCTs, non-randomized controlled clinical trials (CCTs) or prospective cohort studies, with a minimum of 6-month ‘follow-up’ (S)? |
| Liñares et al. (2023) | Efficacy of adjunctive measures in the non-surgical treatment of peri-implantitis. A systematic review. | In patients diagnosed with peri-implantitis (population), which is the efficacy of patient-performed or administered adjunctive measures to non-surgical therapy (intervention) as compared to no adjunct (comparison), in terms of PD and/or BOP reductions (primary outcomes), reported in RCTs or CCTs with at least 6 months of follow-up (study design)? |
| (d) | ||
| Karlsson et al. (2023) | Efficacy of access flap and pocket elimination procedures in the management of peri-implantitis—a systematic review and meta-analysis | #1. In patients requiring treatment of peri-implantitis (P), what is the effect of surgical therapy including access flap or pocket elimination procedures (I), when compared to non-surgical therapy (C), in terms of reduction of PD and/or of BOP (O), as observed in randomized controlled trials with a follow-up of ≥6 months and a sample size of ≥10 patients per arm (S)? |
| #2. In patients requiring treatment of peri-implantitis, what are the long-term outcomes of surgical access flap or pocket elimination procedures based on prospective studies (interventional or observational) with a sample of ≥20 patients and a follow-up of ≥12 months? | ||
| Donos et al. (2023) | The efficacy of bone reconstructive therapies in the management of peri-implantitis. A systematic review and meta-analysis | #1. In patients with peri-implantitis, what is the efficacy of different bone reconstructive therapies compared to access flap surgery in terms of pocket reduction and change in bleeding/suppuration on probing, at a minimum of 12-month of follow-up? |
| #2. In patients with peri-implantitis, what is the long-term (≥12 months) performance of reconstructive therapies in terms of pocket reduction, change in BOP/suppuration? | ||
| Ramanauskaite et al. (2023) | Mechanical and physical implant surface decontamination approaches in conjunction with surgical peri-implantitis treatment: A systematic review | #1. In patients with peri-implantitis (population), what is the efficacy of adjunctive or alternative mechanical/physical measures for implant surface decontamination in conjunction with surgical peri-implantitis treatment (intervention) compared with standard surface instrumentation (comparison) in changing signs of inflammation (outcomes), as reported in RCTs and CCTs with a follow-up period of at least 6 months (study design)? |
| #2. In patients with peri-implantitis (population), what is the efficacy of adjunctive or alternative mechanical/physical measures for implant surface decontamination in conjunction with surgical peri-implantitis treatment (intervention) compared with standard surface instrumentation including additional measures performed for both test and control groups (e.g., local application of antimicrobials and/or additional mechanical/physical measures) (comparison) in changing signs of inflammation (outcomes), as reported in RCTs and CCTs with a follow-up period of at least 6 months (study design)? | ||
| Wilensky et al. (2023) | The efficacy of implant surface decontamination using chemicals during surgical treatment of peri-implantitis: A systematic review and meta-analysis. | In adult patients with peri-implantitis, what is the efficacy of surgical therapy with adjunctive chemical surface decontamination of implant surfaces in comparison with surgical therapy alone or with placebo, in terms of PD reduction and bleeding on probing (BOP)/suppuration on probing (SOP) as reported in RCTs and non-randomized controlled clinical trials (non-RCTs) with a follow-up of at least 6 months? |
| Teughels et al. (2023) | Adjunctive locally and systemically delivered antimicrobials during surgical treatment of peri-implantitis. | In patients with peri-implantitis, what is the efficacy of surgical therapy combined with systemic or local antimicrobials, in comparison with surgical therapy alone, in terms of pocket PD reduction, as assessed in RCTs with at least 6 months of follow-up? |
3.2.4 Relevance of outcomes
For the present guideline, the recommendations of the ‘Implant Dentistry Core Outcome Set and Measurements’ (ID-COSM) initiative were followed (Derks et al., 2022; Needleman et al., 2023; Sanz et al., 2023; Tonetti et al., 2023), specifically the conclusions of the SR dealing with the outcome measures used in clinical studies (Derks et al., 2022). As expected, and since the report of the strongest outcome (dental implant/implant-supported prosthesis survival) was not frequently found, surrogate parameters were selected, in parallel with the previous EFP guidelines on the treatment of periodontitis (Herrera et al., 2022; Sanz et al., 2020).
The primary outcomes selected were parameters capturing the inflammatory component of the peri-implant tissues: PDs and BOP/suppuration on probing (SOP), since they were the most consistently reported outcomes.
The selected secondary outcomes were radiographic marginal bone loss (MBL), composite outcomes including the primary outcomes and MBL, dental implant/implant-supported prosthesis survival/loss, and patient-reported outcome measures (PROMs).
3.2.5 Search strategy
All SRs utilized a comprehensive search strategy of at least two different databases, supplemented by a hand search of periodontology-focused journals and the reference lists of included studies. In all SRs, the electronic and manual search, as well as the data extraction, was undertaken in parallel by two or more investigators.
3.2.6 Quality assessment of included studies
In all SRs, the risk of bias of controlled clinical trials (CCTs) was assessed using the Cochrane risk-of-bias tool (https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials). For observational studies, the Newcastle–Ottawa scale was used (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp).
3.2.7 Data synthesis
Where applicable, the available evidence was summarized by means of a meta-analysis.
3.3 From evidence to recommendation: Structured consensus process
The structured consensus development conference was held during the XVIII European Workshop in Periodontology in La Granja de San Ildefonso Segovia, Spain, between November 6 and 9, 2022. Using the 13 SRs as background information, evidence-based recommendations were formally debated by the guideline panel using the format of a structured consensus development conference. This consisted of small group discussions and open plenary discussions, where the proposed recommendations were presented, voted upon and adopted by consensus (Murphy et al., 1998). Delegates declaring potential conflicts of interest (CoI) abstained from voting and abstentions were recorded. Prior to the in-person meeting, three online meetings were organized (one at the plenary level, and two at the WG level) in September and October 2022, to advance the process of guideline development to a mature stage prior to the face-to-face consensus meeting.
- WG #1. Peri-implant health and Prevention (chairs Iain Chapple and Søren Jepsen).
- WG #2. Management of Peri-implant mucositis (chairs Mariano Sanz and Anton Sculean).
- WG #3. Management of Peri-implantitis—non-surgical (chairs David Herrera, Moritz Kebschull and Maurizio Tonetti).
- WG #4. Management of Peri-implantitis—surgical (chairs Tord Berglundh, Panos N. Papapanou and Frank Schwarz).
With the support of the methodology expert, recommendations and draft background texts were generated and subsequently presented, debated and subjected to a vote in the plenary sessions with all delegates present. During these plenary sessions, the guideline development process and discussions and votes were overseen and facilitated by the independent guideline methodologist (I.K.). The plenary votes were recorded using an electronic voting system, checked for accuracy, and then introduced into the guideline text.
The consensus process was conducted as follows:
3.3.1 Plenary session 1 (online session, 26 September 2022)
Introduction to guideline methodology (presentation, discussion) by the independent guideline methodologist (I.K.) and the chair of the workshop (D.H.).
3.3.2 WG phase 1 (two online sessions, from 28 September to 19 October 2022)
- Initial evaluation of declarations of interest and management of CoI.
- Presentation of the evidence (SR results) by group chairs and reviewers.
- Invitation of all members of the WG to reflect critically on the quality of available evidence by group chairs, considering the GRADE criteria.
-
Structured group discussions:
- initial discussions for the development of draft recommendations and their grading, considering the GRADE criteria;
- initial discussions for the development of draft background texts, considering the GRADE criteria;
- invitation to comment on draft recommendations and background text to suggest reasonable amendments by group chairs;
- collection and merging of amendments by group chairs.
3.3.3 Plenary session 2 (in-person meeting, November 2022)
- Presentation of WG results (draft recommendations and background text) by WG chairs.
- Invitation to formulate questions, statements, and reasonable amendments of the plenum by the independent guideline methodologist /facilitator.
- Answering questions by WG chairs.
- Collection and merging of amendments by an independent moderator.
- Preliminary vote on all suggestions provided by the WGs and all reasonable amendments.
- Assessment of the strength of consensus.
- Recording of abstentions made due to potential CoI.
- Opening debate, where no consensus was reached or reasonable need for discussion was identified.
- Formulation of tasks to be solved within the WGs.
3.3.4 WG phase 2 (in-person meeting, November 2022)
- Discussion of tasks and potential amendments raised by the plenum.
- Formulation of reasonable and justifiable amendments, considering the GRADE framework.
- Initial voting within the WG on recommendations and guideline text in preparation for the plenary session.
3.3.5 Plenary session 3 (in-person meeting, November 2022)
- Presentation of WG results by WG chairpersons.
- Invitation to formulate questions, statements and reasonable amendments of the plenary by the independent moderator.
- Collection and merging of amendments by an independent moderator.
- Preliminary vote.
- Assessment of the strength of consensus.
- Opening debate, where no consensus was reached or reasonable need for discussion was identified.
- Formulation of reasonable alternatives.
- Final vote of each recommendation, recording the consensus and abstentions due to potential CoI.
3.3.6 Plenary session 4 (online meeting, 18 January 2022)
- Presentation of pending recommendations and suggestions received.
- Preliminary vote.
- Assessment of the strength of consensus.
- Opening debate, where no consensus was reached or reasonable need for discussion was identified.
- Formulation of reasonable alternatives.
- Final vote of each recommendation, recording abstentions due to potential CoI.
3.4 Definitions: Rating the quality of evidence, grading the strength of recommendations and determining the strength of consensus
- the underlying quality of evidence, reflecting the degree of certainty/uncertainty of the evidence and robustness of study results
- the grade of the recommendation, reflecting the criteria considered to make the judgement; the strength of consensus, indicating the degree of agreement within the guideline panel and the number of abstentions due to potential CoI.
3.4.1 Quality of evidence
The quality of evidence was assessed using a recommended rating scheme (Balshem et al., 2011; Schunemann et al., 2019).
3.4.2 Strength of recommendations
- relevance of outcomes and quality of evidence for each relevant outcome;
- consistency of study results;
- direct applicability of the evidence to the target population/PICOS specifics;
- precision of effect estimates using CIs;
- magnitude of the effects;
- balance of benefit and harm;
- ethical, legal and economic considerations;
- patient preferences.
| Grade of recommendation gradea | Description | Syntax |
|---|---|---|
| A | Strong recommendation | We recommend (↑↑)/we recommend not to (↓↓) |
| B | Recommendation | We suggest to (↑)/we suggest not to (↓) |
| 0 | Open recommendation | May be considered (↔) |
- a If the group felt that evidence was not clear enough to support a recommendation, statements were formulated, including the need (or not) for additional research.
The grading of the quality of evidence and the strength of a recommendation may therefore differ, but where they do, the justification and context are clearly documented in the background narrative that follows each recommendation table.
3.4.3 Strength of consensus
The consensus determination process followed the recommendations by the German Association of the Scientific Medical Societies (AWMF) and Standing Guidelines Commission, 2012. Where consensus could not be reached, different points of view were documented in the guideline text (see Table 6).
| Unanimous consensus | Agreement of 100% of participants |
| Strong consensus | Agreement of >95% of participants |
| Consensus | Agreement of 75%–95% of participants |
| Simple majority | Agreement of 50%–74% of participants |
| No consensus | Agreement of <50% of participants |
3.5 Editorial independence
3.5.1 Funding of the guideline
The development of this guideline and its subsequent publication were financed entirely by internal funds of the EFP, without any support from industry or other organizations.
3.5.2 Declaration of interests and management of potential conflicts
All members of the guideline panel declared secondary interests using the standardized form provided by the International Committee of Medical Journal Editors (ICMJE 2013).
Management of CoIs was discussed in the WGs and the plenary sessions, following the principles provided by the Guidelines International Network (Schunemann et al., 2015). According to these principles, panel members with relevant, potential CoIs abstained from voting on guideline statements and recommendations within the consensus process. Those abstentions were recorded in each recommendation table.
3.6 Peer review
All 13 SRs underwent a multi-step peer review process. First, the draft documents were evaluated by members of the EFP Workshop Committee and the methodological consultants using a custom-made appraisal tool to assess (i) the methodological quality of the SRs using the AMSTAR 2 checklist (Shea et al., 2017), and (ii) whether all PICOS questions were addressed as planned. Detailed feedback was then provided for the SR authors. Subsequently, all 13 SRs underwent the regular editorial peer review process defined by the Journal of Clinical Periodontology.
The guideline text was drafted by the chairs of the WGs, in close cooperation with the methodological consultant, and was circulated among the members of each guideline group, who served as peer reviewers. The methodological quality was formally assessed by an external consultant using the AGREE framework. The study chairs approved the final guideline text prior to publication in the Journal of Clinical Periodontology.
3.7 Implementation and dissemination plan
For this guideline, a multi-stage dissemination and implementation strategy will be established and implemented by the EFP, supported by a communication campaign.
- Publication of the guideline and the underlying SRs as an Open Access special issue of the Journal of Clinical Periodontology.
- Commentary, Adoption, or Adaptation (Schunemann et al., 2017) by national societies.
- Generation of educational material for dental professionals and patients, and dissemination via the EFP member societies.
- Dissemination via educational programmes at dental conferences.
- Dissemination via the EFP through European stakeholders via National Society members of the EFP.
- Long-term evaluation of the successful implementation of the guideline by a survey of EFP members.
The timeline of the guideline development process is detailed in Table 7.
| Time point | Action |
|---|---|
| April 2018 | Decision by European Federation of Periodontology (EFP) General Assembly to develop comprehensive treatment guidelines for periodontitis and peri-implant diseases |
| May–September 2018 | EFP Workshop Organizing Committee (WOC) assesses merits and disadvantages of various established methodologies and their applicability to the field |
| November 2021 | EFP WOC decides on (i) topics covered by proposed guideline, (ii) working groups and chairs, (iii) systematic reviewers and (iv) outcome measures |
| February 2022 | EFP WOC decides invited systematic reviewers |
| March 2022 | Decision on consensus group, invitations sent to participants, invitations sent to stakeholders |
| March 2022 | Submission of PICO(S) questions by systematic reviewers to group chairs for internal alignment |
| March 30th, 2022 | Online meeting with consultant, WOC and reviewers, to better define PICOS. Final decision by WOC on PICOS |
| April 2022 | Decision on PICO(S) and information sent to reviewers |
| June–August 2022 | Submission of systematic reviews to WOC by the reviewers, initial quality assessment |
| August–September 2022 | Submission to Journal of Clinical Periodontology, peer review and revision process |
| September–December 2022 | Peer review and revision process in Journal of Clinical Periodontology |
| 26 September 2022 | Online plenary meeting |
| 28 September 2022 | Online working group meetings |
| September–October 2022 | Submission of declarations of interest by all delegates |
| 19 October 2022 | Online working group meetings |
| October 2022 | Electronic circulation of reviews |
| 6–9 November, 2022 | Workshop in La Granja with moderated formalized consensus process |
| November 2022–January 2023 | Formal stakeholder consultation, finalize guideline method, report and background text |
| 18 January, 2023 | Online plenary meeting |
| February 2023 | Submission of guideline document to the Journal of Clinical Periodontology |
| April 2023 | Publication of guideline and underlying systematic reviews in the Journal of Clinical Periodontology |
| April–September 2023 | Processes of adaptation/adoption by National Societies |
3.8 Validity and update process
The guideline is valid until 2028. However, the EFP, represented by the members of the Organizing Committee, will continuously assess current developments in the field. Where there are major changes of circumstances, for example, new relevant evidence, this will trigger an update of the guideline to potentially amend the recommendations. It is planned to update the current guideline regularly on demand and consistent with the format of a living guideline.
4 MANAGEMENT OF PERI-IMPLANT DISEASES—PREVENTION, DIAGNOSIS AND TREATMENT SEQUENCE
4.1 Specific approaches in the management of peri-implant diseases
Dental implants and dental implant abutments are class IIb medical devices (The European Commission, 2010), according to the 1993 Medical Device Directive (MDD, 93/42/EEC), which are maintained in the 2017 Medical Device Regulation (MDR, Council Regulation 2017/745) (The European Parliament and the Council of the European Union, 2017). This class of medical devices considers ‘implantable devices and long-term (>30 days) surgically invasive devices’, and applies to most implants used in the orthopaedic, dental, ophthalmic and cardiovascular fields. Implantable devices are ‘partially introduced into the human body through surgical intervention and intended to remain in place after the procedure for at least 30 days’ (The European Commission, 2010). They can be further classified according to their expected ‘duration’, either as short term (normally intended for continuous use for not more than 30 days) or long term (normally intended for continuous use for more than 30 days). In the current MDR regulation, published in 2017 (The European Parliament and the Council of the European Union, 2017) and enforced in May 2022, dental implants and dental implant abutments are considered within the category MDN 1103 (non-active dental implants and dental materials) as ‘non-active implants and long-term surgically invasive devices’ (The European Commission, 2017). Other non-active implants are classified in different categories as ‘non-active cardiovascular, vascular and neurovascular implants’ (MDN 1101), ‘non-active osteo- and orthopaedic implants’ (MDN 1102) and ‘non-active soft tissue and other implants’ (MDN 1104).
When developing a CPG related to dental implants (in the present case, on the management of peri-implant conditions), the CPG structure could be based on similar guidelines on other ‘long-term surgically invasive devices’; however, the clinical use of dental implants has a fundamental difference, since these medical devices are partially inserted in the jaws. Since the oral cavity is one of the most diverse and microbially abundant niches in the human body (Gupta et al., 2017), the intra-oral part of the implant will always be exposed to this contaminated environment. Therefore, dental implants have been specifically designed to withstand biofilm formation on the non-shedding transmucosal abutment surface', which will be covered by the appropriate prosthetic devices to serve as tooth replacements', then subject to the same measures of infection prevention control as natural teeth (OH practices). Another strategy that could have been followed in the development of this guideline was to implement a parallel process to that undertaken for the treatment of periodontal diseases (Herrera et al., 2022; Sanz et al., 2020). However, the major anatomical and histological differences between periodontal and peri-implant tissues (reported in Section 1.1.2) and the histopathological dissimilarities between periodontitis and peri-implantitis lesions (Berglundh et al., 2018; Sahrmann et al., 2020; Schwarz et al., 2018) necessitated a different approach.
The structure of the present guideline, therefore, must recognize the specific features of the ‘implantable medical devices’ and the biological distinctions between the peri-implant and periodontal diseases. Specifically, interventions for the prevention and treatment of peri-implant diseases may be implemented prior to inserting the medical device (dental implant), at the time of placement and restoration (implant/prosthesis placement), as well as post- rehabilitation, in recognition of the high incidence of peri-implant diseases.
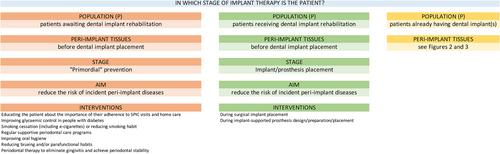
- patients awaiting dental implant rehabilitation;
- patients receiving dental implant rehabilitation;
- patients already rehabilitated using dental implant(s).
- before dental implant placement;
- healthy peri-implant tissues;
- peri-implant mucositis;
- peri-implantitis;
- following treatment of peri-implant mucositis;
- following treatment of peri-implantitis.
- Risk factor control before implant placement
- Risk factor control during implant/prosthesis placement
- Maintenance of peri-implant tissue health
- Treatment of peri-implant mucositis
- Treatment of peri-implantitis (non-surgical)
- Treatment of peri-implantitis (surgical)
- Secondary prevention of peri-implant mucositis
- Secondary prevention of peri-implantitis
4.2 Management according to the stage of implant therapy
- patients awaiting dental implant rehabilitation (pre-operative)
- patients receiving dental implant rehabilitation (peri-operative)
- patients already having dental implant/s (post-operative)
4.2.1 Pre-operative interventions
Due to the high prevalence of peri-implant diseases (described in Section 1), any patient receiving dental implants should be considered at risk of developing some form of peri-implant disease. Once the dental implant/abutment complex is exposed to the oral environment, and once the dental implant has been prosthetically loaded and is in function, biofilms can accumulate on their surface, and the ensuing inflammatory process can lead to the onset of peri-implant diseases. Therefore, interventions to prevent peri-implant diseases should commence during the treatment plan stage and continue during implant placement and prosthetic rehabilitation. These pre-operative interventions should focus on controlling the known risk factors associated with the development of peri-implant diseases, such as smoking, diabetes, uncontrolled or untreated periodontitis, and inadequate OH practices. These interventions are described in Section 5, and the term ‘primordial’ prevention of peri-implant diseases refers to those interventions that can be implemented at the treatment plan stage and target the above risk factors. The concept of ‘primordial’ prevention was first introduced by Strasser (1978), as prevention attained through a self-directed lifestyle that precludes the development of risk factors in a population. More recently, the American Heart Association (Lloyd-Jones et al., 2010) has defined the term on a population-wide basis, where primordial prevention is conceived as a strategy to prevent whole societies from experiencing epidemics, while the corresponding strategy on the individual level is to prevent the development of risk factors, consistent with the use of the term in the present guideline, as described in Section 5.
4.2.2 Peri-operative interventions
There is evidence in the scientific literature that ‘dental implants placed under less than ideal circumstances’ are often encountered in day-to-day practice (Schwarz et al., 2018), which may result in an increased prevalence of peri-implantitis (Berglundh et al., 2018). There is also evidence that prosthetic factors may also increase the risk of onset/progression of peri-implant diseases (Schwarz et al., 2018). In fact, the consensus report from the 2017 Workshop on the Classification of Periodontal and Peri-implant diseases stated that ‘there is some limited evidence linking peri-implantitis to factors such as the post-restorative presence of submucosal cement and the positioning of implants in a manner that does not facilitate OH and maintenance’ (Berglundh et al., 2018).
- placing the dental implant, that is, aiming at optimal implant positioning and considering local factors preventing an ideal placement;
- designing and installing the prosthetic reconstruction, that is, considering local risk factors that may prevent access for OH, or if possible, electing screw-retained restorations.
4.2.3 Post-operative interventions
Once the implants have been exposed to the oral environment, and the prosthetic reconstruction has been installed and is in function, the clinical condition of the peri-implant tissues should guide its management. Given the reported high incidence/prevalence of peri-implant diseases (described in Section 1), patients should be immediately enrolled into a SPIC programme. SPIC programmes should include interventions for primary prevention of peri-implant diseases, such as professional supra- and sub-marginal plaque biofilm removal and OH motivation and coaching, as well as early detection of pathological conditions.
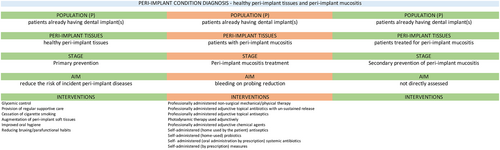
4.3 Diagnosis of peri-implant conditions
Successful implant-supported rehabilitation requires enrolment in a SPIC, where patients are routinely assessed to facilitate early diagnosis of peri-implant diseases.
The 2018 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions (Berglundh et al., 2018; Renvert et al., 2018) has established clear case definitions for peri-implant health (Araujo & Lindhe, 2018), peri-implant mucositis (Heitz-Mayfield & Salvi, 2018) and peri-implantitis (Schwarz et al., 2018).
4.3.1 Diagnosis of healthy peri-implant tissues
- Absence of clinical signs of inflammation.
- Absence of bleeding or suppuration on gentle probing.
- No increase in PD compared to previous examinations.
- Absence of bone loss beyond crestal bone level changes resulting from initial bone remodelling.
The present guideline has also adopted the recent ID-COSM initiative consensus (Tonetti et al., 2023) and the slightly modified definition of peri-implant health, which allows for the presence of a single bleeding spot around the implant.
4.3.2 Diagnosis of peri-implant mucositis
- Presence of bleeding and/or suppuration on gentle probing with or without increased PD compared to previous examinations.
- Absence of bone loss beyond crestal bone level changes resulting from initial bone remodelling.
Following the modification of the ID-COSM initiative consensus (Tonetti et al., 2023), this definition has been updated as follows: presence of bleeding (more than one spot at a location around the implant or presence of a line of bleeding or profuse bleeding at any location) and/or suppuration on gentle probing, in the absence of bone loss beyond crestal bone level changes resulting from initial bone remodelling.
4.3.3 Diagnosis of peri-implantitis
- Presence of bleeding and/or suppuration on gentle probing.
- Increased PD compared to previous examinations.
- Presence of bone loss beyond crestal bone level changes resulting from initial bone remodelling.
- Presence of bleeding and/or suppuration on gentle probing.
- PDs of ≥6 mm.
- Bone levels ≥3 mm apical of the most coronal portion of the intraosseous part of the implant.
4.4 Specific care pathways according to diagnosis of the peri-implant condition
Almost 25 years ago, at the Food and Drug Administration (FDA)/AAP consensus conference in 1996, Lang and co-workers (Lang et al., 1997; Lang et al. 2000; Mombelli & Lang, 1998) proposed the Cumulative Interceptive Supportive Therapy concept for the management of peri-implant diseases. This protocol was based on a combination of early detection, and implementation of preventive and therapeutic interventions, aimed first to prevent the onset, and then to treat peri-implantitis as early as possible to arrest its progression and thus prevent loss of the implant. While the interventions recommended in the current guideline are different, the overall strategy and philosophy are similar.
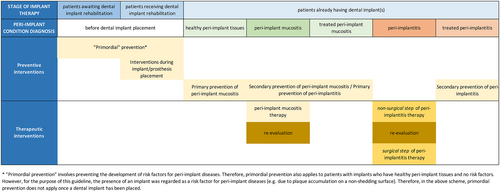
Depending on the clinical diagnosis, distinct care pathways can be followed (Figures 2 and 3). However, the important overarching principle portends that peri-implant mucositis is treatable and leads to the restoration of peri-implant tissue health. Therefore, primary prevention of peri-implant diseases and secondary prevention of peri-implant mucositis (after peri-implant mucositis treatment) share identical interventions. Moreover, since the treatment of peri-implant mucositis is the primary intervention in the prevention of peri-implantitis, this treatment should also be considered a preventive strategy. The maintenance of health and function of dental implants and the associated implant-supported prostheses through prevention and treatment of peri-implantitis is, therefore, the primary aim of this guideline. However, once peri-implantitis has developed, it is well established that treatment will not re-establish intact peri-implant tissue support, even if the inflammation is successfully controlled. Therefore, specific clinical definitions following the treatment of peri-implantitis need to be established.
4.4.1 Specific care pathways in healthy peri-implant tissues
In cases of peri-implant tissue health, interventions for primary prevention should be implemented as part of a SPIC programme, including periodical professional supra-and sub-marginal plaque biofilm removal.
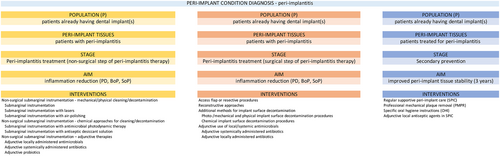
4.4.2 Specific care pathways in peri-implant mucositis
Interventions for the management of peri-implant mucositis are detailed in Section 6 and focus on biofilm control, either self-administered or professionally delivered. Treatment outcomes should be evaluated after 2–3 months, and if relevant end points have not been achieved, re-treatment is recommended. These endpoints reflect the re-establishment of peri-implant health; if peri-implant health is re-established, then the primary prevention of peri-implant diseases and the secondary prevention of peri-implant mucositis are essentially identical. Furthermore, since the treatment of peri-implant mucositis is central to the prevention of the onset of peri-implantitis (Jepsen et al., 2015), this treatment is in fact the most important preventive intervention for peri-implantitis and, as such, represents the main component of professional interventions during SPIC.
4.4.3 Specific care pathways in peri-implantitis
- Peri-implantitis is an irreversible condition; therefore, even after successful peri-implantitis therapy, a diagnosis of ‘stable’ peri-implantitis is assigned at the particular implant.
- Peri-implantitis treatment outcomes depend upon a multitude of factors (implant and prosthetic characteristics, patient factors, local factors, disease severity, bone defect configuration). Consequently, customized interventions specifically targeting one or several of the above factors are used in its management (as reported in the SRs). The treatment outcomes of these interventions are variable.
- Upon diagnosis, a decision must be made whether the affected implant is treatable.
- If so, an initial non-surgical therapy step, which includes sub-marginal instrumentation, is performed.
- Following the non-surgical step, re-evaluation of clinical outcomes, based on a set of pre-established criteria for success, will guide the decision whether to enrol the patient in a secondary prevention SPIC programme, or to proceed with the surgical step, provided the affected implant continues to be deemed treatable.
- The surgical step of peri-implantitis treatment must always include sub-marginal instrumentation after elevating a surgical flap.
- Following evaluation of clinical outcomes after the surgical step, and provided that a set of pre-established criteria for success are met, the patient is enrolled into a secondary prevention SPIC programme. If these criteria are not fulfilled, and the affected implant is still deemed to be maintainable, the implant should be re-treated.
- SPIC programmes for secondary prevention following peri-implantitis treatment may be different from programmes designed for primary prevention.
4.5 Key aspects in the management of peri-implant diseases
- Appropriate interventions for the preservation and/or restoration of peri-implant tissue health should be considered before, in conjunction with, and after the placement of dental implants.
- Risk factor assessment and control, and diagnosis and monitoring of the health/disease status of the peri-implant tissues, are critical in selecting the appropriate care pathway for the individual patient.
- Successful, long-term maintenance of peri-implant tissue health encompasses behavioural modification, health monitoring, appropriate preventive interventions and, when necessary, careful treatment planning and execution.
- Peri-implant tissue health, peri-implant mucositis and peri-implantitis represent a continuum. Changes are driven by inflammatory changes subsequent to microbial biofilm accumulation. Controlling inflammation through removal of the plaque biofilm is key to both preserving health and preventing and treating peri-implant diseases.
- Preventive and treatment interventions are organized into specific needs-based care pathways.
- Prevention aims to attain and preserve peri-implant tissues that are free of clinical inflammation. This is achieved by enabling adequate self-performed and professionally delivered OH measures that need to be customized according to the design of implant-supported restorations.
- SPIC is an essential component of implant dentistry; it is critical for preserving peri-implant tissue health/preventing disease onset and must be offered to every patient who receives dental implants.
- The aim of treatment is to arrest the inflammatory processes within the peri-implant tissues and to control local and systemic risk factors that may sustain it. Disruption of the locally accumulating microbial biofilms is a key target.
- Treatment of peri-implant mucositis is considered a key strategy in the prevention of the onset of peri-implantitis.
- Treatment of peri-implantitis is performed sequentially, and encompasses an initial non-surgical step, followed by a surgical step, depending on the outcomes of the initial treatment. SPIC should always be instituted, particularly upon completion of peri-implantitis treatment.
Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI)
5 RECOMMENDATIONS FOR THE PREVENTION OF PERI-IMPLANT DISEASES
Risk assessment and risk factor control are necessary to prevent the development of peri-implant diseases in patients who are candidates for dental implant(s), and in patients who have received dental implant/s and currently have healthy peri-implant tissues.
The purpose of ‘primordial’ prevention (see Section 4.2.1) in the context of the current workshop is to prevent risk factor development prior to dental implant placement. The goal is to attain and maintain optimal oral health to prevent the development of peri-implant diseases over time. There is no current definition of what the optimal oral and general health status of a patient should be prior to dental implant placement, or of which metrics should be included in such a definition. Therefore, no study directly addressing primordial prevention of peri-implant diseases was found, and any recommendations regarding primordial prevention are based upon indirect evidence and expert-based consensus.
The purpose of primary prevention is to prevent disease onset following dental implant placement and loading. The goal is to achieve an optimal oral condition and to maintain dental implant health over time by controlling risk factors for the disease.
The relationship between primordial, primary, secondary and tertiary prevention is represented in Figure 5, which documents the approach taken by the workshop to interpret the different forms of prevention in the context of peri-implant diseases.
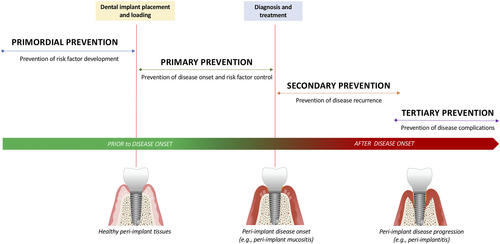
No studies were identified that provided direct evidence for primary prevention. The recommendations are therefore inferred from observational and interventional studies with various working hypotheses that were not originally developed to test the efficacy of a preventive measure on the occurrence of peri-implant diseases. Therefore, the recommendations regarding primary prevention are both evidence-based and expert-based.
In the present guideline, the term SPIC is used to comprise an individually tailored follow-up programme that has been described in the available studies with the terms: (1) supportive care; (2) SPIC; (3) supportive peri-implant therapy; (4) supportive periodontal therapy; (5) supportive periodontal and peri-implant therapy and (6) supportive therapy.
5.1 Recommendations for primordial prevention of peri-implant diseases
The overall objective of this section is to answer the question: in patients awaiting implant placement, does primordial prevention involving the control of lifestyle and behavioural risk factors prevent the development of peri-implant mucositis and peri-implantitis?
R5.1. In patients awaiting implant placement, do the following behaviours or interventions, prior to implant placement, reduce the incidence of peri-implant mucositis and peri-implantitis?
- Educating the patient about the importance of their adherence to SPIC visits and home care
- Improving glycaemic control in people with diabetes
- Smoking cessation (including e-cigarettes) or reducing smoking habit
- Participation in regular supportive periodontal care programmes
- Improving OH
- Reducing bruxing and/or parafunctional habits
- Periodontal therapy to eliminate gingival inflammation and achieve periodontal stability
| PICOS question addressed by a systematic review |
|---|
| R5.1: Expert consensus-based recommendation |
In patients awaiting implant placement, we recommend:
|
| Supporting literature (Carra et al., 2023) and Expert opinion |
| Quality of evidence Very low |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
This recommendation relies upon indirect evidence from studies included in the review and on expert opinion.
R5.2. Prior to and during implant placement, what are the considerations related to implant positioning to reduce the risk of incident peri-implant diseases?
| Additional question addressed by the WG |
|---|
| R5.2: Expert-based recommendation |
We recommend that treatment planning for three-dimensional implant positioning should meet the following conditions:
|
| Supporting literature (Berglundh et al., 2018; Chan et al., 2019; Farina et al., 2017; Jepsen et al., 2015; Kumar et al., 2018; Schuldt Filho et al., 2014; Schwarz et al., 2018; Valles et al., 2018) |
| Quality of evidence Low |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
This question was an additional question that was not specifically addressed by the SR and therefore relies upon indirect evidence and on expert opinion.
R5.3. During implant-supported prosthesis design and placement, are there specific considerations to reduce the risk of incident peri-implant diseases?
| Additional question addressed by the WG |
|---|
| R5.3: Evidence-based recommendation |
In order to facilitate optimal plaque control around implants and to prevent incident peri-implant diseases, we recommend prosthetic treatment planning should provide for:
|
| Supporting literature (De Ry et al., 2021; Grischke et al., 2021; Heitz-Mayfield et al., 2020; Jepsen et al., 2015; Katafuchi et al., 2018; Mattheos, Janda, et al., 2021; Mattheos, Vergoullis, et al., 2021; Serino & Strom, 2009; Soulami et al., 2022; Staubli et al., 2017; Strauss et al., 2022; Yi et al., 2020) |
| Quality of evidence Moderate |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
This question was not addressed by the SR and therefore represents an expert consensus-based recommendation, derived from indirect evidence using the cited supporting literature, which may change in the future as new evidence emerges. Expert opinion based on experience is that implant-supported fixed prostheses should have smooth, polished, convex intaglio surfaces, avoid ‘ridge lap’ designs and, in general, avoid an over-contoured prosthesis, thus facilitating optimal plaque biofilm removal.
5.2 Recommendations for primary prevention of peri-implant diseases
The overall objective of this section is to answer the question: in patients with dental implants and peri-implant tissue health, does primary prevention involving control of lifestyle and behavioural risk factors prevent the development of peri-implant mucositis and peri-implantitis?
R5.4. How should the peri-implant health status be assessed at each clinical examination?
| Additional question addressed by the WG |
|---|
| R5.4: Expert consensus-based recommendations |
We recommend peri-implant probing to assess the presence of BOP, and to monitor changes in PD, and changes in the mucosal margin level. The following are advised:
In addition, we recommend a baseline intra-oral radiograph be obtained at the completion of physiological remodelling to document marginal bone levels. At subsequent visits, if there is an increase in PD in conjunction with BOP/suppuration, we recommend an intra-oral radiograph to evaluate the marginal bone levels. |
| Supporting literature (Berglundh et al., 2018; Jepsen et al., 2015; Lindhe et al., 2008; Renvert et al., 2018; Sanz et al., 2022) |
| Quality of evidence Low |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
This question was not addressed by the SR and therefore represents an expert consensus-based recommendation, derived from indirect evidence using the cited supporting literature.
R5.5. In patients with diabetes and healthy peri-implant tissues, does glycaemic control reduce the risk of incident peri-implant diseases?
| PICOS question addressed by a systematic review |
|---|
| R5.5: Evidence-based recommendation |
| In patients with diabetes who have healthy peri-implant tissues, we recommend glycaemic control to maintain peri-implant health. |
| Supporting literature (Carra et al., 2023) |
| Quality of evidence Low |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
The exposure/risk factor of interest for peri-implantitis is diabetes, and the preventive intervention is glycaemic control (as measured by % of HbA1c).
Available evidence
Number and design of included studies
Eleven observational studies including six case–control studies and five cohort studies (Carra et al., 2023).
Risk of bias
According to the Newcastle–Ottawa scale (NOS), eight studies were at low risk of bias and three studies were at high risk of bias.
Effect sizes and their clinical relevance
Pooled data analyses revealed a significantly lower rate of peri-implantitis (OR = 0.16; 95% CI [0.03; 0.96]; p = .004; I2: 0%; analysis based on two studies including 385 implants), and significantly lower marginal bone level changes over time (−0.36 mm; 95% CI [−0.65; −0.07]; p < .0001; I2: 95%; analysis based on six studies including 591 implants) in patients with good glycaemic control compared with poor glycaemic control. The mean difference (MD) in PD and BOP was not significantly different between the groups. With respect to dental implant survival, diabetes patients with poor glycaemic control were found to have a 7.59 increased risk of dental implant failure compared with patients with good glycaemic control (OR = 7.59; 95% CI [1.63; 35.3]; p = .01; I2: 0%; based on two studies including 524 implants). The estimated mean implant survival was 99% (95% CI [97.8%; 100%]; based on five studies including 253 dental implants) in patients with good glycaemic control and 95.6% (95% CI [91.4%; 99.8%]; based on five studies including 271 dental implants) in patients with poor glycaemic control.
The effect size of these findings is considered clinically relevant, but it must be highlighted that the results are based on a limited number of studies with small sample sizes, that the analyses were performed at the implant level only, and that the definition of good and poor glycaemic control was not consistent among the studies (i.e., good glycaemic control was defined as HbA1c between 6.1% and 8% in five studies, <7% in one study, and <6% in another study; poor glycaemic control was defined as HbA1c level ranging between 8.1% and 10% in 5 studies, as HbA1c > 8% in one study, and as HbA1c ranging between 7% and 9% in another study; three studies also included a group of very poorly controlled type-2 diabetes patients, as HbA1c > 9 or >10%).
Consistency
Consistency was found in the overall results, favouring good glycaemic control over poor glycaemic control. However, the definition of good and poor glycaemic control was not consistent among the available studies.
Balance of benefit and harm
Not assessed. However, glycaemic control in patients with diabetes is advised independently of implant therapy.
Overall certainty of the evidence
No study provided direct evidence. The results are inferred from studies with various working hypotheses that were not originally developed to test the effectiveness of a preventive measure on the occurrence of peri-implant diseases. Further research is needed to provide confidence in the estimated effect of glycaemic control on the risk of peri-implant diseases.
5.1 From evidence to recommendation—additional considerations
Not applicable.
R5.6. In patients with healthy peri-implant tissues, does provision of regular supportive peri-implant care (SPIC) reduce the risk of incident peri-implant diseases?
| PICOS question addressed by a systematic review |
|---|
| R5.6: Evidence-based recommendation |
| We recommend regular supportive peri-implant care in patients who have healthy peri-implant tissues, to reduce the risk of incident peri-implant diseases, emphasizing to the patient the importance of their adherence to SPIC visits and home care. |
| Supporting literature (Carra et al., 2023) |
| Quality of evidence Moderate |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
The risk factor/exposure is a lack of appropriate patient follow-up, including periodontal and peri-implant care, and the preventive intervention is promoting and attaining adequate/regular patient adherence to the supportive periodontal/peri-implant care (SPC/SPIC). Various interventions were employed (tab. 3 in Carra et al., 2023). The term ‘SPIC’ covers the following terms used by the authors of individual studies: (1) supportive care (one study); (2) SPIC (two studies); (3) supportive peri-implant therapy (four studies); (4) supportive periodontal therapy (two studies); (5) supportive periodontal and peri-implant therapy (three studies); and (6) supportive therapy (two studies). For regular supportive care, the interval between the intervention sessions was: (1) tailored (three studies); (2) 3 months (one study); (3) 4 months (one study); (4) 3–6 months (one study); (5) ≤6 months (one study); (6) ≤12 months (three studies); and (7) unknown (four studies).
Available evidence
Number and design of included studies
Fourteen studies, 13 observational studies and 1 randomized clinical trial (RCT) (Carra et al., 2023).
Risk of bias
According to NOS: seven studies were at low risk of bias and six studies were at high risk of bias. According to RoB-II-RCT. one study was at some concern.
Effect sizes and their clinical relevance
Twelve studies compared patients regularly attending the recommended SPIC programme (adherent) versus non-attending patients or those attending SPIC visits irregularly. Pooled data analyses revealed that patients attending SPIC regularly were at significantly lower risk of presenting with peri-implant diseases (including both peri-implant mucositis and peri-implantitis) (OR = 0.42; 95% CI [0.24; 0.75]; p = .003; I2: 57%; analysis based on six studies including 736 patients) during the study follow-up period (ranging from 1 to 20 years). This was also observed for the specific diagnosis of peri-implantitis, both at the patient (OR = 0.45; 95% CI [0.30; 0.68]; p = .0002; I2: 51%; analysis based on six studies including 736 patients) and implant level (OR = 0.26; 95% CI [0.15; 0.46]; p < .0001; I2: 21%; analysis based on six studies including 1337 implants). No significant differences were observed between regular and irregular adherence to SPIC for the diagnosis of peri-implant mucositis.
In a sensitivity analysis excluding those studies that involved patients with a history of periodontitis, dental implants undergoing regular SPIC showed an OR = 0.23 (95% CI [0.08; 0.64]; p = .005; I2: 0%) of developing peri-implantitis compared to dental implants with no SPIC (based on two studies).
When dental implants were used as the statistical unit of analysis, those subjected to regular SPIC demonstrated a lower PD (MD: −0.48 mm; 95% CI [−0.67; −0.29]; p < .0001; I2: 32%; analysis based on five studies including 867 implants) and a reduced risk of exhibiting a MBL > 2 mm (OR: 0.4; 95% CI [0.25; 0.66]; p = .0003; I2: 73%; analysis based on three studies including 689 implants). Irregular SPIC was associated with a 3.76 increased risk of implant failure (95% CI [1.50; 9.45]; p = .005; I2: 0%) compared with regular SPIC.
All studies reporting dental implant survival evaluated study samples that included a proportion of patients with a history of periodontitis. Overall, the estimated mean implant survival was 99.3% (95% CI [98.6%; 100%]) in the regular SPIC group (based on 564 implants) and 97.8% (95% CI [95.6%; 99.9%]) in the irregular SPIC group (based on 454 implants) (follow-up ranging from 4.5 to 20 years after implant loading).
The RCT that was evaluated compared four different SPIC protocols (including a 3-monthly SPIC with curettes, with sonic scalers or air-polishing, and with or without chlorhexidine varnish application) and found no significant differences between the groups in terms of PD, BOP, and survival at 1 year (Ziebolz et al., 2017).
When comparing patients with a history of generalized moderate-to-severe periodontitis presenting with deep residual pockets (>6 mm) during SPC, with patients who had a history of generalized moderate-to-severe periodontitis but without residual deep pockets, a significantly higher occurrence of peri-implantitis (3.5% vs. 15.2%, implant level analysis) was observed when deep residual pockets were present (Cho-Yan Lee et al., 2012).
Consistency
All selected studies were overall consistent, favouring regular SPIC over irregular SPIC.
Balance of benefit and harm
Not assessed. However, the importance and clinical relevance of SPIC should be reinforced, given that regular SPIC carries little risk compared with the benefits it brings.
Overall certainty of the evidence
Moderate. Results are inferred from studies with various working hypotheses that were not originally developed to test the effectiveness of a preventative measure on the occurrence of peri-implant diseases. Further research, including clinical trials with strict inclusion criteria, may have an impact on confidence in the estimated effect of regular versus irregular SPIC on the risk of peri-implant diseases.
5.1 From evidence to recommendation—additional considerations
Not applicable.
R5.7. In patients who smoke and have healthy peri-implant tissues, does the cessation of cigarette smoking reduce the risk of incident peri-implant diseases?
| PICOS question addressed by an SR |
|---|
| R5.7: Expert consensus-based recommendation |
| In patients with healthy peri-implant tissues, we recommend validated smoking cessation interventions (by conformance with guidelines) to reduce the risk of peri-implant diseases. |
| Supporting literature (Carra et al., 2023) |
| Quality of evidence Very low |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
The risk factor is smoking, and the preventive intervention is promotion of smoking cessation advice/strategies.
Available evidence
Number and design of included studies
Four studies, including three case–control studies and one cohort study (Carra et al., 2023). Clear similarities between the three case–control studies conducted by the same research team were noted.
Risk of bias
According to NOS, the three case–control studies were at high risk of bias, and the cohort study was at low risk of bias.
Effect sizes and their clinical relevance
Only one study described the occurrence of peri-implant diseases as a clinical diagnosis, reporting a lower rate of peri-implant mucositis (43.9% vs. 48.6%) and peri-implantitis (19.7% vs. 30.5%) in former smokers compared with current smokers (Costa et al., 2022). The authors observed a direct association between cumulative smoking exposure and the risk for peri-implantitis, as well as with the time span since smoking cessation.
All studies reported significant clinical differences between former smokers, e-cigarette users, waterpipe smokers and current smokers. The former smoker category exhibited less peri-implant mucosal inflammation, PD and MBL compared with the other categories.
Consistency
There is insufficient evidence to determine whether cigarette smoking cessation decreases the risk for peri-implant diseases. There is little evidence to support the contention that using e-cigarettes or the habit of water pipe smoking is associated with a decreased risk for peri-implant diseases compared with cigarette smoking.
Balance of benefit and harm
Not assessed. However, because of the several harmful consequences of smoking, smoking cessation should be advised and promoted for every patient irrespective of implant therapy.
Overall certainty of the evidence
Low. No interventional studies were found to provide direct evidence. The results are inferred from studies with various working hypotheses that were not originally developed to test the effectiveness of smoking cessation on the occurrence of peri-implant diseases. Further research is very likely to have an impact on confidence in the estimate of the effects of cigarette cessation on the reduction of the risk of incident peri-implant diseases. Regarding the use of non-cigarette smoking, any estimate of effect is very uncertain.
5.1 From evidence to recommendation—additional considerations
Not applicable.
R5.8. In patients with healthy peri-implant tissues, does augmentation of peri-implant soft tissues lower the likelihood of incident peri-implant diseases?
| PICOS question addressed by an SR |
|---|
| R5.8: Evidence-based recommendation |
| In patients who have dental implants with an absence or deficiency of keratinized/attached mucosa, and where the patient experiences discomfort on brushing, increasing peri-implant keratinized/attached mucosal width to maintain peri-implant health may be considered. |
| Supporting literature (Carra et al., 2023) |
| Quality of evidence Low |
| Grade of recommendation Grade O—↔ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
The risk factor is the deficiency of PIKM (PIKM < 1, 2 or 3 mm according to the studies), and the preventive intervention is the augmentation of PIKM by a free gingival graft (FGG).
Available evidence
Number and design of included studies
Six of the studies included in the SR and meta-analysis (three RCTs, one NRCT, one case–control and one cohort study) were considered. They compared peri-implant tissue health parameters between sites with a deficiency in PIKM and receiving an FGG to increase PIKM width versus no intervention. No study was specifically designed to assess the impact of FGG on the prevention of peri-implant diseases.
Risk of bias
According to RoB-II-RCT, the three RCTs presented some concerns. According to RoBins-NRTC, the selected study was at moderate risk of bias. According to NOS: the two studies were at low risk of bias.
Effect sizes and their clinical relevance
Indirect evidence based on the evaluation of peri-implant health parameters in the short term showed a non-significantly different PPD between the PIKM-augmented and non-augmented sites but a significantly lower clinical soft tissue inflammation index (BOP/GI) (standardized mean difference [SMD] = −1.18; 95% CI [−1.85; −0.51]; p = .0006; I2: 69%) around the dental implants receiving FGG to augment PIKM. Concerning the mean MBL, based on data from four studies, a significant difference in favour of PIKM-augmented sites (SMD: −0.25; 95% CI [−0.45; −0.05]; p = .01; I2: 62%) was also noted. When excluding from pooled data analysis of cohort and case–control studies, the results were consistent with no statistical heterogeneity. No difference in PPD (SMD: −0.25; 95% CI [−0.63; −0.13]; p = .20; I2: 0%; based on 107 implants), whereas a significant difference in BOP (SMD: −1.5; 95% CI [−1.93; −1.06]; p < .0001; I2: 0%; based on 107 implants) and MBL changes (SMD: −0.33; 95% CI [−0.55; −0.11]; p = .003; I2: 0%; based on two studies, 66 implants) were noted between PIKM-augmented sites versus non-augmented sites.
Only two studies reported the occurrence of PIDs (Frisch et al., 2015; Roccuzzo et al., 2016). The first study defined peri-implantitis as the presence of BOP, PPD ≥ 5 mm, and a radiographic bone loss ≥3.5 mm (Frisch et al., 2015). During a mean follow-up of 12 years, three groups receiving FGG, CTG or no intervention were compared. No statistical differences were found between groups. The second study, a 10-year prospective cohort, observed a significantly higher rate of PIDs for dental implants with PIKM deficiency compared with implants surrounded by PIKM (51.4% vs. 12.7%; p < .0001) (Roccuzzo et al., 2016). The authors also reported a significantly lower soreness for implants surrounded by PIKM or placed in the alveolar mucosa receiving FGG compared with implants surrounded by alveolar mucosa and not receiving FGG (Roccuzzo et al., 2016).
Consistency
Results are based on heterogeneous studies with, most of the time, small sample sizes and short follow-ups. Consistency is low.
Balance of benefit and harm
Not assessed. However, the decision-making process concerning surgical procedures to augment PIKM should consider the general risks associated with periodontal and implant surgery.
Overall certainty of the evidence
Low. No study design provided direct evidence. Results are inferred from studies with various working hypotheses that were not originally developed to test the effectiveness of peri-implant soft tissue augmentation procedures on the prevention of peri-implant diseases over time.
Care must be taken regarding the interpretation of the study results due to the high clinical heterogeneity of the included studies. Most of the studies described clinical peri-implant outcomes in the short term (6–12 months follow-up), whereas only two observational studies reported the occurrence of peri-implant diseases over a 10-year (low risk of bias) and 12-year (high risk of bias) follow-up.
However, a reduced width of keratinized tissue is associated with an increased prevalence of peri-implantitis, plaque accumulation, soft tissue inflammation, mucosal recession, MBL and greater patient discomfort (Ramanauskaite et al., 2022). The effectiveness of increasing PIKM as a preventive measure for peri-implant diseases requires longitudinal studies designed with a long-term follow-up, to evaluate the outcome of interest (i.e., peri-implant diseases).
5.1 From evidence to recommendation—additional considerations
Not applicable.
R5.9. In patients with healthy and thin peri-implant tissues (<2 mm in thickness), does soft tissue augmentation lower the likelihood of incident peri-implant diseases?
| PICOS question addressed by an SR |
|---|
| R5.8: Evidence-based statement |
| We do not know if undertaking procedures to augment soft tissue thickness prevents the development of peri-implant diseases, since there is lack of evidence to support an association between increasing soft tissue thickness and peri-implant tissue health. |
| Supporting literature (Carra et al., 2023; Tavelli et al., 2021; Valles et al., 2022) |
| Quality of evidence—Low |
| Grade of recommendation Grade B—↓ |
| Strength of consensus Consensus (7.8% of the group abstained due to potential CoI) |
Background
Intervention
Peri-implant soft tissue augmentation to increase PIKM thickness includes the following surgical procedures: (1) connective tissue graft (CTF), (2) FGG, (3) the use of xenogenic collagen matrix (XCM) and (4) or acellular dermal matrix allograft.
Available evidence
Number and design of included studies
Eight studies, including one NRCT and six RCTs (Carra et al., 2023).
Risk of bias
According to NOS, one study is at high risk of bias; according to RoB-II-RCT, two studies were at low risk of bias and four studies presented some concern. According to RoBins-NRTC, one study is at moderate risk of bias.
Effect sizes and their clinical relevance
Pooled data analyses were based on four studies, including 179 implants, and found no difference between CTG/FGG versus XCM for mean PPD, MBL and BOP. One CCT with a small sample size (19 patients) observed a 4.3% rate of peri-implantitis in the control group compared with 0% in the test group receiving CTG (partial split-mouth design) (Hosseini et al., 2020). Meta-analysis was performed pooling together two studies comparing CTG versus no intervention (Frisch et al., 2015; Hosseini et al., 2020), and including 37 implants in CTG-augmented sites versus 69 implants in non-augmented sites. It showed no significant difference between the two groups for the rate of incident peri-implantitis (OR = 1.97; 95% CI [0.20; 19.72]; p = .56; I2: 0%).
Consistency
Data are consistent, although based on a very limited number of studies.
Balance of benefit and harm
Not assessed. However, the decision-making process should balance the risks associated with the different surgical procedures aimed at increasing PIKM thickness against the risks of surgery and the additional related costs, in people with peri-implant mucosal health.
Overall certainty of the evidence
No study design provided direct evidence. Care must be taken regarding the interpretation of the results, due to the high clinical heterogeneity of the included studies, in particular the high variability of the timeline at which the augmentation procedure was performed (before or after dental implant placement, after dental implant loading, simultaneously to the dental implant placement, at the stage 2 surgery, etc.). Most of the studies described clinical peri-implant outcomes in the short term (6–12 months follow-up).
5.1 From evidence to recommendation—additional considerations
Not applicable.
R5.9. In patients with healthy peri-implant tissues, does improved oral hygiene prevent incident peri-implant diseases?
| PICOS question addressed by an SR |
|---|
| R5.9: Expert consensus-based recommendation |
| In patients who have dental implants, we recommend specific, individually tailored OH instructions to reduce the risk of incident peri-implant diseases |
| Supporting literature (Carra et al., 2023) |
| Quality of evidence Very low |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
The risk factor is inadequate OH, and the preventive intervention is improving OH behaviours. The following toothbrushes were evaluated: (1) counter-rotational powered toothbrush, (2) sonic toothbrush and (3) manual toothbrush. The following frequencies were evaluated: brushing at least twice/day or brushing at most once/day.
Available evidence
Number and design of included studies
Three studies were selected: two RCTs and one case–control study (Carra et al., 2023).
Risk of bias
According to NOS, one study was at low risk of bias. According to RoB-II-RCT, two studies were at some concern.
Effect sizes and their clinical relevance
Due to the heterogeneity in reporting outcome measures, no analysis of pooled data was possible. One study found a significant difference in favour of a counter-rotational powered toothbrush in terms of peri-implant mucosal inflammation and implant survival compared with manual toothbrushing (Truhlar et al., 2000). One case–control study indicated that the frequency of tooth brushing (at least twice a day vs. at most once a day) had no impact on peri-implant PD, MBL and BOP (Alhakeem et al., 2023).
Consistency
The three studies included were inconclusive regarding the type of toothbrush to use (e.g., powered or manual toothbrush), or the frequency of toothbrushing that was most effective in maintaining peri-implant health.
Balance of benefit and harm
Not assessed in the studies were considered. However, advising patients about OH and promoting OH behaviour improvements (in terms of techniques and frequency) carry little risk compared with the benefit it brings.
Overall certainty of the evidence
Low.
5.1 From evidence to recommendation—additional considerations
Not applicable.
R5.10. In patients with healthy peri-implant tissues, does reducing bruxing/parafunctional habits reduce the risk of incident peri-implant diseases?
| PICOS question addressed by an SR |
|---|
| R5.10: Expert consensus-based statement |
| We do not know whether in patients with healthy peri-implant tissues, controlling bruxing/parafunctional habits reduces the risk of incident peri-implant diseases. |
| Supporting literature (Carra et al., 2023) |
| Quality of evidence Not applicable |
| Grade of recommendation Grade O—↔ Statement, additional research needed |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
There were no studies that investigated the control of bruxing/parafunctional habits in patients with healthy peri-implant tissues in preventing the risk of peri-implant diseases.
5.3 Secondary and tertiary prevention: Recommendations for SPIC
This section aims to answer the following questions: in patients treated for peri-implantitis, what is the efficacy of: (1) supportive care, (2) SPIC with adjunctive local antiseptic agents and (3) of SPIC with a frequency of more than once a year in achieving peri-implant tissue stability.
An SR (Stiesch et al., 2023) was designed to evaluate the efficacy of providing SPIC, as well as specific SPIC protocols and frequency upon peri-implant stability after a minimum recall period of 3 years. Fifteen studies were identified that met the inclusion criteria, which included a minimum of 20 volunteers. No studies were specifically designed to evaluate SPIC provision, protocol or frequency, and all studies were surgical intervention trials that included SPIC as part of their design. Therefore, there were no studies that compared specific SPIC protocols or frequency of provision, or the use of adjunctive therapies versus none, or studies that compared the provision of SPIC versus no SPIC.
There were 10 prospective and 5 retrospective studies, 14 of which provided SPIC using various techniques for professional mechanical plaque removal (PMPR) in combination with (n = 10) or without (n = 4) OH instruction. Disease recurrence/progression outcomes were defined by the authors of the respective studies (n = 13), or were based upon progressive deterioration in BOP, PD or MBL (n = 2). Stability outcomes and disease recurrence were reported at both the implant and the patient levels.
The three PICOS questions documented below could not be answered by the SR, and a meta-analysis was inappropriate due to the high heterogeneity of the data. However, risk of bias was deemed low in 87% of the studies. The WG participants felt there were sufficient data to address the overarching question of whether regular provision of SPIC improved peri-implant tissue stability following surgical treatment of peri-implantitis, in an evidence-based manner; however, most recommendations are based upon expert consensus. There were additional questions deemed to be of importance to clinical practice that were not directly informed by the SR, but for which the workshop formulated recommendations based on the literature base.
Given the paucity of available studies (n = 15), the background study characteristics provided following the recommendation tables are deemed applicable to all recommendations.
R5.11. In patients treated for peri-implantitis, does supportive peri-implant care (SPIC) prevent recurrence of peri-implantitis in the medium to long term (≥3 years)?
| PICOS question addressed by an SR |
|---|
| R5.11: Evidence-based recommendation |
| We recommend the provision of SPIC to reduce the risk of recurrence of peri-implantitis and consequent implant loss, emphasizing to the patient the importance of their adherence to SPIC visits and home care. |
| Supporting literature (Roccuzzo, Imber, et al., 2022; Stiesch et al., 2023) |
| Quality of evidence Low (indirect evidence) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
See background text in R5.16, common for recommendations R5.11–16.
R5.12. In patients treated for peri-implantitis, what is the recommended frequency of supportive peri-implant care (SPIC)?
| PICOS question addressed by an SR |
|---|
| R5.12: Expert consensus-based statement (1) and evidence-based recommendation (2) |
(1) Following non-surgical treatment of peri-implantitis, we suggest SPIC be provided 3–4 months for the first 12 months, commencing 3 months after treatment and thereafter the frequency be tailored according to patient-, implant- and restoration-based risk factors. (2) We suggest that, following surgical treatment of peri-implantitis, SPIC:
|
| Supporting literature (Stiesch et al., 2023) |
| Quality of evidence Low |
| Grade of recommendation Grade B—↑ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
See background text in R5.16, common for recommendations R5.11–16.
R5.13. In patients treated for peri-implantitis, what is the appropriate protocol for supportive peri-implant care provision (SPIC)?
| PICOS question addressed by an SR |
|---|
| R5.13: Expert consensus-based recommendation |
We recommend that the implementation of a patient-centred SPIC protocol should include the following components:
|
| Supporting literature (Carra et al., 2023; Stiesch et al., 2023) |
| Quality of evidence Very low (indirect evidence for some components) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
- a The protocol applies to any patient with dental implants.
Background
See background text in R5.16, common for recommendations R5.11–16.
R5.14. In patients treated for peri-implantitis is there a specific regime for professional mechanical plaque removal (PMPR) that reduces risk of disease recurrence?
| PICOS question addressed by an SR |
|---|
| R5.14: Expert consensus-based statement |
We do not know which specific PMPR regime is most effective in reducing the risk of recurrent peri-implantitis. However, based upon the periodontal literature and indirect evidence, the following approaches for dental implant biofilm removal can be used alone or in combination:
|
| Supporting literature (Stiesch et al., 2023) |
| Quality of evidence No studies were identified to compare different PMPR regimes |
| Grade of recommendation Grade O—↔ Statement, additional research needed |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
See background text in R5.16, common for recommendations R5.11–16.
R5.15. In patients treated for peri-implantitis, is there a specific oral hygiene method that reduces risk of disease recurrence?
| PICOS question addressed by an SR |
|---|
| R5.15: Expert-based consensus statement (1), Evidence-based recommendation (2) |
1. We do not know which specific OH method is most effective in reducing the risk of recurrent peri-implantitis. However, based upon the periodontal literature, indirect evidence and expert opinion, we recommend individually tailored to the patient and prosthesis care including at least:
2. We recommend OH methods be demonstrated by the patient to the oral healthcare professional and periodically reinforced. |
| Supporting literature (Stiesch et al., 2023) |
| Quality of evidence No studies were identified to compare different OH methods |
| Grade of recommendation Grade O—↔ Statement, additional research needed (1); Grade A—↑↑ (2) |
| Strength of consensus Unanimous consensus (10.9% of the group abstained due to potential CoI) |
Background
See background text in R5.16, common for recommendations R5.11–16.
R5.16. In patients treated for peri-implantitis does the professional administration* of adjunctive local antimicrobial agents as part of a supportive peri-implant care (SPIC) programme reduce the risk of disease recurrence?
| Question addressed by an SR |
|---|
| R5.16: Expert-based consensus recommendation |
| We suggest not to use professional applicationa of adjunctive local antimicrobial agents in SPIC to reduce the risk of recurrent peri-implantitis. |
| Supporting literature (Stiesch et al., 2023) |
| Quality of evidence No studies were identified to specifically evaluate local antimicrobial agent use in secondary prevention of peri-implantitis |
| Grade of recommendation Grade B—↓ |
| Strength of consensus Strong consensus (3.8% of the group abstained due to potential CoI) |
- a Professional administration is by the oral healthcare professional within the dental office.
Background
Intervention
- reinforcement of systemic risk factor control (e.g., metabolic, inflammatory and hormonal diseases, medications, tobacco use and stress);
- management of remaining local risk factors (site-related factors, e.g., keratinized tissue width), implant- and prosthesis-related factors;
- reinforcement of self-performed mechanical plaque control regimes (with or without antiseptic agents).
-
PMPR:
- removal of supra- and sub-mucosal biofilm by hand or mechanical instruments;
- removal of supra- and sub-mucosal hard deposits (calculus) by hand or mechanical instruments.
Available evidence
Number and design of included studies
A total of 15 studies were included in this SR (Stiesch et al., 2023). The studies included were of prospective (n = 10) and retrospective (n = 5) design reporting on a single treatment group (n = 9) or multiple treatment groups (n = 6), conducted in a university (n = 12) or private practice (n = 3). All studies that fulfilled the inclusion criteria regarding patient number (≥20 patients) and follow-up time (≥3 years) were focused on the medium- to long-term outcomes of peri-implantitis treatment. None of the studies meeting the inclusion criteria were specifically designed to evaluate or compare different SPIC protocols or SPIC frequencies and only one study was designed to evaluate the effect of SPIC on the secondary prevention of peri-implantitis.
Risk of bias
Most studies (87%) were assessed as having a low risk of bias, two studies (13%) showed some concerns, mainly regarding the inclusion of participants (lack of randomization information), treatment standardization or definition of treatment success and disease recurrence. There was considerable heterogeneity between studies with respect to study design including: peri-implantitis case definitions, outcomes reported, outcome definitions for success and disease recurrence, peri-implantitis treatment methods and supportive care protocols.
Effect sizes and their clinical relevance
Definitions for peri-implantitis, treatment success and recurrence of disease varied considerably across the 15 studies, contributing significantly to the heterogeneity of the data. While all definitions of peri-implantitis included clinical parameters such as BOP, PD and radiographic bone loss, the defined thresholds for bone loss and PD were heterogeneous.
Definitions for success were reported by 13 of the studies but also varied between studies. Therefore, a quantitative assessment of implant- and patient-level success was not possible. In nine studies, success was defined as PD < 5 mm with no BOP or suppuration and no further bone loss. In one study, success was defined as PD < 4 mm with no BOP or suppuration and no mobility. One study defined success as PD reduction, favourable soft tissue parameters and BOP decrease. Another study defined success as no further bone loss of >1.0 mm and no implant removal, and a further study defined success as radiographic evidence of >25% bone fill.
The definition of disease ‘recurrence’ also varied significantly between the studies. In eight studies, ‘further bone loss’ was defined as one important criterion for recurrence, together with implant loss (two studies). In four studies, BOP was a criterion for recurrence and in one study disease recurrence included clinical outcomes not meeting the success criteria.
Consistency
The review found that peri-implant tissue stability reported at the patient level and at the implant level varied widely and that recurrence of peri-implantitis was reported in up to 65.2% of treated implants receiving SPIC in studies with a follow-up of 3 years or more. While the SR (Stiesch et al., 2023) aimed to identify the most effective supportive care protocol in maintaining peri-implant tissue stability after peri-implantitis treatment, no comparison of protocols could be made. Furthermore, as the studies were not specifically designed to evaluate supportive care protocols, detailed information regarding supportive care was lacking. Therefore, it was not possible to make any conclusion regarding the most effective supportive care protocol. However, the protocols included similar preventive and therapeutic principles of supportive periodontal care as described in the EFP S3-level treatment guideline for stages I–III periodontitis (Sanz et al., 2020). Regular removal of plaque from the treated implant was common to all protocols described. Several studies also specified the provision of full-mouth professional plaque removal and the reinforcement of OH instructions.
Balance of benefit and harm
The results of this review confirm that SPIC may result in peri-implant tissue stability after peri-implantitis treatment. However, disease recurrence may occur, requiring additional treatment or, in some cases, implant removal. The undesirable effects of SPIC have not been described in the included studies.
Overall certainty of the evidence
Currently, there is no high-quality evidence available to answer the PICOS of the SR. Based on the available literature, a meta-analysis was not possible. The overall evidence on the effect of SPIC on the secondary prevention of peri-implantitis is based on one RCT, seven prospective and five retrospective clinical trials. Provision of SPIC following peri-implantitis therapy may prevent disease recurrence or progression. Insufficient evidence is available to identify (i) a specific supportive care protocol for secondary prevention of peri-implantitis, (ii) the effect of adjunctive local antiseptic agents in the secondary prevention of peri-implantitis and (iii) the impact of frequency of supportive care provision. Future prospective randomized controlled studies designed to evaluate supportive care protocols are needed.
5.1 From evidence to recommendation—additional considerations
Acceptability
In most of the identified studies, the number of drop-outs was few and the study participants seemed to be compliant. Based on the findings of the SR (Stiesch et al., 2023), it may be assumed that the provision of SPIC with a frequency between 3 and 6 months over a time span of 3 years is acceptable for patients following peri-implantitis treatment.
Feasibility
There were no perceived barriers.
Ethical considerations
As an example, in Germany, neither implant therapy nor SPIC is part of the statutory health insurance. Patients only receive access to SPIC through private health insurance or self-payment.
Economic considerations
As SPIC may prevent peri-implantitis recurrence, it is an important tool to support overall oral health and well-being of patients with implants. The loss of an implant may be associated with bone loss, psychological distress, pain, and costly and time-demanding retreatments, which may require specialist management.
Legal considerations
There were no legal constraints.
6 RECOMMENDATIONS FOR THE MANAGEMENT OF PERI-IMPLANT MUCOSITIS
6.1 Introduction—general recommendations in the management of peri-implant mucositis
R6.1. In patients with peri-implant mucositis, which are the goals/end points of treatment?
| Additional question addressed by the WG |
|---|
| R6.1: Expert consensus-based recommendations |
We recommend that clinicians use as end point of peri-implant mucositis treatment at implant level: ≤1 point of BOPa and absence of suppuration We recommend that clinicians evaluate these end points 2–3 months after the intervention, and in presence of ≥2 BOP sites, or ≥1 sites with profuse BOP, or presence of suppuration, re-treatment should be rendered. |
| Supporting literature (Chan et al., 2019; Monje et al., 2021; Reinedahl et al., 2018; Salvi et al., 2012; Zitzmann et al., 2001) |
| Quality of evidence Low |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
- a BOP should be a spot rather than profuse bleeding using gentle forces (0.2 N) with a manual periodontal probe (0.5 mm tip), provided the contours of the restoration allow for adequate probing. In sites where probing is not feasible, peri-implant mucosal inflammation should be assessed through the modified sulcus bleeding index (Mombelli et al., 1987).
Background
This recommendation is an expert-based recommendation supported by experimental studies (Reinedahl et al., 2018), experimental peri-implant mucositis studies (Chan et al., 2019; Salvi et al., 2012; Zitzmann et al., 2001) and studies evaluating the probe penetration and BOP in healthy periodontal versus peri-implant tissues (Monje et al., 2021). All these studies have assessed the similarities and differences between peri-implant and periodontal tissues, how peri-implant tissues respond to biofilm accumulation, and which is the degree of reversibility when the biofilm is eliminated (experimental peri-implant mucositis model).
R6.2. In patients with peri-implant mucositis, what is the effect of oral hygiene as an adjunct to professional mechanical plaque removal (PMPR)?
| PICOS question addressed by an SR |
|---|
| R6.2: Expert consensus-based recommendation |
| In patients with peri-implant mucositis, we recommend self-performed effective OH along with PMPR |
| Supporting literature (Verket et al., 2023) |
| Quality of evidence No clinical studies were identified. |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
There are no available clinical studies with an arm with OH alone without PMPR. Furthermore, for obvious ethical reasons, there are no studies without implementing OH measures. However, there is indirect evidence from experimental mucositis studies demonstrating that OH can revert the inflammatory signs in the peri-implant mucosa. This evidence has concluded that experimental peri-implant mucositis is caused by biofilm accumulation and that it may be reversible by means of OH reinforcement alone (Chan et al., 2019; Salvi et al., 2012; Zitzmann et al., 2001). Due to this microbial aetiology, there is a clear rationale to combine professionally administered non-surgical mechanical/physical therapy with patient-performed OH reinforcement in the treatment of peri-implant mucositis. This combination results in biofilm disruption and leads to improved clinical outcomes.
Available evidence
There are no RCTs, nor observational studies (with n = 30 patients or more), or single arms from RCTs (with n = 10 patients or more) evaluating the efficacy of OH reinforcement alone as treatment for peri-implant mucositis. Similarly, there are no RCTs where professionally administered non-surgical mechanical/physical instrumentation was implemented without OH reinforcement.
Risk of bias
Not applicable.
Effect sizes and their clinical relevance
Not applicable.
Consistency
Not applicable.
Balance of benefit and harm
Not applicable.
Overall certainty of the evidence
Not applicable.
6.1 From evidence to recommendation—additional considerations
Acceptability
Self-performed OH measures are generally well accepted by individuals.
Feasibility
Non-surgical mechanical/physical treatment of peri-implant mucositis can be performed by dental hygienists, general dentists as well as specialist.
Ethical considerations
Not applicable.
Economic considerations
Not applicable.
Legal considerations
Not applicable.
R6.3. In patients with peri-implant mucositis, what is the efficacy of oral irrigators adjunctively used to PMPR?
| PICOS question addressed by an SR |
|---|
| R6.3: Evidence-based recommendation |
| In patients with peri-implant mucositis the self-use of oral irrigation devices with water may be considered as an adjunct to PMPR. |
| Supporting literature (Gennai et al., 2023) |
| Quality of evidence Low (two RCTs, one with low and the other with moderate risk of bias) |
| Grade of recommendation Grade O—↔ need for further research |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Oral irrigators can be used regularly as adjuvants to PMPR in addition to regular OH practices.
Available evidence
Number and design of included studies
The SR (Gennai et al., 2023) included two RCTs evaluating the effect of oral irrigators used by the patient adjunctively to PMPR compared with PMPR, demonstrating significant BOP reduction at 3 months in patients with peri-implant mucositis.
Risk of bias
The overall risk of bias of the included studies was judged as ‘moderate’ (RoB 2 tool), with one study with a low risk of bias and one with a moderate risk of bias.
Effect sizes and their clinical relevance
These two RCTs show imprecision in the effect estimates, the results are not consistent and publication bias could not be assessed.
Consistency
The reported results are not consistent.
Balance of benefit and harm
It could not be assessed.
Overall certainty of the evidence
Two RCTs have evaluated the adjunctive self-use of oral irrigators, one using 0.06% chlorhexidine (CHX) as the irrigating fluid and the other water, one study was at low and the other at moderate risk of bias. Furthermore, the imprecision of the effect estimates, the lack of consistency of the results and the potential risk of publication advises downgrading the quality of the evidence.
Acceptability
Oral irrigators are usually well accepted by patients.
Feasibility
There are no perceived barriers.
Ethical considerations
There are no perceived ethical considerations.
Economic considerations
There is an additional cost on buying the irrigator.
Legal considerations
There are no perceived legal considerations.
R6.4. In patients with peri-implant mucositis, what is the effect of any single mode of PMPR, compared with other single modes of PMPR?
| PICOS question addressed by an SR |
|---|
| R6.4: Evidence-based recommendation |
| In patients with peri-implant mucositis, ultrasonics with plastic coated tips or air-polishing devices with glycine powder or titanium curettes or chitosan brushes may be considered as a single mode of PMPR. |
| Supporting literature (Verket et al., 2023) |
| Quality of evidence Low (two RCTs demonstrating positive effects within the single mode of PMPR, but without differences among them) |
| Grade of recommendation: Grade O—↔ (need for further research) |
| Strength of consensus Consensus (5.3% of the group abstained due to potential CoI) |
Background
Intervention
PMPR aims at reducing soft tissue inflammation by removing hard and soft deposits from the surface of the dental implant and/or its supra-structure without scratching the surface of the smooth transmucosal element (implant collar, abutment). Several modalities including ultrasonics with carbon fibre or plastic tip, air-polishing, curettes of plastic, carbon or titanium or rotating/oscillating brushes and lasers have been used within PMPR. The end point of treatment is to eliminate inflammation, evaluated by BOP and suppuration.
Available evidence
Two RCTs comparing two single modes of mechanical therapies were identified (Verket et al., 2023). One is a 12-month parallel group RCT (n = 37 patients) comparing glycine powder air polishing and ultrasonic with plastic coated tips. The mean BOP reductions were 31.8% and 35.1%, respectively at 12 months, without statistically significant differences between both modes of therapy. The other is a 6-month split-mouth RCT (n = 11 patients) comparing titanium curettes and chitosan brushes after a period of OH. The mean reduction in BOP severity (modified sulcus bleeding index), was 0.84 and 0.61, respectively. The mean disease resolution at implant level (up to one spot BOP) was 50% and 35% at 6 months.
Risk of bias
Study quality assessment identified some concerns of risk of bias in one study and high risk of bias in the other.
Effect sizes and their clinical relevance
One study reported disease resolution/treatment success in 8.3%–16.7% at 6 months, and BOP severity of 0.70–0.74. In this study, OH instruction was performed before the baseline examination. Another study reported BOP extent at 12.1%–18.6% at 12 months.
Consistency
Evidence was consistent in the two studies with limited reduction in BOP. The only patient-reported outcome showed no difference in pain during treatment when titanium curettes were compared with chitosan brush.
Balance of benefit and harm
An overall consideration of the benefit versus harm of professionally administered non-surgical mechanical/physical therapy supports the recommendation.
Overall certainty of the evidence
Low.
6.1 From evidence to recommendation—additional considerations.
Acceptability
Patients usually accept and understand the need for treatment.
Feasibility
Non-surgical mechanical/physical treatment of peri-implant mucositis can be performed by dental hygienists, general dentists as well as specialist.
Ethical considerations
Not applicable.
Economic considerations
Not applicable.
Legal considerations
Not applicable.
R6.5. In patients with peri-implant mucositis, what is the effect of combinations of PMPR procedures, compared to single modes?
| PICOS question addressed by an SR |
|---|
| R6.5: Evidence-based recommendations |
In patients with peri-implant mucositis, we suggest not to add air-polishing devices to conventional PMPR (curettes, ultrasonics or both), even though these devices have shown efficacy when used as a single mode of treatment. In patients with peri-implant mucositis, we suggest not to add diode lasers with conventional PMPR (curettes, ultrasonics or both). |
| Supporting literature (Verket et al., 2023) |
| Quality of evidence Moderate (three RCTs, n = 313 patients) |
| Grade of recommendation Grade B—↓ |
| Strength of consensus Consensus (15.4% of the group abstained due to potential CoI) |
Background
Intervention
Professionally administered PMPR therapy aims at reducing soft tissue inflammation by removing hard and soft deposits from the surface of dental implants and/or its supra-structure. Combinations of PMPR therapy have been used and include laser adjunctive to ultrasonics and curettes, and air-polishing adjunctive to ultrasonics. The end point of treatment is absence of inflammation, that is, BOP and suppuration.
Available evidence
Number and design of included studies
Three RCTs addressed the PICOS question (n = 313 patients). Two RCTs analysed the effect of laser therapy adjunctive to ultrasonics and curettes (n = 289), and one RCT analysed the effect of air-polishing adjunctive to ultrasonics (n = 24), all with a 3-month follow-up. One study compared ultrasonics with carbon fibre tip plus glycine powder air polishing versus ultrasonics alone (n = 24). The results on mean BOP severity were 1.1 and 1.0, respectively. The second study (n = 220) compared ultrasonics with carbon fibre tip and titanium-coated curettes with and without diode laser (980 nm) application. Results were 34.5% and 30.9% disease resolution, respectively. BOP extent at 3 months was 23.2% and 26.8%, respectively. The third study (n = 69) compares ultrasonic with plastic tips and plastic curettes with and without diode laser (810 nm). The reported BOP extent was 0.26 and 0.57 respectively at 3 months, this difference being statistically significant.
Risk of bias
Study quality assessment identified some concerns of risk of bias in two studies, and a third had a high risk of bias.
Effect sizes and their clinical relevance
One RCT reported disease resolution/treatment success in 30.9%–34.6% and 23.2%–26.8% BOP extent at 3 months. Another RCT reported BOP extent of 0.26 and 0.57 in favour of adjunctive laser at 3 months, which was statistically significant. The third RCT reported BOP severity of 1.0 and 1.1 at 3 months.
Consistency
Evidence was consistent in the studies with a reduction in BOP, but statistically significant only in one of the RCTs with laser therapy adjunctive to ultrasonics and curettes. No patient-reported outcomes were reported.
Balance of benefit and harm
An overall consideration of the benefit versus harm of professionally administered non-surgical mechanical/physical therapy supports the recommendation.
Overall certainty of the evidence
Moderate.
6.1 From evidence to recommendation—additional considerations.
Acceptability
Patients usually accept and understand the need for treatment.
Feasibility
Non-surgical mechanical/physical treatment of peri-implant mucositis can be performed by dental hygienists, general dentists as well as specialist.
Ethical considerations
Not applicable.
Economic considerations
Additional costs associated with adjunctive laser therapy may not be justified.
Legal considerations
Not applicable.
R6.6. In patients with peri-implant mucositis, what is the effect of repeating PMPR procedures, compared to a single administration of PMPR?
| PICOS question addressed by an SR |
|---|
| R6.6: Expert consensus-based recommendation |
| In patients with peri-implant mucositis, we recommend repeating PMPR if the end points of therapy have not been achieved within 3 months after the administration of PMPR. These end points and the evaluation times should be modified according to the patient's OH, risk factor profile and the cleansability of the prosthesis. |
| Supporting literature No studies evaluating the impact of repeated PMPR on peri-implant mucositis outcomes were identified (Verket et al., 2023) |
| Quality of evidence No evidence from clinical studies identified. |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
If the end point of professionally administered non-surgical mechanical/physical therapy is not met following an intervention, it may be advisable to repeat the treatment.
Available evidence
There are no available RCTs or any observational study (with n = 30 patients or more), or single arms from RCTs (with n = 10 patients or more) evaluating the effect of repeated PMPR in the treatment of peri-implant mucositis.
Risk of bias
Not applicable.
Effect sizes and their clinical relevance
No RCTs were available, but in one of the included trials (Riben-Grundstrom et al., 2015), results were reported at multiple time points after providing repeated mechanical instrumentation. After an initial reduction of 20.9%–28.6% in BOP extent, the effect of further repetitions was limited (1.9%–6.3%, and 0.0–11.3%, respectively).
Consistency
Not applicable.
Balance of benefit and harm
Not applicable.
Overall certainty of the evidence
Not applicable.
6.1 From evidence to recommendation—additional considerations
Acceptability
Not applicable.
Feasibility
Non-surgical mechanical/physical treatment of peri-implant mucositis can be performed by dental hygienists, general dentists as well as specialist.
Ethical considerations
Not applicable.
Economic considerations
Not applicable.
Legal considerations
Not applicable.
R6.7. In patients with peri-implant mucositis, which is the effect of modifying the implant-supported prosthesis to enable oral hygiene access?
| Question not addressed by the SR |
|---|
| R6.7: Expert consensus-based recommendations |
| In patients with peri-implant mucositis where the implant-supported prosthesis does not allow for proper self-performed and/or professional cleansability, we recommend cleaning/removal/modification of the prosthesis. |
| Supporting literature (de Tapia et al., 2022; de Tapia, Mozas, et al., 2019) |
| Quality of evidence High (one RCT with low risk of bias, n = 45) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Modification of the implant-supported prosthesis to improve accessibility for OH and biofilm removal in surfaces of dental implants and restorative components.
Available evidence
There is one RCT (n = 45) (de Tapia, Mozas, et al., 2019) evaluating the adjunctive effect of modifying the prosthesis to enable adequate OH. An additional publication reports on the 30-month follow-up of the same study (de Tapia et al., 2022).
Risk of bias
Low risk of bias.
Effect sizes and their clinical relevance
Results at 6 months demonstrated reductions in the modified bleeding index of 1.14 and 0.50 for test and control groups, respectively; these differences were statistically significant; and, at 6 months, disease resolution was 66.6% and 9.6%, respectively.
Consistency
Not applicable.
Balance of benefit and harm
There is a clear benefit and minimal harm in the prosthesis modification to improve access for biofilm control.
Overall certainty of the evidence
Limited due to the scarcity of the available evidence.
6.1 From evidence to recommendation—additional considerations
Acceptability
Well accepted intervention, although patients may complain for a short time of food entrapment.
Feasibility
Prosthesis modification should be implemented by general dentists as well as specialist.
Ethical considerations
Not applicable.
Economic considerations
Not applicable.
Legal considerations
Not applicable.
R6.8. In patients with peri-implant mucositis, what is the efficacy of locally administered antibiotics adjunctive to PMPR?
| PICOS question addressed by an SR |
|---|
| R6.8: Evidence-based recommendation |
| In patients with peri-implant mucositis, we recommend not to use locally administered antibiotics. |
| Supporting literature (Dommisch et al., 2023; Renvert et al., 2006) |
| Quality of evidence No direct evidence available |
| Grade of recommendation Grade A—↓↓ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Professional administration of topical antibiotics, with sustained drug release, following non-surgical mechanical/physical therapy in patients with peri-implant mucositis.
Available evidence
Number and design of included studies
No study could be identified when considering the inclusion criteria outlined in the SR (Dommisch et al., 2023). However, one RCT (n = 32) evaluated the adjunctive effect of minocycline microspheres in the treatment of peri-implant mucositis/incipient peri-implantitis (bone loss less or equal to three threads). Results showed a significant added effect in reducing BOP and PD at 6 months. However, BOP relapsed after 9 months (Renvert et al., 2006).
Risk of bias
Not applicable.
Effect sizes and their clinical relevance
Not applicable.
Consistency
Not applicable.
Balance of benefit and harm
Harm versus benefit considerations on the use of antibiotics need to be undertaken. The use of antibiotics should always meet the antibiotic stewardship guideline.
Overall certainty of the evidence
Not applicable.
6.1 From evidence to recommendation—additional considerations
Acceptability
Not applicable.
Feasibility
Not applicable.
Ethical considerations
The use of antibiotics should always meet the antibiotic stewardship guideline.
Economic considerations
High economic costs and limited availability of products in European countries need to be considered.
Legal considerations
Not applicable.
R6.9. In patients with peri-implant mucositis, what is the efficacy of other locally administered agents adjunctive to PMPR?
| PICOS question addressed by an SR |
|---|
| R6.9: Evidence-based recommendation |
| In patients with peri-implant mucositis, we suggest not to use locally administered agents (antiseptics, ‘postbiotics’, desiccant gel) as adjuncts to PMPR |
| Supporting literature (Dommisch et al., 2023) and (Butera et al., 2022; Lombardo et al., 2019; Sahrmann et al., 2019) and expert opinion |
| Quality of evidence Low |
| Grade of recommendation Grade B—↓ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Professional administration of topical antiseptics/agents (hydrogen peroxide, chlorhexidine, delmopinol hydrochloride, sodium hypochlorite, chitosan, acids, ‘postbiotics’) following non-surgical mechanical/physical therapy in patients with peri-implant mucositis. ‘Postbiotics’ are products of the metabolic activity of the micro-organism, which, by exerting an antioxidant action, lead to a positive effect on the host (Zolkiewicz et al., 2020); in contrast with probiotics, they do not include live microorganisms.
Available evidence
Number and design of included studies
Two RCTs were selected by the SR (Dommisch et al., 2023). One of them (n = 37 patients) assessed the professional administration of 0.12% CHX in 119 implants over the time periods of 1, 3 and 6 months in non-smokers. In the control group, CHX was not professionally applied. Outcome measures compared PD, BOP and visible plaque index (PlI). Disease resolution, SOP and PROMs were not reported. In both the control and test groups, significant reductions in PD, BOP and visible PlI were observed when comparing values at baseline with values at the 3- and 6-month follow-up. The inter-group comparison revealed no differences when comparing test and control groups (Menezes et al., 2016). The second one (n = 46 patients) tested the professional administration of 0.95% NaOCl in 68 implants over a time period of 1, 3 and 6 months. In the control group, NaOCl was not applied. Outcome measures compared reduction in BOP, PD and a modified PlI. In addition, disease resolution was evaluated. Significant reductions in BOP, PD and the modified PlI for oral implants in both the test and control groups were observed at the 6-month follow-up. The inter-group comparison did not show differences among groups regarding BOP, disease resolution, PD or the modified PlI (Iorio-Siciliano et al., 2020). Changes in SOP as well as PROMs were not reported. Thus, the main finding of the RCTs identified in the SR was that 0.12% CHX or 0.95% NaOCl did not additionally improve clinical outcomes.
Apart from the evidence included in the SR, three additional RCTs were considered, evaluating the adjunctive effect of an antiseptic (CHX chip), a ‘postbiotic’ (‘Lactobacillus Ferment’) and a desiccant gel/liquid (concentrated aqueous mixture of hydroxybenzenesulphonic and hydroxymethoxybenzene acids, together with sulphuric acid) as adjunctives to mechanical therapy, compared with mechanical therapy plus application of 1% CHX gel (Butera et al., 2022; Lombardo et al., 2019; Sahrmann et al., 2019).
Risk of bias
For CHX and NaOCl, study quality assessment using the RoB 2 tool identified a low risk of bias for both studies included (Iorio-Siciliano et al., 2020; Menezes et al., 2016). For the other three RCTs, risk of bias was not specifically evaluated, since they were not included in the SR, but their overall quality of evidence was considered as low.
Effect sizes and their clinical relevance
For CHX, based on one RCT (n = 37 patients), no additional effect of 0.12% CHX was demonstrated regarding reductions in BOP, PD and PlI. For NaOCl, based on one RCT (n = 46 patients), no additional effect of 0.95% NaOCl was identified regarding reductions in BOP, PD and PlI. In the case of the individual RCTs, the magnitude of the effect (comparison vs. a negative control or a placebo) could not be determined as the control groups were 1% CHX gel. For the ‘postbiotic’ gel, based on one RCT (n = 20 patients), no additional effect was demonstrated (Butera et al., 2022). For the desiccant solution, based on one RCT (n = 23 patients), significant differences between groups were only observed for plaque indices (Lombardo et al., 2019). For the CHX chip, based on one RCT (n = 32 patients), significant additional benefits in BOP were observed in the test group, but the statistically significant differences observed at baseline precluded a strong conclusion on the adjunctive effect (Sahrmann et al., 2019). The effect of the 1% CHX was heterogeneous, which is beneficial in two studies (Butera et al., 2022; Lombardo et al., 2019), but not in the third (Sahrmann et al., 2019).
Consistency
Not applicable.
Balance of benefit and harm
In both identified studies, the adjunctive professional administration of 0.12% CHX or 0.95% NaOCl did not cause unintentional side effects that suggest harm to the patient (Iorio-Siciliano et al., 2020; Menezes et al., 2016). Thus, formulations of both CHX and NaOCl may be considered as a professional treatment adjunctive to non-surgical mechanical/physical therapy in the treatment of peri-implant mucositis. Future studies are needed to further investigate the efficacy of the given and other concentrations of CHX and NaOCl. For CHX, several adverse effects such as taste alteration, mouth numbness, xerostomia, and tooth discoloration have been reported (Poppolo Deus & Ouanounou, 2022). For NaOCl, the occurrence of potential adverse effects is uncertain for various concentrations. Potential adverse side effects must be considered to balance benefits and harms. For the ‘postbiotic gel’, potential unintentional side effects were not reported (Butera et al., 2022); based on the composition of the postbiotic gel, potential side effects, such as allergic reactions, cannot be excluded. For the CHX chip, numerous unintentional side effects are listed in the product information, but they are reported to be not frequent and usually mild. For the desiccant, no unintentional side effect was reported (Lombardo et al., 2019); however, potential side effects of sulphuric acid are listed by the company, and thus, the application is not recommended in patients if allergic to sulphur in any form and in the case of pre-existing skin disorders.
Overall certainty of the evidence
The certainty is weak lack of studies.
6.1 From evidence to recommendation—additional considerations
Acceptability
In general, the application of antiseptics is well accepted by patients when understanding the pathogenesis of peri-implant mucositis.
Feasibility
CHX gels, CHX chips, desiccant materials, ‘postbiotics’ and NaOCl formulations can be professionally applied by the general dentist or specialist. Their adjunctive use is not clinically demanding or time-consuming. For the NaOCl formulation (PeriSolv®, RLS Global AB, Mölndal, Sweden); CHX chip (PerioChip, Karr Dental, Wollerau, Switzerland), the ‘postbiotic’ (Biorepair Parodontgel Intensive, Coswell SPA, Funo di Argelato, BO, Italy), and the desiccant liquid (HybenX® Oral Tissue decontaminant™, EPIEN Medical Inc., Saint Paul, MN, USA), specific brands were tested and the information provided may only be valid for those products, which may not be available in all markets.
Ethical considerations
Based on the available evidence, no evaluation of ethical aspects could be performed.
Economic considerations
CHX gels, CHX chips, desiccant materials, “postbiotics” and NaOCl formulations are associated with additional costs to the patient as well as to the dental professional team. The application of any antiseptic treatment adjunctive to non-surgical mechanical/physical therapy may lead to additional costs for the patients depending on individual health insurance plans in the individual countries. As examples, the additional costs associated with the use of the desiccant material, in Germany, are approximately €100 for two syringes of 1 mL each, and for the use of CHX chips is approximately €300 for 20 applications. No information on cost-effectiveness could be retrieved from the RCTs (Butera et al., 2022; Iorio-Siciliano et al., 2020; Lombardo et al., 2019; Menezes et al., 2016; Sahrmann et al., 2019).
Legal considerations
The NaOCl formulation (PeriSolv®) is approved as Class I medical device in the European Union, and the desiccant material (HybenX®) has also been approved as Class I medical device in the European Union and Canada. The implications of the use in other geographical locations or the use for indications besides the ones approved are unclear.
R6.10. In patients with peri-implant mucositis, what is the efficacy of locally administered photodynamic therapy adjunctive to PMPR?
| PICOS question addressed by an SR |
|---|
| R6.10: Evidence-based recommendation |
| In patients with peri-implant mucositis, we suggest not to use photodynamic therapy adjunctively to PMPR. |
| Supporting literature (Dommisch et al., 2023) |
| Quality of evidence Low (five RCTs) |
| Grade of recommendation Grade B—↓ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Application of antimicrobial photodynamic therapy (aPDT) adjunctive to non-surgical mechanical/physical therapy in patients with peri-implant mucositis.
Available evidence
Number and design of included studies
For the application of aPDT adjunctive to sub-marginal instrumentation, five RCTs (in total, n = 204 patients) analysed an estimated number of 231 implants over a time period of 3 months (Dommisch et al., 2023). Of these five studies on adjunctive application of aPDT, four included patients with habitual tobacco intake (cigarette smokers, smoke-less tobacco chewers and vaping individuals). In the control groups, aPDT was not applied. In the test groups, the intervention varied in terms of a range in the applied wavelength between 660 and 670 nm, power density between 100 milliwatts (mW) and 150 mW. One study did not report on treatment modalities. Outcome measures compared BOP, PD and PlI. Disease resolution and PROMs were not reported. In the synthesis of data, three studies were evaluated for changes in BOP and PD and four studies for changes in PlI, comparing test and control groups. For BOP and PD, no difference was identified between test and control groups, whereas for PI, a significant difference was shown in favour of aPDT adjunctive to sub-marginal instrumentation (Dommisch et al., 2023). High heterogeneity and a high level of asymmetry were evident (Dommisch et al., 2023). Two RCTs were excluded due to the lack of reporting mean and standard deviation or assessing a modified bleeding index instead of BOP. Changes in SOP as well as PROMs were not reported. The main findings were that aPDT did not additionally improve clinical outcomes for changes in BOP, PD or PlI.
Risk of bias
For aPDT, study quality assessment using the RoB 2 tool identified a low risk of bias for one study, whereas some concerns indicated a risk of bias in four studies on aPDT.
Effect sizes and their clinical relevance
For aPDT, based on three RCTs (204 patients) included in the meta-analysis, no additional effect of the adjunct application of aPDT was demonstrated regarding reduction in BOP and PD (Dommisch et al., 2023). A significant reduction of PlI was identified in the meta-analysis; however, clinically, this reduction was not related to the reduction of surrogate parameters for disease resolution (reduction or absence of BOP, reduction in PD) (Dommisch et al., 2023).
Consistency
For aPDT, the identified RCTs included male patients only, and from these five RCTs four focused on patients with habitual tobacco intake (cigarette smokers, smoke-less tobacco chewers and vaping individuals). The analysis of data revealed high heterogeneity among the studies (Dommisch et al., 2023). This inconsistency among the studies may be explained by the heterogeneity of reported outcome parameters as well as regarding the variation of tobacco intake habits, even though only male patients were evaluated. In addition, the intervention varied in terms of a range in the applied wavelength between 660 and 670 nm, power density between 100 mW and 150 mW, and choice of photosensitizer (phenothiazine chloride, methylene blue) in the respective test groups.
Balance of benefit and harm
For the additional application of aPDT adjunctive to sub-marginal instrumentation, no benefit was identified in the meta-analysis (Dommisch et al., 2023). Potential harm of aPDT adjunctive to sub-marginal instrumentation has not been studied to date. However, potential adverse effects cannot be entirely ruled out due to various wavelength, power density and photosensitizer available on the market.
Overall certainty of the evidence
The overall certainty regarding the additional effect of aPDT is weak. The quality of evidence is low.
6.1 From evidence to recommendation—additional considerations
Acceptability
The adjunct application of aPDT is accepted by patients when understanding the pathogenesis of peri-implant mucositis.
Feasibility
The application of aPDT can only be performed by a trained operator and appropriate eye protection must be used by the dental professional team and the patient.
Ethical considerations
Not applicable.
Economic considerations
The application of aPDT causes comparatively high costs for the dental team with regard to the acquisition and maintenance of the corresponding equipment. For the patient, aPDT adjunctive to sub-marginal debridement may lead to additional costs depending on individual health insurance plans in the individual countries. No information on cost-effectiveness could be retrieved from the five selected RCTs. Additional costs associated with adjunctive laser therapy may not be justified.
Legal considerations
Not applicable.
R6.11. In patients with peri-implant mucositis, what is the efficacy of patient self-administered antiseptics adjunctive to PMPR?
| PICOS question addressed by an SR |
|---|
| R6.11: Evidence-based recommendation |
| In patients with peri-implant mucositis, the time limited self-administration of oral rinse antiseptics (chlorhexidine and herbal-based) adjunctive to PMPR may be considered. |
| Supporting literature (Gennai et al., 2023) |
| Quality of evidence Moderate (six RCTs, using different antiseptic agents, CHX and herbal-based). |
| Grade of recommendation Grade O—↔ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Application of antiseptics adjunctive to PMPR in patients with peri-implant mucositis.
Available evidence
Number and design of included studies
The SR (Gennai et al., 2023) included five RCTs evaluating the effect of antiseptics used by the patient adjunctively to sub-marginal instrumentation compared with sub-marginal instrumentation alone or combined with a negative control or a placebo in terms on BOP reduction at 3 months in patients with peri-implant mucositis.
In these five RCTs, self-administered antiseptics as adjuvant to PMPR were used in the format of gels (0.5% CHX) or mouth rinses. In this latter delivery format (mouth rinses), the following active agents have been tested: CHX at different concentrations (0.03%, 0.12% or 0.2%) alone or combined with cetylpyridinium chloride (CPC) (0.05%); herbal-based mouth rinses; delmopinol (0.2%).
Risk of bias
The overall risk of bias of the included studies was judged as ‘low’ (RoB 2 tool), with all five studies with a low risk of bias.
Effect sizes and their clinical relevance
For CHX gel: 29 patients received PMPR at the implant sites, they were instructed to brush around the implant twice daily using a chlorhexidine gel (0.5%) (n = 15) or a placebo gel (n = 14) for a period of 4 weeks, and there were significant reductions in the mean number of sites with BOP from baseline to 1 month for both test and control groups (p < .05), with little apparent change between 1 and 3 months (p > .1); there was no statistically significant difference in the changes in BOP between the test and control groups at 1 month or at 3 months (p > .1).
For CHX mouth rinses, four RCTs with 166 patients compared the efficacy of self-administered CHX mouth rinses versus distilled water/saline or placebo, for 2 weeks, 1 month or 1 year, and the results showed significant reductions over time of BOP, with conflicting results in terms of superiority versus control. Statistically significant differences in BOP or in modified gingival index (MGI) were noted after 3 months, while no statistically significant differences in terms of BOP were reported at 1 month or with the usage of 0.05% CHX plus 0.05% CPC at 1 year (Pulcini et al., 2019).
For herbal mouth rinses, 2 RCTs with 62 patients were managed with self-administered herbal-based mouth rinses for 2 weeks or NaCl/distilled water. At 3 months, statistically significant differences in BOP and MGI, between test and control groups, were reported, with better performance in the herbal mouth rinse groups.
For delmopinol, one RCT analysed the efficacy of 1-month self-performed delmopinol mouth rinse versus placebo, with 59 patients. Both treatments showed reduction on BOP with no differences among test and control groups.
Consistency
Conflicting results were reported when using CHX.
Balance of benefit and harm
In the included studies, some antiseptics have been associated with undesirable side effects, such as transient anaesthetic sensation in the oral mucosa (delmopinol) or higher levels of staining on the teeth or tongue (CHX). Moreover, other rarer side effects cannot be excluded.
Overall certainty of the evidence
Low.
6.1 From evidence to recommendation—additional considerations
Acceptability
Antiseptics are widely accepted by the population.
Feasibility
There are no perceived barriers.
Ethical considerations
The issue has not been addressed. There are no perceived ethical considerations.
Economic considerations
For dentifrices, it may not be relevant since it is always combined with mechanical tooth brushing. For mouth rinses use, the extra cost should be taken into consideration.
Legal considerations
It should also be noted that the evidence base contains studies using products that may no longer be available.
R6.12. In patients with peri-implant mucositis, what is the efficacy of patient self-administered probiotics adjunctive to PMPR?
| PICOS question addressed by an SR |
|---|
| R6.12: Evidence-based recommendation |
| In patients with peri-implant mucositis, the professionally guided self-administration of probiotics may be considered as adjunctive to PMPR. |
| Supporting literature (Gennai et al., 2023) |
| Quality of evidence Moderate (six RCTs) |
| Grade of recommendation Grade O—↔ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Adjunctive probiotic tablets containing Lactobacillus reuteri. In two trials, the adjunctive measurement was combined with a 0.12% CHX mouth rinse, 15 days before starting probiotics intake. The most frequent posology was one tablet per day for 1 month. In contrast, the shortest posology was two tables per day for 3 weeks and the longest, twice per day for 3 months.
Available evidence
Number and design of included studies
The SR (Gennai et al., 2023) included six RCTs evaluating the effect of systemic probiotic used by the patient, adjunctively to sub-marginal instrumentation, compared with sub-marginal instrumentation alone or combined with a negative control or a placebo, in terms of BOP reduction at 3 months in patients with peri-implant mucositis.
Risk of bias
The overall risk of bias of the included studies was judged as ‘low’ (RoB 2 tool), with three studies with a low risk of bias and three with a moderate risk of bias.
Effect sizes and their clinical relevance
- Statistically significant greater reduction in BOP (%) for antiseptics than controls (nstudies = 6; npatients = 260; weighted mean difference [WMD] = 12.11%; 95% CI [3.20; 21.03]; p = .008; I2 = 93.3%).
- Statistically significant greater reduction in plaque (%) for antiseptics than controls (nstudies = 6; npatients = 260; WMD = 14.20%; 95% CI [3.46; 29.94]; p = .01; I2 = 92.4%).
- No statistically significant differences in PD reductions.
- Complete disease resolution was only reported in one study (32% after 135 days, without differences between test and control groups).
At 6 months, no statistically significant differences were found when comparing probiotics versus control groups for any study outcome. No adverse events were reported due to the adjunctive use of L. reuteri tablets.
Consistency
All studies reported the same tendency.
Balance of benefit and harm
No adverse events have been reported. Clear benefits observed at 3 months, although they were not sustained at 6 months.
Overall certainty of the evidence
Moderate.
6.1 From evidence to recommendation—additional considerations
Acceptability
Systemic probiotics are still not widely accepted by the population.
Feasibility
There are no perceived barriers.
Ethical considerations
There are no perceived ethical considerations.
Economic considerations
There are no perceived economic considerations, although an extra economic cost is derived from the prescription of the probiotics.
Legal considerations
There are no perceived legal considerations.
R6.13. In patients with peri-implant mucositis, what is the efficacy of the oral administration of systemic antibiotics when used adjunctively to PMPR?
| PICOS question addressed by an SR |
|---|
| R6.13: Evidence-based recommendation |
| Due to concerns about patient's health and the impact of systemic antibiotic use to public health, in patients with peri-implant mucositis we recommend not to use |
| Supporting literature (Gennai et al., 2023; Sanz et al., 2020) and antibiotic stewardship. |
| Quality of evidence Low (three RCTs) |
| Grade of recommendation Grade A—↓↓ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
- Azithromycin (500 mg the first day and 250 mg from the second to fourth day).
- Amoxicillin (500 mg, thrice daily for 1 week).
Available evidence
Number and design of included studies
The SR (Gennai et al., 2023) included three RCTs evaluating the effect of systemic antibiotics prescribed as oral administration adjunctively to sub-marginal instrumentation. In one study, amoxicillin was compared with sub-marginal instrumentation combined with probiotics. In another study, the adjunctive administration of azithromycin was compared with instrumentation alone. In the third study, the adjunctive use of azithromycin plus a 0.12% CHX mouth rinse was compared with instrumentation plus a 0.12% CHX mouth rinse. Outcomes evaluated in these three studies were the percentage of BOP, PlI and PD.
Risk of bias
The overall risk of bias in the included studies was judged as ‘moderate’ (RoB 2 tool), with all the three studies with a moderate risk of bias.
Effect sizes and their clinical relevance
- Statistically significant greater reduction in BOP (%) for antibiotics than controls (nstudies = 3; npatients = 101; WMD = 5.97%; 95% CI [1.34; 10.59]; p = .012; I2 = 58.1%).
- Statistically significant greater reduction in plaque (%) for antiseptics than controls (nstudies = 3; npatients = 101; WMD = 14.74%; 95% CI [3.83; 25.65]; p = .008; I2 = 83.2%).
- Statistically significant differences in the reduction in PD (mm) for the use of systemic antibiotics than controls only for one study (nstudies = 1; npatients = 28; MD = 1.8 mm; 95% CI [1.37; 2.23]; p < .001).
- Complete disease resolution was rarely reported. One study reported at 3 months an OR of 4.5 (95% CI [1.2; 17.0]; p < .05) of favourable treatment in favour of systemic azithromycin in comparison with the control group.
- Statistically significant greater reduction in BOP (%) for antibiotics than controls (nstudies = 2; npatients = 71; WMD = 20.79%; 95% CI [15.24; 26.34]; p < .001; I2 = 30.60%).
- Statistically significant greater reduction in plaque (%) for antiseptics than controls (nstudies = 2; npatients = 7; WMD = 13.97%; 95% CI [4.10; 23.84]; p = .006; I2 = 30.6%).
- Only one study using amoxicillin reported statistically significant differences with control group (nstudies = 1; npatients = 28; MD = 2.60 mm; 95% CI [2.20; 3.00]; p < .001).
No studies reported a longer follow-up than 6 months.
Consistency
All studies reported the same tendency.
Balance of benefit and harm
In one study that collected side effects, no adverse events were observed after antibiotic intake. No specific concerns can be raised for antibiotics as adjunctive use for treating peri-implant mucositis.
Overall certainty of the evidence
Moderate.
6.1 From evidence to recommendation—additional considerations
Acceptability
The population widely accepts antibiotics. Nevertheless, there is an issue related to the need of diminishing the usage of antibiotics due to the potential risks associated with antibiotic resistance.
Feasibility
There are no perceived barriers.
Ethical considerations
The issue has not been addressed. There are no perceived major ethical considerations. Yet it must be reiterated the need of containing prescription of antibiotics for the population at large.
Economic considerations
The specific economic considerations can be stated.
Legal considerations
No specific legal consideration can be stated.
7 RECOMMENDATIONS FOR NON-SURGICAL MANAGEMENT OF PERI-IMPLANTITIS
7.1 Introduction—general recommendations in the non-surgical step of peri-implantitis treatment
The management of peri-implantitis is a relatively new area of research and clinical practice. Although key differences impacting care between peri-implantitis and periodontitis have been identified, the theoretical foundation of peri-implantitis treatment is based on the successful approaches developed for the treatment of periodontitis. Therefore, a step-by-step approach may be appropriate, as it has been suggested for the treatment of periodontitis (Sanz et al., 2020), and described in Section 4 of the present CPG. Thus, the interventions included in the SRs of WG #3 (Cosgarea et al., 2023; de Waal et al., 2023; Liñares et al., 2023) are part of the non-surgical step of peri-implantitis treatment.
This stepwise approach mirrors the one used in periodontal therapy (Sanz et al., 2020), and the included interventions are also similar to those proposed for periodontitis. The main objective of the non-surgical step of peri-implantitis treatment is to control peri-implant biofilms and inflammation, and therefore the central intervention would be sub-marginal instrumentation. In addition, interventions focusing on supramarginal biofilm control or on risk factor control are also part of the non-surgical step of peri-implantitis treatment.
After delivery of treatment, progress in controlling inflammation and suppuration should be monitored, and the outcomes should be re-assessed. While in periodontitis treatment, end points of therapy have been well established, and success of steps 1 and 2 of treatment is a reasonable expectation (Suvan et al., 2020), comparable evidence for the treatment of peri-implantitis is still scarce. The rationale for using a stepwise approach and for a non-surgical phase of peri-implantitis treatment, therefore, comes from (i) attempting biofilm and inflammation control with relatively simple approaches before escalating treatment complexity and invasiveness; (ii) the fact that subjects with peri-implantitis frequently present with poorly controlled periodontitis that requires a concomitant stepwise treatment approach; and (iii) the ability to deliver any surgical treatment at a later step and in a subject with better biofilm and risk factor control.
R7.1. Is peri-implantitis treatable?
| Additional question addressed by the WG |
|---|
| R7.1: Expert consensus-based recommendation |
In patients with peri-implantitis, we recommend therapy to retain an individually acceptable implant/prosthesis as the first line of treatment. We recommend that peri-implantitis therapy starts with a non-surgical step, followed by re-evaluation and, depending on the outcomes, progress to the surgical step or to SPIC. |
| Supporting literature (Cosgarea et al., 2023; de Waal et al., 2023; Liñares et al., 2023) and Expert opinion |
| Quality of evidence Moderate—indirect evidence derived from 15 RCTs, with at least 6-month follow-up (10 with low, 3 with some concerns and 2 with high risk of bias) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus: Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
The interventions for treating peri-implantitis differ among studies, but they most commonly include sub-marginal instrumentation and peri-implant biofilm control (Cosgarea et al., 2023; de Waal et al., 2023; Liñares et al., 2023) in both test and control groups.
Available evidence
Number and design of included studies
In the SRs prepared for the present project (Cosgarea et al., 2023; de Waal et al., 2023; Liñares et al., 2023), 15 RCTs with at least 6-month follow-up were considered as valid for developing recommendations. For the present recommendation, outcomes from both test and control groups are considered.
Risk of bias
Ten presented with low risk of bias, three with some concerns and two with high risk.
Effect sizes and their clinical relevance
The observed improvements after treatment are significant in magnitude and consistent across the considered RCTs. Taken together, the evidence is unlikely to arise from the placebo or the Hawthorne effect. Still, it is not possible to assess the relative contribution of the different components that have been tested.
Consistency
Not applicable.
Balance of benefit and harm
Benefits were observed in both the test and control groups. Of 17 test groups, statistically significant benefits were observed in 11 for PD reduction and 9 for BOP. Of 17 control groups, statistically significant benefits were observed in 11 for PD reduction and 7 for BOP. The percentage of disease resolution was provided by seven test groups (ranging 0%–65%) and seven control groups (ranging 14%–55%). Limited evidence of harm was presented.
Overall certainty of the evidence
Moderate.
7.1 From evidence to recommendation—additional considerations
Acceptability
The interventions for treating peri-implantitis seem to be acceptable for patients, health providers and health authorities, although no direct evidence is available.
Feasibility
The interventions for treating peri-implantitis are feasible, although some of them may need specific training.
Ethical considerations
The interventions for treating peri-implantitis may negatively impact equity, if public services are not covering the cost, and those will need to be directly covered by patients.
Economic considerations
Limited evidence is available, see Section 1.
Legal considerations
Not applicable.
R7.2. Which interventions should be provided as part of the non-surgical step of peri-implantitis treatment?
| Additional question addressed by the WG |
|---|
| R7.2: Expert consensus-based recommendation |
We recommend that the following interventions should be provided as part of the non-surgical step of peri-implantitis:
|
| Supporting literature (Cosgarea et al., 2023; de Waal et al., 2023; Liñares et al., 2023) and Expert opinion |
| Quality of evidence Low—indirect evidence derived from 15 RCTs, with at least 6-month follow-up (10 with low, 3 with some concerns and 2 with high risk of bias) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus: Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
- OH instructions and motivation, see Section 5.
- Risk factor control, see Section 6.
- Prosthesis cleaning/removal/modification, including controlling biofilm retentive factors and evaluation of the components of the prosthesis, whenever needed and feasible. If renewal is necessary, additional evaluation of the overall treatment planning should be made, considering the added costs and the cost-effectiveness ratio (Karlsson et al., 2022).
- Supramarginal and sub-marginal instrumentation. For the latter, for the present work, instrumentation performed with curettes and/or sonic/ultrasonic devices was considered as the basic/control intervention. Additional or alternative methods to clean/decontaminate the implant surface are discussed in the following recommendations.
- Concomitant periodontal therapy as needed. If periodontal diseases are detected, they should be properly managed, in particular periodontitis, which is a recognized risk factor for peri-implantitis (Berglundh et al., 2018; Schwarz et al., 2018). Concomitant treatment of periodontitis should follow available guidelines (Sanz et al., 2020).
Available evidence
Number and design of included studies
In the SRs prepared for the present project (Cosgarea et al., 2023; de Waal et al., 2023; Liñares et al., 2023), 15 RCTs with at least 6 months of follow-up were considered as valid for developing recommendations. For the present recommendation, both test and control groups are considered.
Risk of bias
Ten presented with a low risk of bias, three with some concerns and two with high risk.
Effect sizes and their clinical relevance
Not applicable.
Consistency
Not applicable.
Balance of benefit and harm
Benefits were observed in both test and control groups (see background text of previous recommendation). Limited evidence of harm was presented.
Overall certainty of the evidence
Low.
7.1 From evidence to recommendation—additional considerations
Acceptability
The interventions for treating peri-implantitis seem to be acceptable for patients, health providers and health authorities, although no direct evidence is available.
Feasibility
The interventions for treating peri-implantitis are feasible, although some of them may need specific training.
Ethical considerations
The interventions for treating peri-implantitis may negatively impact equity if public services are not covering the cost, as in these situations they will need to be directly covered by patients.
Economic considerations
Limited evidence is available, see Section 1.
Legal considerations
Not applicable.
R7.3. Which are the end points of the non-surgical step of peri-implantitis treatment, and when and how should they be evaluated?
| Additional question addressed by the WG |
|---|
| R7.3: Expert consensus-based recommendations |
1. To assess the outcome of the non-surgical step of peri-implantitis treatment, we recommend monitoring residual inflammation/suppuration and probing depths. Patient satisfaction, good OH and prosthesis cleansability should also be considered. 2. We recommend using, at implant level, residual probing depths ≤5 mm with no BOP at more than one pointa and no suppuration, as therapy endpoints. 3. If they are not achieved, we recommend considering additional treatment. 4. We recommend evaluating the outcome (re-evaluation) of the non-surgical step of therapy after 6–12 weeks; it may be prudent to monitor cases frequently during healing. |
| Supporting literature (Cosgarea et al., 2023; de Waal et al., 2023; Liñares et al., 2023) and Expert opinion |
| Quality of evidence Low—indirect evidence derived from 15 RCTs, with at least 6 months of follow-up (10 with low, 3 with some concerns and 2 with high risk of bias) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus: Unanimous consensus (0% of the group abstained due to potential CoI) |
- a BOP present at a single spot, not line or profuse bleeding, is compatible with control of inflammation. It should be assessed at six sites per implant using gentle forces (0.2 N) with a standard manual periodontal probe (0.5 mm tip).
Background
Intervention
The group identified follow-up intervals and outcomes among those described in test and control groups of the 15 RCTs included in the three SRs (Cosgarea et al., 2023; de Waal et al., 2023; Liñares et al., 2023). In addition, the findings of the ID-COSM project (see Section 2) were also considered (Derks et al., 2022).
Available evidence
Number and design of included studies
In the SRs prepared for the present project (Cosgarea et al., 2023; de Waal et al., 2023; Liñares et al., 2023), 15 RCTs with at least 6 months of follow-up were considered as valid for developing recommendations.
Risk of bias
Ten presented with low risk of bias, three with some concerns and two with high risk.
Effect sizes and their clinical relevance
Not applicable.
Consistency
Not applicable.
Balance of benefit and harm
Not applicable.
Overall certainty of the evidence
Low.
7.1 From evidence to recommendation—additional considerations
Acceptability
The evaluation of the outcomes after the non-surgical step of peri-implantitis treatment seems to be acceptable for patients, health providers and health authorities, although no direct evidence is available.
Feasibility
The evaluation of the outcomes after the non-surgical step of peri-implantitis treatment seems to be feasible.
Ethical considerations
Not applicable.
Economic considerations
Not applicable.
Legal considerations
Not applicable.
7.2 Non-surgical sub-marginal instrumentation—mechanical/physical cleaning/decontamination
The SR by Cosgarea and co-workers (Cosgarea et al., 2023) focused on mechanical/physical approaches for implant surface cleaning/decontamination. Three PICOS questions were formulated, one to understand the efficacy of sub-marginal instrumentation versus no treatment or supramarginal instrumentation (PICOS #3) and two PICOS questions aimed to evaluate different mechanical/physical decontamination methods (e.g., air- polishing, sonic/ultrasonic devices, lasers), alone or in combination, compared with non-surgical sub-marginal instrumentation with/without placebo decontamination (non-aiming at mechanical/physical decontamination, for example, scalers to remove hard deposits with adjunctive saline irrigation) with (PICOS #2) or without (PICOS #1) other concomitant interventions.
The review initially identified nine RCTs, but for the consensus report seven RCTs were finally considered, five (Abduljabbar et al., 2017; Alpaslan Yayli et al., 2022; Roccuzzo, Klossner, et al., 2022; Schwarz et al., 2005, 2006) assessing various types of laser therapies (i.e., Nd:YAG, diode laser, Er,Cr:YSGG and Er:YAG), and two (Merli et al., 2020; Sahm et al., 2011) assessing an air-abrasive decontamination system. Two presented a high risk of bias, and the other five a low risk of bias.
R7.4. What is the efficacy of sub-marginal instrumentation in the non-surgical step of peri-implantitis treatment?
| PICOS question addressed by an SR |
|---|
| R7.4: Expert consensus-based recommendation |
| In patients with peri-implantitis, we recommend performing non-surgical supra- and sub-marginal instrumentation with curettes and/or sonic/ultrasonic devices. |
| Supporting literature (Cosgarea et al., 2023; de Waal et al., 2023; Liñares et al., 2023) and Expert opinion |
| Quality of evidence Moderate—indirect evidence derived from 15 RCTs, with at least 6 months of follow-up (10 with low, 3 with some concerns and 2 with high risk of bias) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus: Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
For the present CPG development process, the control intervention to evaluate non-surgical sub-marginal instrumentation approaches was defined as those approaches not aiming at mechanical/physical decontamination, which includes scalers or sonic/ultrasonic devices to remove hard deposits with/without adjunctive irrigation with an inactive solution (i.e., saline). For answering the proposed question, studies comparing control decontamination with no treatment or supragingival instrumentation were searched for. Since no direct evidence was found, indirect evidence derived from the control groups of the selected studies was used: in some control groups, in addition to sub-marginal instrumentation, additional interventions were included (that were also part of the treatment protocol in the test group), such as adjunctive decontamination with chlorhexidine digluconate as subgingival irrigation (0.1%–0.2%), as subgingival application (1% chlorhexidine digluconate gel) or as mouth rinsing (2 weeks with 0.1%–0.2% chlorhexidine digluconate) (Sahm et al., 2011; Schwarz et al., 2005, 2006).
Available evidence
Number and design of included studies
No study was found answering this question.
Risk of bias
Not applicable
Effect sizes and their clinical relevance
Due to the lack of studies, indirect evidence was used, analysing the clinical impact in control groups in the 15 RCTs identified in the three SRs (Cosgarea et al., 2023; de Waal et al., 2023; Liñares et al., 2023). Of 17 control groups, statistically significant benefits were observed in 11 for PD reduction and in seven for BOP. The percentage of disease resolution was provided for seven control groups, and it ranged 14%–55%. Limited evidence of harm was presented.
Consistency
Most control groups found a statistically significant impact of the treatment; this was similar to that reported in test groups.
Balance of benefit and harm
No proper evaluation of PROMs was carried out.
Overall certainty of the evidence
Moderate.
7.1 From evidence to recommendation—additional considerations
Acceptability
There is no evidence so far for clinicians' or patients' acceptability.
Feasibility
Implementation of therapy may be negatively influenced by the lack of retrievability and/or shape of the prosthetic suprastructure.
Ethical considerations
No data are available to address ethical considerations.
Economic considerations
Cost-effectiveness has not been evaluated in these studies.
Legal considerations
So far, if the manufacturer's indications are respected, there are no legal considerations.
R7.5. What is the efficacy of lasers in the sub-marginal instrumentation of the non-surgical step of peri-implantitis treatment?
| PICOS question addressed by an SR |
|---|
| R7.5: Evidence-based recommendation |
| We suggest not to use lasers, either adjunctively or as monotherapy, for non-surgical sub-marginal peri-implant instrumentation. |
| Supporting literature (Cosgarea et al., 2023) |
| Quality of evidence Low—five RCTs (n = 178 patients, n = 225 implants) with a minimum follow-up of 6 months (two studies at high risk and three studies with low risk of bias) |
| Grade of recommendation Grade B—↓ |
| Strength of consensus: Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Lasers have received significant attention as a method for sub-marginal instrumentation as they may enhance biofilm removal and/or surface decontamination. Lasers are a wide class of biomedical instruments, each one of them working based on specific principles. In the selected studies, different lasers have been tested, either alone as monotherapy (three studies) or as an adjunct to conventional sub-marginal instrumentation (two studies).
Available evidence
Five RCTs (n = 178 patients, n = 225 implants) with a minimum follow-up of 6 months, with various types of laser (Nd:YAG, diode laser, Er,Cr:YSGG and Er:YAG) assessed the sub-marginal peri-implant instrumentation with lasers alone or in combination with additional chlorhexidine irrigation (Abduljabbar et al., 2017; Alpaslan Yayli et al., 2022; Roccuzzo, Klossner, et al., 2022; Schwarz et al., 2005, 2006). Two of them used Er:YAG laser as monotherapy, one study used Nd:YAG laser as monotherapy, two studies used diode laser adjunctive to mechanical decontamination with curettes, of which one study also had a group using Er,Cr:YSGG laser as an adjunctive treatment.
Risk of bias
Two studies were at high risk, and three studies at low risk of bias.
Effect sizes and their clinical relevance
Due to heterogeneity in the treatment protocol, no meta-analysis was carried out. All studies showed improvements in both test and control groups in PD and BOP, at 3 and/or 6 months compared with baseline. In general, studies showed no additional benefit from the application of lasers at 6 months, in terms of either PD or BOP reductions. Only in one study did the adjunctive application of a Er,Cr:YSGG laser show statistically significantly larger PD reductions at 6 months, compared with sub-marginal instrumentation alone (Alpaslan Yayli et al., 2022). An Er:YAG laser as monotherapy (Schwarz et al., 2005, 2006) led to statistically significant differences in BOP. Their magnitude, however, was small.
Consistency
Positive results for the primary outcomes were observed in all five RCTs, for both control and test groups.
Balance of benefit and harm
No proper evaluation of PROMs was carried out in the studies.
Overall certainty of the evidence
Low.
7.1 From evidence to recommendation—additional considerations
Acceptability
None of the included studies provides evidence of superior patients' acceptance of laser application as compared with mechanical instrumentation with curettes. There is no evidence so far for clinicians' acceptability.
Feasibility
Implementation of therapy may be negatively influenced by the lack of retrievability and/or shape of the prosthetic suprastructure.
Ethical considerations
No data are available to address ethical considerations.
Economic considerations
Cost-effectiveness has not been evaluated in these studies.
Legal considerations
So far, the manufacturer's indications are respected, there are no legal considerations.
R7.6. What is the efficacy of sub-marginal instrumentation with air-polishing in the non-surgical step of peri-implantitis treatment?
| PICOS question addressed by an SR |
|---|
| R7.6: Evidence-based recommendation |
| We suggest not to use air polishing for non-surgical sub-marginal peri-implant instrumentation. |
| Supporting literature (Cosgarea et al., 2023; Renvert et al., 2011) |
| Quality of evidence Very low—two RCTs (n = 64 patients, n = 75 implants) with a minimum follow-up of 6 months, with low risk of bias |
| Grade of recommendation Grade B—↓ |
| Strength of consensus: Consensus (13.7% of the group abstained due to potential CoI) |
Background
Intervention
To overcome challenges with conventional sub-marginal instrumentation, alternative approaches have been assessed. Among them, air-polishing systems have been tested both as monotherapy and as adjuncts to conventional sub-marginal instrumentation.
Available evidence
Number and design of included studies
Two RCTs (npatients = 64, nimplants = 75) assessed the sub-marginal peri-implant instrumentation with air-polishing (Merli et al., 2020; Sahm et al., 2011). One used air-polishing as monotherapy (Sahm et al., 2011), while the other combined ultrasonics and air-polishing (Merli et al., 2020).
Risk of bias
Both studies had low risk of bias.
Effect sizes and their clinical relevance
Due to the heterogeneity of the treatment protocols, no meta-analysis was carried out. Both studies on air-abrasive decontamination showed PD and BOP reductions but no statistically significant differences. Inter-group differences for BOP were observed with air-polishing as monotherapy (Sahm et al., 2011).
Consistency
Not feasible to be assessed.
Balance of benefit and harm
One study reported higher levels of pain values during treatment and after 1 week for the glycine powder group as compared with mechanical instrumentation with ultrasonics (Merli et al., 2020). Cases of subcutaneous emphysema have been reported after the use of air-polishing devices (Alonso et al., 2017; Bassetti et al., 2014; Bruckmann et al., 2022). Among members of the expert panel, three groups had experienced such adverse events.
Overall certainty of the evidence
Very low.
7.1 From evidence to recommendation—additional considerations
Acceptability
Patient perception and acceptance were assessed in one study, showing no statistically significant differences (Merli et al., 2020).
Feasibility
Implementation of therapy may be negatively influenced by the lack of retrievability and/or shape of the prosthetic suprastructure. Sometimes sub-marginal delivery may not be possible due to the size of the nozzle.
Ethical considerations
Consider that the additional clinical benefit, if present, is small; that there is a potential risk of harm (subcutaneous emphysema); and that no clear benefit in terms of patient acceptability has been demonstrated.
Economic considerations
Cost-effectiveness has not been evaluated in these studies.
Legal considerations
So far, the manufacturer's indications are respected, and there are no legal considerations.
7.3 Non-surgical sub-marginal instrumentation—chemical approaches for cleaning/decontamination
The SR by de Waal and co-workers evaluated chemical approaches for implant cleaning/decontamination, aiming to answer the following PICOS question: ‘in adult patients with peri-implantitis (P), what is the efficacy of sub-marginal instrumentation combined with chemical surface decontamination (I) in comparison with sub-marginal instrumentation with or without placebo (C), in terms of changes in PDs and/or BOP (O), as reported in RCTs, non-randomized CCTs or prospective cohort studies, with a minimum of 6-month follow-up (S)?’
Three RCTs were identified: two with low risk of bias and one with some concerns. Two RCTs assessed the benefits of aPDT as an adjunct to sub-marginal instrumentation, using either toluidine blue (Wang et al., 2019) or methylene blue (Alasqah, 2022) as photosensitizers. One RCT assessed the efficacy of a desiccant material consisting of a gel of concentrated aqueous mixture of hydroxybenzenesulphonic and hydroxymethoxybenzene acids and sulphuric acid (Merli et al., 2020).
R7.7. What is the efficacy of adjunctive antimicrobial photodynamic therapy in the non-surgical step of peri-implantitis treatment?
| PICOS question addressed by an SR |
|---|
| R7.7: Evidence-based recommendation |
| We suggest not to use antimicrobial photodynamic therapy, adjunctively to sub-marginal instrumentation or as monotherapy, in non-surgical peri-implantitis therapy. |
| Supporting literature (de Waal et al., 2023) |
| Quality of evidence Very low—for adjunctive use, two 6-month RCTs, one with some concerns and one with low risk of bias; as monotherapy, no studies were considered. |
| Grade of recommendation Grade B—↓ |
| Strength of consensus: Unanimous consensus (1.9% of the group abstained due to potential CoI) |
Background
Intervention
aPDT involves the local application of light and a photosensitizing compound. Photosensitizers are generally applied sub-marginally (in the peri-implant pocket). Photons with specific energy (wavelength) interact with the specific photosensitizer and release electrons that catalyse an oxidative reaction, which has an antibacterial effect. The rationale for application of this method in the control of peri-implantitis is based on its potential antibacterial effect on the microbial biofilm associated with the implant (Vohra et al., 2014).
Available evidence
Number and design of included studies
Two RCTs assessing aPDT as adjunct to sub-marginal instrumentation, using either toluidine blue (66/66 patients) (Wang et al., 2019) or methylene blue (25/26 patients and 30/33 implants) (Alasqah, 2022), with appropriate wavelengths for the photosensitizers (635 nm for toluidine blue, 670 nm for methylene blue). As expected, no studies were found assessing aPDT as monotherapy, since aPDT cannot remove biofilm.
Risk of bias
One study was considered at low risk of bias, and the other had some concerns in terms of bias.
Effect sizes and their clinical relevance
Although both studies reported some favourable results in terms of PD reduction for aPDT as adjunct to sub-marginal instrumentation, over sub-marginal instrumentation alone, results were inconsistent and/or showed no differences for other outcome variables (BOP, MBL and/or CAL). No meta-analysis could be performed due to the limited number of studies identified and their heterogeneity.
Consistency
Substantial heterogeneity was observed in study design, interventions (laser type, photosensitizer and pre-treatment), populations studied and reported results of the studies.
Balance of benefit and harm
No adverse effects were reported.
Overall certainty of the evidence
Due to the heterogeneity in study design, interventions, populations studied and reported outcomes, the certainty of evidence is very low.
7.1 From evidence to recommendation—additional considerations
Acceptability
There are insufficient data to support or refute the use of aPDT as adjunct to sub-marginal instrumentation in the non-surgical treatment of peri-implantitis.
Feasibility
The adjunctive use of aPDT following sub-marginal instrumentation is not clinically demanding or time-consuming but requires the availability of a laser.
Ethical considerations
There is no evidence for ethical considerations. The studied photosensitizers are generally considered as safe.
Economic considerations
The additional cost associated with aPTD may not be justified.
Legal considerations
There are no obvious legal considerations.
R7.8. What is the efficacy of an adjunctive antiseptic desiccant solution in the non-surgical step of peri-implantitis treatment?
| PICOS question addressed by an SR |
|---|
| R7.8: Evidence-based recommendation |
| We suggest not to use a desiccant antiseptic gel, adjunctively to sub-marginal instrumentation or as monotherapy, in non-surgical peri-implantitis therapy. |
| Supporting literature (de Waal et al., 2023) |
| Quality of evidence Very low—one RCT with 6 months follow-up, with low risk of bias, on adjunctive use. No studies as monotherapy were considered. |
| Grade of recommendation Grade B—↓ |
| Strength of consensus: Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
In some studies, patients diagnosed with chronic periodontitis were treated with a desiccant material, consisting of a gel or liquid of concentrated aqueous mixture of hydroxybenzenesulphonic and hydroxymethoxybenzene acids, together with sulphuric acid. Results were promising regarding improvements in clinical parameters, microbiological variables and inflammatory mediators when compared with subgingival instrumentation alone (Isola et al., 2018; Lombardo et al., 2015). The same principles were used for its application as an adjunct to sub-marginal instrumentation in the treatment of peri-implantitis.
Available evidence
Number and design of included studies
One factorial design RCT with two control and two test groups (16 of 16 patients and 16 of 16 implants) assessed the adjunctive desiccant antiseptic gel and the method of sub-marginal instrumentation (Merli et al., 2020). No studies were found testing efficacy as monotherapy.
Risk of bias
The study was considered at low risk of bias.
Effect sizes and their clinical relevance
PD and CAL reduction were greater in patients treated with the desiccant material, regardless of the sub-marginal instrumentation method (ultrasonic scaler alone or combined with glycine powder air-polishing). The magnitude of the additional improvements in PD was 0.5 mm. There were no significant differences for any of the other outcomes reported.
Consistency
Not applicable.
Balance of benefit and harm
No adverse effects were reported. However, since the product is an acid, a negative impact on the surrounding tissues may happen (caustic effect on the soft tissues).
Overall certainty of the evidence
Due to the limited number of studies, the certainty of the evidence is very low.
7.1 From evidence to recommendation—additional considerations
Acceptability
There are insufficient data to support the use of desiccant material as an adjunct to sub-marginal instrumentation in the non-surgical treatment of peri-implantitis.
Feasibility
The adjunctive use of desiccant material following sub-marginal instrumentation is not clinically demanding or time-consuming. Currently, there is only one brand name/manufacturer for this material (HybenX®, EPIEN Medical Inc., Saint Paul, MN, USA).
Ethical considerations
There is no evidence for ethical considerations.
Economic considerations
There are additional costs associated with the use of the desiccant material (e.g., in Germany the cost are ca. €100 for two syringes of 1 mL each).
Legal considerations
The product has been approved as Class I medical device in the European Union and Canada. The implications of the use in other geographical locations or the use for indications besides the ones approved are unclear.
7.4 Non-surgical sub-marginal instrumentation—Adjunctive therapies
The SR by Liñares and co-workers (Liñares et al., 2023) explored the added value of adjunctive therapies by answering the following PICOS question: ‘in patients diagnosed with peri-implantitis (population), which is the efficacy of patient-performed or administered adjunctive measures to non-surgical therapy (intervention) as compared to no adjunct (comparison), in terms of PD and/or BOP reductions (primary outcomes), reported in RCTs or CCTs with at least 6 months of follow-up (study design)?’
Initially, eight studies were identified, but for the consensus development, five RCTs were finally considered: two on local antimicrobials, two on systemic antimicrobials and one on probiotics. Two studies presented some concerns and three studies a low risk of bias. The other studies were excluded due to different reasons: non-sustained release for local antimicrobials; inadequate control group (treated with aPDT) and inclusion criteria (abscess) for systemic antimicrobials; and antibiotic intake in test and control groups, when assessing probiotics.
R7.9. Do adjunctive locally administered antimicrobials improve the clinical outcome of subgingival instrumentation?
| PICOS question addressed by an SR |
|---|
| R7.9: Evidence-based recommendation |
| We suggest not to use locally administered antimicrobials, adjunctively to sub-marginal instrumentation or as monotherapy, in non-surgical peri-implantitis therapy. |
| Supporting literature (Liñares et al., 2023; Renvert et al., 2006, 2008) |
| Quality of evidence Low—two RCTs for chlorhexidine ‘chips’ with low risk of bias and two RCTs for minocycline microspheres |
| Grade of recommendation Grade B—↓ |
| Strength of consensus: Consensus (1.9% of the group abstained due to potential CoI) |
Background
Intervention
Locally delivered antimicrobials may be used as an adjunct to subgingival instrumentation in patients with periodontitis, particularly in non-responding and recurrent sites (Herrera et al., 2020). The same principle may apply for non-surgical therapy of peri-implantitis.
Available evidence
Number and design of included studies
Two placebo-controlled RCTs with 6-month follow-up assessed the adjunctive effect of locally applied chlorhexidine ‘chips’ to the non-surgical sub-marginal instrumentation (Machtei et al., 2012, 2021). These studies used an intense regime with multiple, repeated applications during the observation period. In addition, although they were not included in the SR, two RCTs evaluating locally applied minocycline microspheres were considered in the discussions (Renvert et al., 2006, 2008).
Risk of bias
Two RCTs with low risk of bias.
Effect sizes and their clinical relevance
Results of two studies evaluating multiple applications of a biodegradable matrix containing chlorhexidine were pooled for meta-analyses, showing a statistically significant improved PD reduction (WMD = 0.2 mm; 95% CI [0.0; 0.5]; p = .031; I2 = 0.0%; p = .570). No or very limited information was available for BOP or disease resolution.
Consistency
Not feasible due to the limited information available.
Balance of benefit and harm
No increase in adverse effects was observed. PROMs were not reported. Harm versus benefit considerations on the use of locally delivered antibiotics need to be considered.
Overall certainty of the evidence
Low.
7.1 From evidence to recommendation—additional considerations
Acceptability
No specific information is available; however, local antimicrobials are normally easy to use by the practitioners. Conversely, some patients/clinicians may not be willing to use antimicrobial products.
Feasibility
Some of the evaluated products may not be commercially available in some countries. For chlorhexidine ‘chips’, only one brand/manufacturer is available (PerioChip®, Dexcel Pharma, Or Akiva, Israel). For minocycline microspheres, the brand tested in the considered studies was Arestin® (OraPharma, Bridgewater, NJ, USA).
Ethical considerations
No applicable.
Economic considerations
Economic costs and cost-effectiveness should be considered before their use. Economic cost may be relatively high (for chlorhexidine ‘chips’, one chip may cost around €30, while for minocycline microspheres, one cartridge costs around $100, especially if multiple applications are needed). Some additional information is presented in Section 1.
Legal considerations
Some of the evaluated products have not been registered for use in some countries, and/or may not have been approved for this specific indication.
R7.10. Do adjunctive systemically administered antibiotics improve the clinical outcomes of non-surgical treatment?
| PICOS question addressed by an SR |
|---|
| R7.10: Expert consensus-based recommendation |
| Due to concerns about patients' health and the impact of systemic antibiotic use on public health, its routine use as an adjunct to non-surgical treatment in patients with peri-implantitis is not recommended. |
| Supporting literature (Liñares et al., 2023) |
| Quality of evidence Low—two RCTs, one with some concerns, and another with low risk of bias. |
| Grade of recommendation Grade A—↓↓ |
| Strength of consensus—Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
The adjunctive use of systemic antimicrobials has been extensively evaluated in the treatment of periodontitis (Teughels et al., 2020). The same principles may apply for its adjunctive use in the non-surgical step of the treatment of peri-implantitis.
The expert group evaluated, first, the adjunctive benefit of systemic antibiotics to sub-marginal instrumentation alone. The effect was both statistically significant and clinically relevant. In the included studies (Blanco et al., 2022; Shibli et al., 2019), the effect tended to be more pronounced at cases with initially deeper lesions and to improve over time up to 1 year. At least in one study (Blanco et al., 2022), the benefit included improvements in MBLs. The size of the benefit may allow achievement of the stipulated treatment end points in a significant number of cases and hence avoid surgical intervention. The clinical recommendation that antibiotics cannot be recommended as a routine is, therefore, based on the general principles of antibiotic stewardship and the public health objective of limiting unnecessary use of antibiotics in dentistry. Rationale for limitation is twofold: the public health considerations related to spread of antibiotic resistance and the potential individual harms related to dysbiosis of the individual patient microbiome. The panel felt that clinicians should avoid use of systemic antibiotics for the management of peri-implantitis and limit it to cases at the end of the severity spectrum (e.g., deep pockets ≥7 mm, extensive suppuration) and/or with multiple and/or strategically affected implants that could respond well and be retained over time (the suggested protocol in these cases would be metronidazole 500 mg/8 h/7 days). However, the use of systemic antimicrobials should be avoided in palliative care of lost implants.
Available evidence
Two studies were included in the SR (Liñares et al., 2023), both showing statistically significant benefits in PD reduction at 6 months and up to 12 months after the prescription of systemic antimicrobials. These results were more pronounced when the deepest site of each implant was considered for the analysis. A significant effect for the use of systemic antimicrobials in radiographic bone gain (≈1.2 mm) was observed on rough-surface implants (Blanco et al., 2022). However, no changes in MBLs were reported on machined implants (Shibli et al., 2019).
In both studies, PD reductions improved from 3 to 12 months, suggesting that, if at the re-evaluation (6–12 weeks) the recommended endpoints are not achieved at implant level (i.e., residual PD ≤ 5 mm with no BOP at more than one site point and no suppuration), but a clear improvement in PD reduction is detected, it may be adequate to wait longer before a decision to perform additional treatment is made.
Number and design of included studies
RCTs (n = 2) with a double-blind, placebo-controlled, parallel design with follow-up up to 12 months (Blanco et al., 2022; Shibli et al., 2019). One evaluated amoxicillin plus metronidazole (n = 40 patients/40 implants) (Shibli et al., 2019), and the other, metronidazole alone (n = 32 patients/62 implants) (Blanco et al., 2022).
Risk of bias
Risk of bias was low for one study, while the other study presented some concerns.
Effect sizes and their clinical relevance
Systemic antimicrobials showed a greater PD reduction when compared with mechanical debridement alone at 6 months and up to the 12 months follow-up (≈1.5 mm). These results were more pronounced when the deepest site of each implant was considered for the analysis.
Consistency
Not applicable.
Balance of benefit and harm
One study assessed the potential side effects of systemic antibiotics, with six subjects (38%) in the test group (systemic metronidazole) and five (31%) in the control group (placebo) reporting either gastrointestinal disorders, headaches or metallic taste, without significant differences among groups. Global concerns regarding the overuse of antibiotics and the development of antibiotic resistance must be considered. Benefit versus harm analysis includes considerations on the overall use of antibiotics for the individual patient and public health. Systemic antibiotic regimens have shown long-lasting impacts on the faecal microbiome, including an increase in genes associated with antimicrobial resistance.
Overall certainty of the evidence
Limited evidence is available.
7.1 From evidence to recommendation—additional considerations
Acceptability
Due to concerns for patient's health and the impact of systemic antibiotic use on public health, its routine use as an adjunct to sub-marginal peri-implant instrumentation in patients with peri-implantitis is not recommended.
Feasibility
Adjunct systemic antimicrobials to non-surgical peri-implant therapy are a feasible procedure since these antimicrobials may be prescribed in most countries. Moreover, the procedure does not demand high clinical skills.
Ethical considerations
Important concerns are related to patient's health and the impact of systemic antibiotic use to public health.
Economic considerations
Although economic considerations have not been analysed in the included studies, some indications can be given. The cost of systemic antimicrobials is low, particularly in comparison to other potential adjuncts (e.g., local antimicrobials or probiotics). Although there is not enough evidence to provide any strong recommendation, the prescription of systemic antimicrobials in specific cases may reduce the need for additional treatment, including surgical procedures, reducing added costs and morbidity.
Legal considerations
There are no specific legal considerations.
R7.11. What is the efficacy of adjunctive probiotics in the non-surgical step of peri-implantitis treatment?
| PICOS question addressed by an SR |
|---|
| R7.11: Evidence-based recommendation |
| We suggest not to use probiotics as an adjunct to sub-marginal instrumentation, in non-surgical peri-implantitis therapy. |
| Supporting literature (Liñares et al., 2023) |
| Quality of evidence Very low—one RCT with some concerns in risk of bias |
| Grade of recommendation Grade B—↓ |
| Strength of consensus: Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Probiotics are defined as ‘live microorganisms which, when administered in adequate amounts, confer a health benefit on the host’ (Hill et al., 2014). Probiotics have been proposed to modulate oral microbiota and host immune response (Gatej et al., 2018; Invernici et al., 2020). While it has been suggested that probiotics may not be used as an adjunct to subgingival instrumentation in the treatment of stages I–III periodontitis (Sanz et al., 2020), regarding peri-implantitis, available studies reveal contradictory results.
Available evidence
Number and design of included studies
One placebo-controlled RCT assessed the adjunctive effect of probiotics to non-surgical sub-marginal instrumentation (Laleman et al., 2020), with a preparation containing L. reuteri, to be applied both locally and systemically.
Risk of bias
Some concerns.
Effect sizes and their clinical relevance
No adjunctive effect of the use of probiotics was observed on PD or BOP.
Consistency
Not applicable.
Balance of benefit and harm
No proper evaluation of PROMs was carried out, although the extrapolation from the periodontal field suggests that this formulation is safe, and patients do not frequently report adverse effects.
Overall certainty of the evidence
Very low.
7.1 From evidence to recommendation—additional considerations
Acceptability
No specific information is available. However, probiotics are normally easy to use by the practitioners. Conversely, some patients/clinicians may not be willing to use these products.
Feasibility
Adjunctive probiotics to non-surgical peri-implant therapy are a feasible approach since these products can be prescribed in many countries. Moreover, the procedure does not demand high clinical skills.
Ethical considerations
Not applicable.
Economic considerations
There is an additional cost associated with the use of probiotics that is borne by the patient.
Legal considerations
There are no specific legal considerations.
8 RECOMMENDATIONS FOR THE SURGICAL MANAGEMENT OF PERI-IMPLANTITIS
8.1 Introduction—general recommendations in the surgical step of peri-implantitis treatment
The purpose of a surgical approach in the management of peri-implantitis is to provide access to the implant to facilitate surface decontamination. The goal is to achieve the resolution of the inflammatory lesion. Target sites for surgical treatment are those presenting with persisting signs of pathology after non-surgical therapy, that is, deep pockets together with BOP/SOP.
A standard surgical procedure includes, in addition to flap elevation and removal of inflamed tissue, cleaning/ decontamination of the implant surface using, for example, small pieces of gauze soaked in saline and removal of mineralized deposits with curettes.
Additional procedures in the surgical treatment of peri-implantitis may include: (i) the management of peri-implant osseous defects using reconstructive approaches, (ii) additional methods for implant surface decontamination and (iii) the adjunctive use of local/systemic antibiotics.
R8.1. What is the importance of adequate self-performed oral hygiene in the context of surgical treatment of peri-implantitis?
| Additional question addressed by the WG |
|---|
| R8.1: Expert consensus-based recommendation |
| We recommend not to perform surgical treatment of peri-implantitis in patients not achieving and maintaining adequate levels of self-performed OH. |
| Supporting literature Expert opinion |
| Quality of evidence Not applicable |
| Grade of recommendation Grade A—↓↓ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Studies have shown the detrimental effects of surgical treatment of periodontitis in patients with insufficient levels of self-performed OH (Sanz et al., 2020). Since bacterial biofilms are considered the primary etiological factor for both periodontitis and peri-implantitis, the importance of adequate self-performed levels of OH needs to be emphasized also in the context of surgical treatment of peri-implantitis. Similar to the periodontal scenario, studies on surgical treatment of peri-implantitis have also indicated unfavourable outcomes in patients not achieving and maintaining adequate levels of self-performed OH (De Waal et al., 2015; Koldsland et al., 2018).
R8.2. What is the level of professional expertise required for surgical treatment of peri-implantitis?
| Additional question addressed by the WG |
|---|
| R8.2: Expert consensus-based recommendation |
| We recommend that dental teams offering implant therapy also possess the professional expertise to manage peri-implantitis. Since surgical treatment of peri-implantitis is complex, we recommend that it is provided by dentists with specific training or by specialists. |
| Supporting literature Expert opinion |
| Quality of evidence Not applicable |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Recognition of peri-implantitis as a disease entity is relatively recent and the armamentarium of surgical approaches is constantly evolving. The dental team must be continuously updated on the most effective treatment modalities. Treatment of peri-implantitis lies within the scope of the speciality of periodontology.
R8.3. What are the end points of successful surgical therapy of peri-implantitis?
| Additional question addressed by the WG |
|---|
| R8.3: Expert consensus-based recommendations |
|
| Supporting literature Expert opinion |
| Quality of evidence Not applicable |
| Grade of recommendation Grade A—↑↑ (1,2); Grade B—↑ (3) |
Strength of consensus (1) Strong consensus (0% of the group abstained due to potential CoI) (2) Strong consensus (0% of the group abstained due to potential CoI) (3) Strong consensus (0% of the group abstained due to potential CoI) |
Background
Studies (e.g., Carcuac et al., 2017, 2020; Karlsson et al., 2019) demonstrate that progression of peri-implantitis occurs in the presence of clinical signs of inflammation, and is manifested through reduction of peri-implant bone levels. In contrast, shallow peri-implant PDs and absence of BOP/SOP have been associated with stable peri-implant support in longitudinal studies.
R8.4. What considerations should be made about the implant-supported prosthesis when performing surgical treatment of peri-implantitis?
| Additional question addressed by the WG |
|---|
| R8.4: Expert consensus-based recommendations |
|
| Supporting literature Not applicable |
| Quality of evidence Not applicable |
| Grade of recommendation Grade A—↑↑ (1); Grade B—↑ (2) |
Strength of consensus (1) Unanimous consensus (0% of the group abstained due to potential CoI) (2) Consensus (0% of the group abstained due to potential CoI) |
Background
Adequate levels of self-performed OH are a prerequisite for successful outcomes of surgical treatment for peri-implantitis. Studies have shown that inadequate access for OH around implants is associated with higher risk for peri-implantitis (Serino & Strom, 2009; Tormena et al., 2020); therefore, adjustment of the implant-supported prosthesis with the aim to facilitate access for OH is an important measure prior to surgical treatment of peri-implantitis.
8.2 Indications of the surgical treatment of peri-implantitis and efficacy of access/resective approaches
R8.5. When is surgical treatment of peri-implantitis indicated?
| PICOS question addressed by an SR |
|---|
| R8.5: Expert consensus-based recommendation |
| In peri-implantitis patients in whom end points of non-surgical therapy (PD ≤ 5 mm and ≤ 1 point of BOP) have not been achieved, we recommend performing surgical therapy. |
| Supporting literature (Donos et al., 2023; Karlsson et al., 2023) |
| Quality of evidence Moderate |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Surgical therapy of peri-implantitis may consist of different approaches, including simple access flap, pocket elimination or reconstructive procedures. All modalities incorporate flap elevation, removal of inflamed tissues and implant surface debridement/decontamination.
Available evidence
Number and design of included studies
Data from 13 prospectively collected studies (649 patients) with a follow-up ranging from 1 to 5 years addressed access flap and resective surgery. Seven RCTs assessed the efficacy of reconstructive surgery (194 patients) compared with access flap surgery. The respective datasets were evaluated in two SRs (Donos et al., 2023; Karlsson et al., 2023). All studies reported on reduction of PD and BOP. Clinically relevant end points (e.g., PD < 6 mm), PROMs, health economic parameters and adverse events were not consistently reported.
Risk of bias
The 13 studies on access flap and resective surgery were generally found to be at low RoB, while multiple studies evaluating reconstructive measures were judged to show high RoB.
Effect sizes and their clinical relevance
For access flap and resective surgery, the estimated reduction of PD was 2.2 mm (95% CI [1.8; 2.7]). Reconstructive surgery resulted in similar PD reduction (additional effect relative to access flap alone: −0.39 95% CI [−1.16; 0.24]) at 12 months. For access flap and resective surgery, reduction of standardized mean %BOP was estimated at 27.0 (95% CI [19.8; 34.2]) and an overall bone gain of 0.2 mm (95% CI [0.0; 0.5]) was noted. Reconstructive surgery resulted in an additional bone gain of 0.75 mm (95% CI [−1.39; −0.11]) over access flap alone at 12 months (CI is presented with negative values, since in the original analyses positive values indicated more gain for access flap and negative for reconstructive procedures). Over 5-year observation periods, disease recurrence/progression was observed at 32%–44% of treated implants. Corresponding implant loss was low in the short term but after 5 years ranged from 14% to 21%.
Consistency
Results were consistent across studies for changes of PD and MBL. Reduction of BOP was heterogenous across studies. Data were generated in various clinical settings, including university centres and private clinics.
Balance of benefit and harm
In general, considerable improvements in clinical and radiographic parameters were noted. However, disease recurrence and implant loss were not uncommon events after 5 years. Data on PROMs and adverse events were rarely reported.
Overall certainty of the evidence
The certainty of evidence is graded as moderate based on the lack of direct comparisons between surgical and non-surgical therapy of peri-implantitis.
8.1 From evidence to recommendation—additional considerations
Acceptability
PROMs were rarely reported. Limited data suggest a high degree of patient satisfaction at 1 year following surgical therapy. Adverse events reported were mostly related to the use of systemic antibiotics.
Feasibility
Related procedures are clinically demanding.
Ethical considerations
Some decontamination procedures and grafting materials evaluated in the studies included have not been tested for safety.
Economic considerations
Health economic parameters were not evaluated in the identified studies. In general, surgical therapy of peri-implantitis is a costly procedure. Some decontamination procedures and grafting materials may generate additional costs in the absence of documented benefit.
Legal considerations
Some decontamination procedures and grafting materials evaluated in the studies included have not been tested for safety and are considered off-label.
R8.6. What is the efficacy of surgical treatment of peri-implantitis using access flap or resective procedures (resection of hard/soft peri-implant tissues aiming at reducing or eliminating pockets)?
| PICOS question addressed by an SR |
|---|
| R8.6: Evidence-based recommendation |
| In peri-implantitis patients in whom endpoints of non-surgical therapy (PD ≤5 mm and ≤ 1 point of BOP) have not been achieved, we recommend performing access flap or resective surgery as both modalities are effective. |
| Supporting literature (Karlsson et al., 2023) |
| Quality of evidence Moderate |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Surgical therapy of peri-implantitis includes flap elevation, removal of inflamed tissues and implant surface debridement/decontamination. In access flap procedures, soft tissue flaps are simply repositioned, while resective approaches aim at apically displacing flaps through soft tissue and/or hard tissue recontouring.
Available evidence
Number and design of included studies
Thirteen studies (n = 649 patients), with a follow-up range from 1 to 5 years (only two studies with a 5-year follow-up), were included (Karlsson et al., 2023). One study was an RCT comparing surgical therapy to non-surgical intervention. All datasets were prospective and a total of 10 originated from control arms within RCTs, while the remaining two were case series. All studies reported on reduction of PD and BOP. Clinically relevant end points (e.g., PD < 6 mm), PROMs, health economic parameters and adverse events were not consistently reported.
Risk of bias
The 13 studies were generally found to be at low RoB. In the two evaluations covering longer follow-ups (≥5 years; Carcuac et al., 2020; Heitz-Mayfield et al., 2018), loss to follow-up exceeded 20% and the overall rating was downgraded to ‘fair’.
Effect sizes and their clinical relevance
Based on 18 studies (n = 661 implants), the estimated reduction of PD was 2.2 mm (95% CI [1.8; 2.7]). Based on 8 studies (n = 477), reduction of standardized mean BOP% was estimated at 27.0 (95% CI [19.8; 34.2]). Based on 12 studies (n = 637), a standardized mean bone gain of 0.2 mm (95% CI [0.0; 0.5]) was estimated. Over 5-year observation periods, disease recurrence/progression was observed at 32%–44% of treated implants. Corresponding implant loss was low in the short term but after 5 years ranged from 14% to 21%.
Consistency
Results were consistent across studies in regard to changes in PD and MBL. Reduction of BOP was heterogenous across studies. Data were generated in various clinical settings, including university centres and private clinics.
Balance of benefit and harm
In general, considerable improvements in clinical and radiographic parameters were noted. However, disease recurrence and implant loss were not uncommon events after 5 years. Data on PROMs (two studies) and adverse events (three studies) were rarely reported.
Overall certainty of the evidence
The certainty of evidence is graded as moderate based on the lack of direct comparisons between surgical and non-surgical therapy of peri-implantitis.
8.1 From evidence to recommendation—additional considerations
Acceptability
PROMs were reported in two studies, only. Limited data suggest a high degree of patient satisfaction at 1 year after surgical therapy. Adverse events reported in three studies were mostly related to the use of systemic antibiotics.
Feasibility
Related procedures are clinically demanding.
Ethical considerations
Some decontamination procedures evaluated in the studies included have not been tested for safety.
Economic considerations
Health economic parameters were not evaluated in the identified studies. In general, surgical therapy of peri-implantitis is a costly procedure. Some decontamination procedures may generate additional costs in the absence of documented benefit.
Legal considerations
Some decontamination procedures evaluated in the studies included have not been tested for safety and are considered off-label.
8.3 Management of peri-implant osseous defects using reconstructive approaches
R8.7. Do reconstructive procedures used in the management of osseous defects (e.g., bone substitute materials) as part of surgical treatment of peri-implantitis result in superior outcomes when compared with access flap alone?
| PICOS question addressed by an SR |
|---|
| R8.7: Evidence-based recommendation |
| In the surgical management of osseous defects in peri-implantitis patients, access flap with or without reconstructive procedures may be considered; no evidence demonstrating superiority of any specific surgical technique was identified. |
| Supporting literature (Donos et al., 2023) |
| Quality of evidence Low |
| Grade of recommendation Grade O ↔ (need for further research) |
| Strength of consensus Consensus (19.0% of the group abstained due to potential CoI) |
Background
Intervention
Reconstructive procedures aim to regenerate the bony defect, achieve re-osseointegration and limit peri-implant soft tissue recession (Jepsen et al., 2019). Reconstructive therapy of peri-implant bone defects includes the use of bone grafts, bone replacement grafts, barrier membranes, bioactive agents (growth factors, autologous platelet concentrates and amelogenin) or combinations thereof.
Available evidence
Number and design of included studies
Seven RCTs assessed the efficacy of reconstructive surgery (total of 200 implants in 194 patients) compared with access flap surgery (total of 188 implants in 184 patients) (Donos et al., 2023). Different types of reconstructive surgeries were documented, including the use of titanium granules, amelogenin, deproteinized bovine bone mineral (DBBM or DBBM graft with 10% collagen) alone or combined with a native bilayer collagen membrane, or a beta-tricalcium phosphate graft formulated with prolonged release of local doxycycline.
Risk of bias
Based on RoB 2, there was concern for four studies in one domain (predominantly due to bias in measurement of the outcome), while three studies were considered at high risk of bias, mainly due to the combination of missing outcomes and bias in selection of the reported results.
Effect sizes and their clinical relevance
Meta-analysis (4 studies; 262 patients and 272 implants) showed an estimated MD in PD changes between access flap surgery and reconstructive surgery of −0.39 (95% CI [−1.16; 0.24]; p = .325, I2 = 66.4%) at 12 months. No evidence of small-study effects was detected. Among the five studies that reported on BOP changes at 12 months, one study showed a statistically significant improvement for reconstructive therapy as compared with access flap surgery. No differences were indicated in relation to the change in SOP. At 12 months, implant survival was similar between the two treatment procedures, ranging from 85.7% to 100% for access flap and from 95% to 100% for reconstructive therapy. Meta-analysis for changes in radiographic mean bone levels (4 studies; 262 patients and 272 implants) showed a statistically significant benefit of reconstructive compared with access flap surgery of −0.75 mm (95% CI [−1.39; −0.11]; p = .022; I2 = 83.4%). The CI is presented with negative values, since in the original analyses positive values indicated more gain for access flap and negative for reconstructive procedures. Irrespective of the surgical approach and biomaterial employed, resolution of peri-implantitis is unpredictable and a significant difference between the two treatment approaches was not consistently shown.
Consistency
Overall, inconsistency in the direction of effect was noticed for the included studies, as only one showed a significant improvement in PD change and one in BOP change, when reconstructive procedures were employed.
Balance of benefit and harm
A similar number of adverse events and complications was associated with reconstructive and access flap surgeries. In the long-term, a number of implants are expected to develop disease recurrence, which may require additional surgical procedures or could lead to implant loss.
Overall certainty of the evidence
The certainty of evidence is low based on the quality of the studies (RoB) and inconsistency of outcomes.
8.1 From evidence to recommendation—additional considerations
Acceptability
Only two studies considered PROMs, with no significant differences in terms of pain scores, number of tablets taken and satisfaction.
Feasibility
Related procedures are clinically demanding.
Ethical considerations
Some decontamination procedures applied in the studies have not been tested for safety.
Economic considerations
No study addressed health economic outcomes on this topic (Donos et al., 2023). Reconstructive surgery represents an additional financial burden for the patient, which should be discussed with the patient.
Legal considerations
Not applicable.
R8.8. What are the specific prerequisites (e.g., dimensions of intra-bony defects) for a reconstructive approach?
| PICOS question addressed by an SR |
|---|
| R8.8: Evidence-based recommendations |
| We suggest that reconstructive procedures preferably be applied at intra-osseous defects with a depth of ≥3 mm. |
| Supporting literature (Donos et al., 2023) |
| Quality of evidence Low |
| Grade of recommendation Grade B—↑ |
| Strength of consensus Consensus (13.3% of the group abstained due to potential CoI) |
Background
Intervention
See previous section.
Available evidence
Number and design of included studies
None of the identified studies in the SR was designed to investigate the site prerequisites for a reconstructive surgery (Donos et al., 2023). Nevertheless, the five RCTs of the network meta-analysis included ≥3 mm, angular peri-implant bone defects, which showed significant improvements in clinical and radiographic parameters from baseline to 12 months post-reconstructive therapy. Deeper defects are more likely to result in radiographic defect fill and 3- and 4-wall defects result in higher reduction in PD and BOP.
Risk of bias
Based on RoB 2, the risk of bias varied from low to high in the relevant studies.
Effect sizes and their clinical relevance
Not applicable.
Consistency
Despite the three identified studies showed consistency on the impact of defect morphology on the treatment outcome, none of these studies was designed to answer this question.
Balance of benefit and harm
Not applicable.
Overall certainty of the evidence
Low.
8.1 From evidence to recommendation—additional considerations
Acceptability
Not applicable.
Feasibility
Not applicable.
Ethical considerations
Not applicable.
Economic considerations
Not applicable.
Legal considerations
Not applicable.
R8.9. What are the preferred materials to be used in reconstructive procedures?
| PICOS question addressed by an SR |
|---|
| R8.9: Evidence-based recommendation |
| Bone grafts with or without barrier membranes may be considered in reconstructive procedures. |
| Supporting literature (Donos et al., 2023) |
| Quality of evidence Low |
| Grade of recommendation Grade O ↔ |
| Strength of consensus Consensus (19.0% of the group abstained due to potential CoI) |
Background
Intervention
A variety of bone substitutes, barriers and bioactive agents have been proposed for reconstructive procedures.
Available evidence
Number and design of included studies
Five RCTs and six prospective case series assessed the efficacy of reconstructive peri-implantitis therapy (Donos et al., 2023).
Risk of bias
Based on RoB 2, two of the five included RCTs were at high risk of bias, some concerns were raised for two studies and one was at low risk of bias. Based on ROBINSI, one CCT was at serious risk of bias, three prospective cohort studies were considered at serious risk of bias and two prospective cohort studies were at critical risk of bias (Donos et al., 2023).
Effect sizes and their clinical relevance
Regardless of the biomaterials applied, reconstructive therapy led to a mean PD reduction ranging from 2.0 to 4.5 mm and to a mean reduction in BOP ranging from 44.8% to 86% at 12 months after therapy. Studies reporting on SOP showed a significant reduction at 12 months and 5 years post-surgery. Based on one study (45 patients and 75 implants), included in the network meta-analysis (4 studies; 160 patients and 190 implants) (Donos et al., 2023), an improved PD reduction was shown when a xenogeneic rather than an autologous graft was applied in combination with a collagen membrane. Implant survival at 12 months ranged from 92% to 100%, but when considering composite outcomes for peri-implantitis resolution the range reported by the included studies was considerably wider (0%–91% at 12 months).
Consistency
All reconstructive procedures improved clinical and radiographic outcomes as compared with baseline regardless of the biomaterials employed.
Balance of benefit and harm
None of the different reconstructive approaches was associated with early side effects or adverse events beyond what would be expected for this type of surgical procedure. Notably, the use of a combination of membrane and bone graft was associated with an increased risk for flap dehiscence in two studies.
Overall certainty of the evidence
Low.
8.1 From evidence to recommendation—additional considerations
Acceptability
Based on one study, the use of a graft alone was associated with significantly less pain at 2 weeks as compared with the combined use of a graft and collagen membrane.
Feasibility
Not applicable.
Ethical considerations
Not applicable.
Economic considerations
No study addressed health economic outcomes on this topic. However, it should be noted that reconstructive surgery represents an additional financial burden for the patient.
Legal considerations
Not applicable.
R8.10. What is the preferable mode of healing (submerged versus transmucosal) to be used in reconstructive procedures?
| Additional question addressed by the WG |
|---|
| R8.10: Expert consensus-based recommendation |
| We do not know whether a submerged or transmucosal healing protocol would influence the outcomes of reconstructive procedures. |
| Supporting literature (Donos et al., 2023) |
| Quality of evidence Very low |
| Grade of recommendation Grade O ↔ |
| Strength of consensus Strong consensus (1.9% of the group abstained due to potential CoI) |
Background
Intervention
In reconstructive procedures, submerged and transmucosal healing have been documented.
Available evidence
Number and design of included studies
No focused question in the current SR (Donos et al., 2023) was formulated to address this topic. Nevertheless, none of the included studies compared submerged with unsubmerged healing protocol.
Risk of bias
Not applicable.
Effect sizes and their clinical relevance
Not applicable.
Consistency
Not applicable.
Balance of benefit and harm
The main advantage of submerged healing would be to achieve primary wound closure and to promote an aseptic healing environment, which are crucial factors for stabilizing the blood clot, improving graft stability and maximizing the regenerative potential of the intra-bony compartment. On the other hand, unsubmerged healing eliminates the need for prosthesis removal, reduces treatment time, costs and possibly the overall complexity of treatment.
Overall certainty of the evidence
Very low.
8.1 From evidence to recommendation—additional considerations
Acceptability
It should be noted that a submerged healing protocol may result in the need of temporary tooth replacement.
Feasibility
Not applicable.
Ethical considerations
Not applicable.
Economic considerations
No study addressed health economic outcomes on this topic. It should be noted that unsubmerged healing eliminates the need of prosthesis removal, thus reducing treatment time and possibly costs.
Legal considerations
Not applicable.
8.4 Additional methods for implant surface decontamination
R8.11. Do photo−/mechanical and physical implant surface decontamination procedures improve outcomes of surgical treatment?
| PICOS question addressed by an SR |
|---|
| R8.11: Evidence-based recommendations (1, 2) and statement (3) |
1. We suggest not to use air-polishing or Er:YAG laser for implant surface decontamination during surgical treatment of peri-implantitis. 2. Titanium brushes may be considered as an alternative/adjunct to standard decontamination. 3. There is insufficient evidence to make any recommendation regarding the use of implantoplasty. |
| Supporting literature (Ramanauskaite et al., 2023) |
| Quality of evidence Low |
| Grade of recommendation Grade B—↓ (1); Grade O—↔ (2); Statement (3) |
Strength of consensus (1) Consensus (7.8% of the group abstained due to potential CoI) (2) Consensus (0% of the group abstained due to potential CoI) (3) Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
As substantial evidence supports the bacterial aetiology of peri-implantitis, removal of the biofilm from contaminated implant surfaces is a crucial treatment step in obtaining disease resolution (Berglundh et al., 2018; Lang et al., 2011; Schwarz et al., 2018).
Available evidence
Number and design of included studies
In total, five RCTs (4 two-armed and 1 three-armed; 183 patients/242 implants) with follow-up periods ranging from 6 to 24 months were included (Ramanauskaite et al., 2023). One RCT investigated the alternative use of air polishing with erythritol powder in conjunction with non-reconstructive surgical peri-implantitis therapy compared with standard instrumentation (Hentenaar et al., 2022); another RCT, with three arms, assessed the efficacy of titanium brushes (test 1) and air polishing with glycine powder (test 2) as alternative decontamination measures for implant surface decontamination compared with standard instrumentation in conjunction with non-reconstructive surgical peri-implantitis therapy (control) (Toma et al., 2019); two RCTs investigated the efficacy of Er:YAG laser compared with either standard instrumentation (Schwarz et al., 2012) or debridement with piezoelectric scaler and stainless-steal scaler (Wang et al., 2021) during reconstructive therapy and as an adjunct to implantoplasty; and one RCT evaluated the added value of a titanium brush, on top of ultrasonic decontamination and hydrogen peroxide in regenerative surgery (de Tapia, Valles, et al., 2019).
Risk of bias
Based on RoB 2, two RCTs were judged to have an overall low risk of bias, two RCTs had an overall high risk of bias and one RCT had an unclear risk of bias.
Effect sizes and their clinical relevance
Based on two RCTs with 6- to 12-month follow-ups, the adjunctive/alternative use of an air-abrasive device with glycine or erythritol powders did not result in improved BOP reductions compared with the control during surgical therapy of peri-implantitis (Hentenaar et al., 2022; Toma et al., 2019). One RCT indicated a significantly higher PD reduction following the alternative use of air polishing with glycine powder and titanium brushes compared with the standard decontamination (Toma et al., 2019). Based on one RCT, after 6 months, alternative use of titanium brush resulted in significantly higher BOP reduction compared with either air polishing or the standard instrumentation (i.e., curettes to remove hard deposits plus gauze soaked in saline/saline irrigation) (Toma et al., 2019).
During reconstructive therapy, a titanium brush resulted in significantly greater reduction of the deepest PD values compared with the control group (i.e., mechanical and chemical implant surface decontamination) (de Tapia, Valles, et al., 2019). An Er:YAG laser resulted in significantly higher PD reductions after 6 months in one RCT, but was not associated with improved BOP reductions over respective control measures (i.e., implantoplasty and standard instrumentation or debridement with piezoelectric scaler and stainless-steal scaler) as shown in two RCTs (Schwarz et al., 2012; Wang et al., 2021).
Consistency
Two RCTs reported on no benefit of air polishing either with erythritol or glycine powder on the reduction of BOP values (Hentenaar et al., 2022; Toma et al., 2019). A beneficial effect of the use of a titanium brush was reported in two RCTs in terms of BOP (Toma et al., 2019) and PD reductions (de Tapia, Valles, et al., 2019). Two RCTs consistently reported on no benefits of Er:YAG laser on changing BOP values after 6 and 12 months in conjunction with reconstructive therapy and as an adjunct to implantoplasty (Schwarz et al., 2012; Wang et al., 2021). Inconsistencies were found between the studies with respect to the PD changes following Er:YAG laser application. In fact, significantly higher PD reduction following the use of Er:YAG laser was reported after 6 months in one RCT (Wang et al., 2021), whereas after 24 months another RCT indicated no benefits of ER:YAG laser in reducing PD values (Schwarz et al., 2012).
Balance of benefit and harm
Harms have not been explicitly reported and evaluated in two RCTs. A slight pigmentation of peri-implant soft tissues was observed in one out of 30 patients treated with implantoplasty. One RCT reported on adverse events observed in one out of 16 patients associated with persistence of suppuration and swelling following air polishing. Another RCT reported on membrane exposure during the healing, following reconstructive therapy of peri-implantitis, however, without providing the number of implants/patients experiencing this complication.
Overall certainty of the evidence
The evidence was graded as low due to a low number of studies with a considerable heterogeneity.
8.1 From evidence to recommendation—additional considerations
Acceptability
None of the studies investigated PROMs.
Feasibility
Certain decontamination protocols may be considered as technically demanding.
Ethical considerations
Certain decontamination protocols have not been tested for safety.
Economic considerations
Economic aspects could not be assessed due to the lack of reporting.
Legal considerations
Not applicable.
R8.12. Do chemical implant surface decontamination procedures improve outcomes of surgical treatment?
| PICOS question addressed by an SR |
|---|
| R8.12: Evidence-based recommendation |
| We suggest not to use chlorhexidine or photodynamic therapy for implant surface decontamination during surgical therapy of peri-implantitis. |
| Supporting literature (Wilensky et al., 2023) |
| Quality of evidence Very low (due to uncertainty of evidence) |
| Grade of recommendation Grade B—↓ |
| Strength of consensus Consensus (1.7% of the group abstained due to potential CoI) |
Background
Intervention
Adjunctive antimicrobial chemical therapy is an approach used to improve the standard implant surface decontamination methods. CHX has antiseptic properties that kill bacteria. PDT functions by light activation of a photosensitizing dye to generate reactive oxygen species that destroy those bacteria.
Available evidence
Number and design of included studies
Evidence was available for PDT from two RCTs (n = 43) and for CHX from two RCTS (n = 130) (Wilensky et al., 2023). Both with a follow-up of ≥6 and up to 12 months. Only RCTs reporting mean PD changes and BOP changes were included.
Risk of bias
For PDT, the risk of bias was low to unclear, and for CHX it was unclear to high risk of bias.
Effect sizes and their clinical relevance
No benefits were observed with the adjunctive application of CHX; no improvement was observed for PDT in terms of PD reduction, and only minor reductions in BOP (MD = 7.4).
Consistency
For PDT, heterogeneity was low, and for CHX it was medium to high.
Balance of benefit and harm
One study did not report on adverse events, while three studies reported no to minor adverse effects. One study reported gastrointestinal problems in five patients who were taking systemic antibiotics. One study reported no adverse effects, and another study reported two patients with one complication.
Overall certainty of the evidence
The GRADE analysis showed a very low certainty of evidence for both adjunctive treatments in all the tested parameters.
8.1 From evidence to recommendation—additional considerations
Acceptability
None of the studies reported patient-reported outcomes and there is no evidence supporting one approach over the other, including the standard therapy.
Feasibility
While CHX solution is affordable and easily available, PDT results in additional costs without any documented clinical benefit.
Ethical considerations
The lack of efficacy together with possible side effects, such as hypersensitivity, suggests that these treatments are not justified.
Economic considerations
The additional costs associated with adjunctive PDT therapy are not justified.
Legal considerations
PDT is an off-label use during surgery, with no clear benefits.
8.5 Adjunctive use of local/systemic antimicrobials
R8.13. Do adjunctive systemically administered antibiotics improve clinical outcomes of surgical treatment of peri-implantitis?
| PICOS question addressed by an SR |
|---|
| R8.13: Evidence-based recommendation |
| Due to concerns about patients' health and the impact of systemic antibiotic use on public health and inconsistent evidence, its use as adjunct to surgical therapy of peri-implantitis is not recommended. |
| Supporting literature (Teughels et al., 2023) |
| Quality of evidence Low |
| Grade of recommendation Grade A—↓↓ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Tissue destruction at peri-implantitis sites is more pronounced than periodontitis around teeth due to anatomical differences, larger size of the inflammatory lesion and extent of the lesion to the bone crest. Therefore, clinicians are tempted to use systemic antibiotics in addition to the surgical treatment of peri-implantitis.
Available evidence
Number and design of included studies
Two RCTs including 49 patients (25 test and 24 control) and 39 patients (20 test and 19 control) and followed for 1 year showed inconsistent results in terms of PD, BOP and bone level changes: one assessed the systemic application of amoxicillin, 750 mg, twice per day for 10 days, and starting 3 days prior to surgery (Carcuac et al., 2016); the other evaluated the systemic application of azithromycin, 500 mg at the day of surgery, and 250 mg, once per day, during 4 additional days (Hallstrom et al., 2017).
Risk of bias
Some concerns (Carcuac et al., 2016) and high risk (Hallstrom et al., 2017), as evaluated with RoB 2.
Effect sizes and their clinical relevance
Disease resolution (based on <5 mm PDs, no BOP and no additional bone loss >5 mm) was consistent between studies and favoured systemic antibiotics: 56% test versus 29.2% control (Carcuac et al., 2016); 46.7% test versus 25% control group (Hallstrom et al., 2017). Two implant losses occurred in the control group of the first study (Carcuac et al., 2016).
Consistency
See previous section.
Balance of benefit and harm
The potential benefit of the use of systemic antibiotics needs to be balanced with the overall risks, which include adverse events (e.g., allergic reactions) and antibiotic resistance.
Overall certainty of the evidence
Low.
8.1 From evidence to recommendation—additional considerations
Acceptability
Due to concerns about patients' health and the impact of systemic antibiotic use on public health and inconsistent evidence, its use as adjunct to surgical therapy of peri-implantitis is not recommended.
Feasibility
Not applicable.
Ethical considerations
Harms related to the intake of systemic antibiotics must be balanced with potential benefits.
Economic considerations
Not applicable.
Legal considerations
Not applicable.
R8.14. Do adjunctive locally administered antibiotics improve clinical outcomes of surgical treatment of peri-implantitis?
| PICOS question addressed by an SR |
|---|
| R8.14: Evidence-based statement |
| There is insufficient evidence to make any recommendation on the use of local antibiotics as adjuncts in the surgical treatment of peri-implantitis. |
| Supporting literature (Teughels et al., 2023; Wilensky et al., 2023) |
| Quality of evidence Very low |
| Grade of recommendation Statement |
| Strength of consensus Unanimous consensus (2.1% of the group abstained due to potential CoI) |
Background
Intervention
Tissue destruction at peri-implantitis sites is more pronounced than periodontitis around teeth due to anatomical differences, larger size of the inflammatory lesion and extent of the lesion to the bone crest. Therefore, clinicians are tempted to use antibiotics in addition to the surgical treatment of peri-implantitis.
Available evidence
Number and design of included studies
Two RCTs were identified: one assessing local minocycline application at the time of surgery in 50 patients (25 test and 25 control), and repeated at 1, 3 and 6 months, with all patients also receiving systemic amoxicillin thrice per day, 500 mg, for 3 days (Cha et al., 2019); and another evaluating local doxycycline application in 27 patients (14 test and 13 control), formulated in a bone graft, at the time of surgery (Emanuel et al., 2020).
Risk of bias
High risk of bias for both RCTs.
Effect sizes and their clinical relevance
Not applicable.
Consistency
Not applicable.
Balance of benefit and harm
The potential benefit of the use of local antibiotics needs to be balanced with the overall risks, which include adverse events (e.g., allergic reactions) and antibiotic resistance.
Overall certainty of the evidence
Very low.
8.1 From evidence to recommendation—additional considerations
Acceptability
Not applicable.
Feasibility
Related products may not be available in all European countries.
Ethical considerations
Harms related to the intake of local antibiotics must be balanced with potential benefits.
Economic considerations
Additional costs related to the medical product must be considered.
Legal considerations
Not applicable.
AUTHOR CONTRIBUTIONS
David Herrera, Tord Berglundh, Frank Schwarz, Iain Chapple, Søren Jepsen, Anton Sculean, Moritz Kebschull, Panos N. Papapanou, Maurizio S. Tonetti, Mariano Sanz and the methodological consultant (Ina Kopp) substantially contributed to the conception and design of the project, to the interpretation of data, and to the drafting and critical review of the manuscript. The EFP workshop participants (listed previously) significantly contributed by critically reviewing the guideline document and by participating in the workshop discussions. All authors approved the final version of the manuscript.
ACKNOWLEDGEMENTS
The authors express their gratitude to all reviewers involved in the preparation of the SRs and sincerely thank those organizations that participated in the guideline development process: Council of European Dentists, European Dental Hygienists Federation, European Dental Students' Association, European Society for Endodontology, Platform for Better Oral Health in Europe.
CONFLICT OF INTEREST STATEMENT
Individual potential conflict of interest forms were completed by all participants and are available on file at the EFP and extracted in the Supporting Information, available online. Potential conflicts of interest in the previous 36 months, reported by the chairs of the workshop (in alphabetic order) are listed here:
Tord Berglundh (Chair) reports—Grants or contracts from any entity: Dentsply Implants IH, Osteology Foundation (University institution grants). Consulting fees: Dentsply Implants IH (Personal). Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events: Dentsply Implants IH, Spanish Society of Periodontology (SEPA), French Society of Periodontology (Lecture, personal). Support for attending meetings and/or travel: Dentsply Implants IH, Spanish Society of Periodontology (SEPA), French Society of Periodontology, EFP (Travel support when participating as an invited speaker). Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: National Board of Health and Welfare, Sweden (National guidelines in Dentistry) (University institution grant); Swedish Quality Registry for Caries and Periodontal Disease (Personal fee).
Iain Chapple (Chair) reports—Grants or contracts from any entity: Grant from GSK (PhD studentship & other grants); Two grants from DEBRA (Epidermolysis Bullosa Charity), one grant from Unilever (Research Grants); NIHR Biomedical Research Centre Grant (Research infrastructure grant). Royalties or licences: Quintessence (Book Royalties). Consulting fees: J&J, GSK, Unilever, Philips (Consultancy Fees). Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events: J&J, GSK, Unilever, Philips (Sponsored Lecture Fees); Lectures to Swiss, German, Italian, Portuguese, Turkish, Polish, Danish, Austrian, British societies, IAP Colombia (Expenses paid for meetings). Support for attending meetings and/or travel: P&G, J&J (Support to participate in international conference). Patents planned, issued, or pending: 65 patents (Patents on saliva diagnostics). Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: European Workshop Committee of the EFP—co-chair; Invited member of EFP Executive Board (Unpaid); Chair of FDI WG on Oral & Systemic Health (Unpaid); Scientific Advisor to the British Society of Periodontology Executive Board (Unpaid). Other financial or non-financial interests: non-financial (wife runs Oral Health Innovations, which has the licence for PreViser and DEPPA risk assessment software in the UK).
David Herrera (Chair) reports—Grants or contracts from any entity: Dentaid, Kulzer, Lacer, ZIZ Dental, Isdin, Affinity (Research contract via university); University of Bristol, University of Pisa (Research contract via university). Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events: Oral-B, Straumann, Klockner, Dexcel, Dentaid, Colgate, Lacer Spanish Society of Periodontology (SEPA), EFP, Saudi Society of Periodontology (SSP), Ukrainian Association of Periodontology (UAPerio), Iberopanamerican Federation of Periodontology (FIPP), Dansk Parodontologisk Selskab (DPS) (Personal honoraria for lectures); Dentaid (Manuscript writing). Support for attending meetings and/or travel: Oral-B, Straumann, Klockner, Dexcel, Dentaid, Colgate (Support for attending conferences and/or travel when participating as invited speaker). Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: Patron of the Fundación SEPA de Periodoncia e Implantes Dentales (Unpaid); Chair of the European Workshop Committee of the EFP (Unpaid). Receipt of equipment, materials, drugs, medical writing, gifts, or other services: Dentaid, Lacer, ZIZ Dental, Isdin, Affinity (Receipt of equipment, materials, pharmaceuticals, in association with research contracts).
Søren Jepsen (Chair) reports—Grants or contracts from any entity: Osteology Foundation (Research Contract with University), Innovationsfond of GBA, German Ministry of Health (Research Contract with University), University of Bristol (Research Contact with University), BMBF and Sirona/Dentsply (Research Contact with University). Consulting fees: CP Gaba, P&G. Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events: Osteology Foundation, ITI Foundation, Geistlich, Straumann, TePe (Personal honoraria for lectures). Support for attending meetings/travel: EFP, DG PARO, DGI, Osteology Foundation (support for attending meetings/travel when participating as invited speaker). Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: German Society of Dentistry and Oral Medicine (DGZMK), Advisory Council on continuing dental education, EFP Workshop Committee, ARPA Science Foundation.
Moritz Kebschull (Chair) reports—Grants or contracts from any entity: National Institutes for Health Research, Genolytic, Bredent, Unilever (To University of Birmingham). Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events: Camlog, Curaden, Dexcel, Geistlich, Hu-Friedy, NSK, Procter&Gamble, Resolve Biosciences, Unilever (For lecturing, to me); Several universities and public bodies, learned societies (For lecturing, to me). Patents planned, issued, or pending: With commercial partner Genolytic (filed) (Perio diagnostics). Leadership or fiduciary role in another board, society, committee or advocacy group, paid or unpaid: EFP, BSP, DG PARO (Unpaid). Receipt of equipment, materials, pharmaceuticals, medical writing, gifts, or other services: Geistlich, Hu-Friedy, NSK, Straumann (Support for courses/material and instruments/devices).
Panos N. Papapanou (Chair) reports—Grants or contracts from any entity: National Institutes of Health/National Institute of Aging (NIH/NIA R1 AG7615; 9/3/221–8/31/226) (A Longitudinal Study of Periodontal Infections and Alzheimer's Disease: The WHICAP Ancillary Study of Oral Health); Colgate-Palmolive, Inc., Piscataway, NJ, USA (Cerebrospinal fluid microbiome, oral health and cognitive aging). Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events: New York Society of Periodontics (Lecture honorarium); Straumann (Lecture honorarium). Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: Editor in Chief, Journal of Clinical Periodontology (EiC honorarium from Wiley and EFP).
Mariano Sanz (Chair) reports—Grants or contracts from any entity: Straumann, Nobelbiocare, Sweden & Martina, Dentsply Implants, TiCare Implants, Klockner Implants, DENTAID, Sunstar, Geistlich Pharma (Research contract via university); Osteology Foundation, Oral Reconstruction Foundation, ITI Foundation (Member). Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events: Straumann, Nobelbiocare, Sweden & Martina, Dentsply Implants, TiCare Implants, Klockner Implants, Dentaid, Sunstar, Geistlich Pharma, Osteology Foundation, ITI, ORF (Personal honoraria for lectures); Dentaid (Personal honoraria for manuscript writing). Support for attending meetings and/or travel: Straumann, Nobelbiocare, Sweden & Martina, Dentsply Implants, TiCare Implants, Klockner Implants, Dentaid, Sunstar, Geistlich Pharma, Osteology Foundation, ITI, ORF (Support for attending meetings and/or travel when participating as invited speaker). Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: President ORF, Fellow ITI, Honorary Member OF (Unpaid).
Frank Schwarz (Chair) reports—Grants or contracts from any entity: Osteology Foundation, ITI, Oral Reconstruction Foundation (Contract via University); Camlog, Straumann, Geistlich, Dentsply (Contract via University); BMBF and Dentsply (Contract via University—Grant to investigate surgical peri-implantitis therapy by combination of a plasma and a water jet). Consulting fees: Henry Schein (Personal Honoraria). Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events: Osteology Foundation, ITI, Oral Reconstruction Foundation (Personal lecture fees); Camlog, Straumann, Geistlich (Personal lecture fees). Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: Osteology Foundation (Annual compensation fee); EAO (unpaid). Receipt of equipment, materials, drugs, medical writing, gifts or other services: Straumann, Camlog, Geistlich, Dentsply (Receipt of equipment, materials in association with research contracts via university).
APPENDIX
Workshop Participants: Mario Aimetti, Juan Blanco, Nagihan Bostanci, Philippe Bouchard, Darko Božić, Nurcan Buduneli, Elena Calciolari, María Clotilde Carra, Raluca Cosgarea, Jan Cosyn, Bettina Dannewitz, Monique Danser, Beatriz de Tapia, Yvonne de Waal, Jan Derks, Henrik Dommisch, Nikos Donos, Kenneth Eaton, Peter Eickholz, Bahar Eren Kuru, Elena Figuero, Moshe Goldstein, Filippo Graziani, Jasmin Grischke, Fernando Guerra, Lisa Heitz-Mayfield, Karin Jepsen, Lise-Lotte Kirkevang, Odd Carsten Koldsland, France Lambert, Antonio Liñares, Bruno Loos, Phoebus Madianos, Paula Matesanz, Paulo Melo, Ana Molina, Virginie Monnet Corti, Eduardo Montero, Frauke Müller, Luigi Nibali, Andrés Pascual, Ioannis Polyzois, Marc Quirynen, Ausra Ramanauskaite, Gitana Rederiene, Stefan Renvert, Mario Roccuzzo, Philipp Sahrmann, Giovanni Salvi, Nerea Sánchez, Ignacio Sanz-Sánchez, Lior Shapira, Andreas Stavropoulos, Meike Stiesch, Ieva Tamošiūnaitė, Wim Teughels, Cristiano Tomasi, Leonardo Trombelli, Spyros Vassilopoulos, Anders Verket, Nicola West, Asaf Wilensky.
Methodological Consultant: Ina Kopp.
Workshop Organization: European Federation of Periodontology.
Scientific societies involved in the guideline development process: European Dental Hygienists Federation; European Society for Endodontology.
Other organizations involved in the guideline development process: Council of European Dentists; European Dental Students' Association; Platform for Better Oral Health in Europe.





