Outcome measures and methods of assessment of soft tissue augmentation interventions in the context of dental implant therapy: A systematic review of clinical studies published in the last 10 years
Funding information: No financial support or sponsorship was received.
This manuscript was simultaneously and jointly published in the Journal of Clinical Periodontology and Clinical Oral Implants Research
Abstract
Aim
To identify and report outcome measures and methods of assessment on soft tissue augmentation interventions in the context of dental implant therapy reported in clinical studies published in the last 10 years.
Materials and Methods
The protocol of this Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) 2020-compliant systematic review was registered in PROSPERO (CRD42021252214). A literature search was conducted to identify articles that met the pre-established eligibility criteria. Data of interest, with an emphasis on outcome measures, were extracted. For each outcome, specific methods and timing of assessment were described in detail. Following a critical qualitative analysis of the data, outcome measures were categorized. Primary outcomes were identified, and the frequency of reporting in the selected articles was calculated. Additionally, risk-of-bias assessments were performed for individual articles and primary outcomes.
Results
Ninety-two articles, of which 39 reported randomized controlled trials (RCTs), 20 non-RCTs, and 33 case series studies, were selected. Outcome measures were categorized into either investigator-evaluated outcome measures (i.e., clinical, digital imaging, aesthetic, histological, biomarker, and safety) or patient-reported outcome measures (PROMs). Clinical outcomes were the most frequently reported type of outcome. Considering all categories, the most frequently reported primary outcomes were facial mucosa thickness assessed with clinical methods (22.83%), facial keratinized mucosa width assessed with clinical methods (19.57%), facial mucosal margin position/recession assessed with clinical methods (18.48%), facial mucosa thickness assessed with digital imaging methods (11.96%), facial soft tissue volume assessed with digital imaging methods (9.78%), and supracrestal tissue height assessed with clinical methods (9.78%). No distinguishable patterns of association between specific types or quality (level of bias) of clinical studies and the choice of primary outcomes were observed.
Conclusions
Clinical research on peri-implant soft tissue augmentation has progressively increased in the last 10 years. Although clinical outcome measures were the most frequently reported outcomes in the selected literature, trends in the field are indicative of a shift from traditional clinical assessment methods to the use of digital technologies. PROMs were generally under-reported but should be considered an integral methodological component in future clinical studies.
Clinical Relevance
Scientific rationale for study: Assessment and interpretation of outcomes is an essential aspect of clinical research. This systematic review was conducted as part of the Implant Dentistry Core Outcome Sets and Measurements initiative.
Principal findings: Ninety-two articles published between January 2010 and April 2021 were selected. Outcomes were categorized into clinical, digital imaging, aesthetic, histological, biomarker, safety, and patient-reported outcome measures. Clinical outcomes were the most frequently reported type of outcome measure.
Practical implications: Standardization of core research outcomes on soft tissue augmentation interventions in the context of implant therapy can be beneficial to reduce bias in future clinical studies and contribute to optimize the translation of scientific findings into patient care.
1 INTRODUCTION
Peri-implant soft tissue augmentation interventions are indicated before or after insertion of the final implant-supported prosthesis in sites presenting unfavourable structural features that are associated with or may predispose to the occurrence of inflammatory peri-implant pathosis, soft tissue deformities, and/or sub-optimal aesthetics (Thoma et al., 2018). Depending on their primary therapeutic goal, peri-implant soft tissue augmentation interventions may be broadly classified in two categories: peri-implant soft tissue phenotype modification (Tavelli et al., 2021), and treatment of peri-implant marginal mucosa defects (PMMDs), also known as peri-implant soft tissue dehiscences (Zucchelli et al., 2019).
The peri-implant phenotype has been defined as the morphological and dimensional features characterizing the clinical presentation of the tissues that surround and support osseointegrated implants (Avila-Ortiz et al., 2020). The peri-implant phenotype is constituted by a soft tissue and a bone component. While the peri-implant bone phenotype is primarily determined by the bone thickness (BT), the peri-implant soft tissue phenotype includes three key elements with different clinical and therapeutic implications: the keratinized mucosa width (KMW), the mucosal thickness (MT), and the supracrestal tissue height (STH). On the other hand, PMMDs are alterations of the peri-implant soft tissue architecture characterized by an apical discrepancy of the mucosal margin with respect to its ideal position with or without exposure of transmucosal prosthetic components or the implant fixture surface (Gamborena & Avila-Ortiz, 2021).
Over the past decade, a variety of surgical modalities for peri-implant soft tissue phenotype modification (i.e., augmentation of KMW, MT, and/or STH) and correction of PMMDs using different techniques and graft materials have consolidated or emerged. In parallel, a variety of true and surrogate endpoints of interest (Chambrone & Armitage, 2016) and assessment methods to monitor the results of therapy have also been developed. Research outcomes, also known as endpoints or events, are variables that are recorded during a study to assess the impact that a given intervention or exposure has on the health of a given population. Assessment and interpretation of outcomes is an essential component of research, as this allows testing the validity of the hypothesis (Sanz & Vignoletti, 2014). Standardization of core research outcomes can help in guiding future research, decreasing potential biases, and allowing for more reliable inter-study comparisons and pooled data analyses for the advancement of science and, ultimately, the enhancement of patient care.
The primary objective of this systematic review, which was conducted as part of the Implant Dentistry Core Outcome Sets and Measurements (ID-COSM) initiative, was to identify and report outcome measures and methods of assessment on soft tissue augmentation interventions performed in the context of dental implant therapy reported in clinical studies published in the last 10 years.
2 MATERIALS AND METHODS
The protocol of this review was previously registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the identification code CRD42021252214. This review adheres to the guidelines of the Preferred Reporting Items of Systematic Reviews and Meta-Analyses 2020 statement (Page et al., 2021).
2.1 Focused research question and PICO outline
“In adult patients receiving any soft tissue augmentation intervention prior to or after the insertion of the final implant-supported prosthesis, which outcome measures and methods of assessment have been used to monitor the results of therapy in clinical studies published in the last 10 years?”
- Population: Adult human subjects in need of or who have one or more dental implants.
- Intervention: Any soft tissue augmentation intervention performed in the context of dental implant therapy prior to or after insertion of the final implant-supported prosthesis.
- Comparison: Absence of treatment or control treatment.
- Outcomes: Any outcome measures and methods of assessment reported after peri-implant soft tissue augmentation, independently of the total follow-up time.
2.2 Eligibility criteria
Randomized controlled trials (RCTs), non-RCTs, prospective cohort studies, and pre–post case series studies (i.e., clinical studies with no control group but involving several visits over time) in the field of implant dentistry with a minimum of 10 subjects per study group were eligible. The difference between RCTs and non-RCTs was whether randomization was used for group allocation (RCTs) or not (non-RCTs). For inclusion, studies must have involved at least one peri-implant soft tissue augmentation intervention and a subsequent outcome assessment.
2.3 Information sources and search strategy
Three electronic databases were searched, namely National Library of Medicine (MEDLINE/PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), and EMBASE using specific strategies (Table 1). The electronic search included articles published between 1 January 2011 and 30 April 2021. Additionally, a thorough hand search was performed by screening articles published in relevant scientific journals (i.e., Journal of Periodontology, Journal of Clinical Periodontology, Journal of Dental Research, Clinical Oral Investigations, The International Journal of Periodontics and Restorative Dentistry, International Journal of Oral Implantology, Journal of Oral and Maxillofacial Surgery, The International Journal of Oral & Maxillofacial Implants, Clinical Oral Implant Research, and Clinical Implant Dentistry and Related Research) as well as recent systematic reviews on this topic (Thoma et al., 2014, 2018; Rotundo et al., 2015; Bassetti et al., 2016, 2017; Lin et al., 2018; Cairo et al., 2019; Seyssens et al., 2021; Tavelli et al., 2021) published between 1 January 2011 and 30 April 2021. Per consensus agreement with the ID-COSM steering committee, the grey literature was not searched.
PubMed n = 6088 records |
|
CENTRAL n = 3387 records |
dental implant OR peri-implant AND “keratinized tissue” OR “tissue thickness” OR “connective tissue graft” OR “free gingival graft” OR “soft tissue augmentation” OR “collagen matrix” OR “xenogeneic collagen matrix” OR “acellular dermal matrix” OR “dermal matrix allograft” |
EMBASE n = 5392 records |
dental implant OR peri-implant AND “keratinized tissue” OR “tissue thickness” OR “connective tissue graft” OR “free gingival graft” OR “soft tissue augmentation” OR “collagen matrix” OR “xenogeneic collagen matrix” OR “acellular dermal matrix” OR “dermal matrix allograft” |
- Note: Search strategies in CENTRAL and EMBASE were modelled based on the search strategy designed for PubMed using the filters: Humans and date of publication from 1 January 2011 to 30 April 2021.
2.4 Selection process
Two reviewers (Emilio Couso-Queiruga and Miha Pirc) independently performed the hand search and read the title and abstract of the entries obtained from the literature search. Inter-examiner calibration was achieved by open discussion and comparison after independent assessment of the first 200 records. After completing the screening process, both reviewers read individually through the full-text version of the potentially eligible studies. Final article selection was dictated by the eligibility criteria (see Section 2.2). When disagreement regarding the inclusion of a specific article occurred, both reviewers had an open discussion. If no agreement was achieved, another co-author (Gustavo Avila-Ortiz) made the final decision. Following article selection, Cohen's kappa coefficient (κ) was calculated to determine the degree of inter-examiner agreement.
2.5 Data extraction
Data extraction was preliminarily performed by two independent examiners (Emilio Couso-Queiruga and Miha Pirc). Examiners were calibrated by using a random selection of five articles to ensure consistency in the data extraction process and the terminology employed. Final data accuracy and consistency was independently verified by a third author (Gustavo Avila-Ortiz). Any missing information that could contribute to this review was requested from the corresponding author(s) via email communication.
2.6 Data synthesis
Extracted data were organized into evidence tables. In addition to the reported outcomes measures and their assessment methods, supplemental data included the year of publication and author(s), country(ies) and setting(s) in which the study was conducted, study design, initial and final number of participants, gender and age distribution, and description of intervention(s). For each outcome, specific methods and timing of assessment were described in detail. Following a critical qualitative assessment of the data, outcomes identified in the selected literature were categorized. The frequency of use as either primary or secondary outcome in the selected literature was calculated.
2.7 Risk-of-bias assessment
The risk-of-bias (RoB) analyses of each included article were independently performed by two authors (Emilio Couso-Queiruga and Miha Pirc). RCTs and non-RCTs (quasi-RCTs) were assessed with the RoB-1 tool from version 5.1 of the Cochrane handbook (Higgins et al., 2011), and case series were assessed using the National Institute of Health (NIH) quality assessment tool for before–after (pre–post) studies with no control group (NIH, 2021). Independently of the study type, primary outcome measures were specifically assessed using domain 4 of the RoB-2 tool from the current version of the Cochrane handbook (Sterne et al., 2019). Additional quality aspects, such as pertinence/significance, accuracy, and reproducibility of each primary outcome measure, were taken into consideration. Disagreement between reviewers was resolved by open discussion. In case no agreement could be achieved, the final decision was made by another co-author (Gustavo Avila-Ortiz).
3 RESULTS
3.1 Article selection
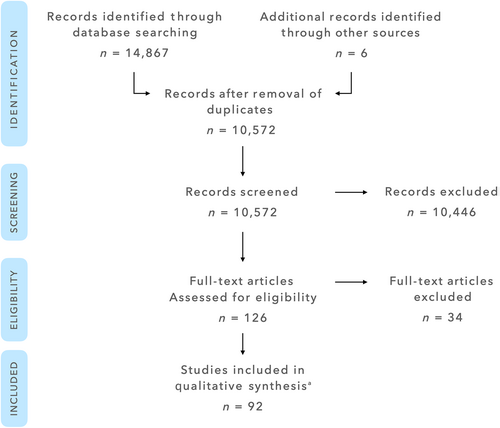
The initial database search yielded a total of 14,867 entries, of which 6088 were found in PubMed, 3387 in CENTRAL, and 5392 in EMBASE. Six additional articles were identified through manual search. Following removal of duplicates, 10,572 entries remained. After title and abstract screening, 126 articles were selected for full-text review. Of these 126 articles, 34 were excluded after full-text review. The list of excluded articles and reasons for exclusion are displayed in Table S1. Thus, the final selection comprised 92 articles (see list of selected articles in Supporting Information). A flow chart illustrating the article selection process is depicted in Figure 1. Inter-examiner agreement kappa score for title/abstract review and for full-text review were 0.93 (95% confidence interval [CI]: 0.855–1.0) and 0.78 (95% CI: 0.662–0.921), respectively.
3.2 Study characteristics
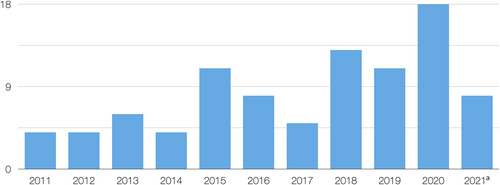
Of the 92 selected articles, 4 were published in 2011, 4 in 2012, 6 in 2013, 4 in 2014, 11 in 2015, 8 in 2016, 5 in 2017, 13 in 2018, 11 in 2019, 18 in 2020, and 8 between 1 January and 30 April 2021 (Figure 2). Fifty-seven studies were conducted in an academic (university) setting, 20 were carried out exclusively in a private clinic (of which 2 were multi-centre), 6 multi-centre studies were conducted in both academic and private practice settings, and 1 in a military setting; in 8 articles, this information was not clearly reported. Thirty-nine studies were RCTs, 20 were non-RCTs, and 33 were case series, none of which was reported as being retrospective. For specific bibliographic details, see list of selected articles in Supporting Information. Although some investigations were reported by the authors as cohort studies, critical assessment of the information provided in these articles made it evident that, technically, they were either cases series or clinical trials (RCTs or non-RCTs). Hence, no proper cohort studies were present in the final article selection. Within this selection of 92 articles, a total of 81 distinct clinical studies were identified because some articles were follow-up studies of previous publications or only reported additional data from an already included study.
Aside from specific data on the initial and final number of participants, as well as gender and age distribution, specific details pertaining to the type of interventions performed, details on the methods used for the measurement, and timing of assessment of the outcomes reported in the selected articles, as well as additional comments, are available online in the data collection form in Supporting Information.
3.3 RoB assessment
3.3.1 RoB assessment of individual studies
- RCTs
- Non-RCTs
- Case series
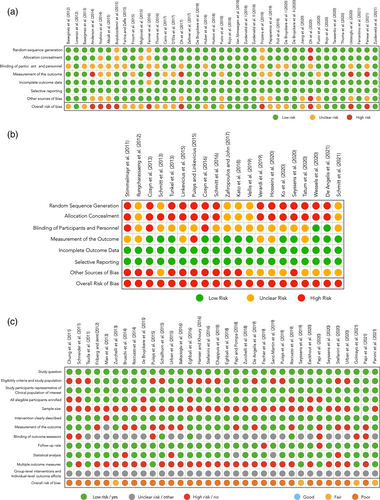
Except four studies in which the RoB was fair (Zucchelli et al., 2013; Seyssens et al., 2019; Golmayo et al., 2021; Parvini et al., 2021), all case series were categorized as “poor” (Figure 3c).
3.3.2 RoB assessment of primary outcomes
- RCTs
- Non-RCTs
- Case series
The RoB associated with the measurement of the primary outcome was categorized as low in 18 case series, high in 14 case series, and unclear in 1 case series (Figure 3c).
The primary outcome was clearly reported in 54 (58.70%) of the 92 selected articles. In the remaining 38 articles, the primary outcome was inferred based on our best judgement (specific comments are included in the data collection form in Supporting Information). Interestingly, examiner calibration for the primary outcome was reported only in 28 of the selected articles (30.43%), 15 of them being RCTs, 5 non-RCTs, and 8 case series. However, beyond that observation, no distinguishable patterns of association between specific types or methodological quality of studies and the choice of primary outcomes were observed.
3.4 Qualitative assessment: Categorization of outcomes in selected studies
After a critical assessment of the papers selected in this systematic review, outcome measures and methods of assessment were categorized into investigator-evaluated outcome measures and patient-reported outcome measures (PROMs) (Sanz & Vignoletti, 2014) as follows1:
3.4.1 Investigator-evaluated outcome measures
Clinical outcomes
- Facial KMW: Reported in 45 articles (48.91%). Specifically, using a periodontal probe in 44 articles (47.83%) and using intra-oral photographs in 1 article (1.09%). Primary outcome in 19 articles (19.57%).
- Plaque index (PI) (Loe, 1967) or modified PI (Mombelli et al., 1987) using a periodontal probe: Reported in 45 articles (48.91%).
- Probing depth (PD) using a periodontal probe: Reported in 45 articles (48.91%).
- Facial mucosal margin position/recession: Reported in 33 articles (35.87%). Specifically, using a periodontal probe or a Castroviejo calliper either intra-orally or on stone casts in 26 articles (28.26%), and using intra-oral photographs in 7 articles (7.61%). Primary outcome in 17 articles (18.48%).
- Bleeding on probing using a periodontal probe: Reported in 29 articles (31.52%).
- Facial MT via transmucosal bone sounding (e.g., endodontic file, periodontal probe, or anaesthesia needle) or using a calliper: Reported in 27 articles (29.35%). Primary outcome in 21 articles (22.83%).
- Implant survival rate: Reported in 16 articles (17.39%).
- Inter-proximal papilla height: Reported in 12 articles (13.04%). Specifically, using a periodontal probe intra-orally or on stone casts in seven articles (7.61%) and using intra-oral photographs in five articles (5.43%).
- Gingival index (Loe, 1967) using a periodontal probe: Reported in 11 articles (11.96%).
- STH using transmucosal bone sounding (e.g., endodontic file, periodontal probe, or anaesthesia needle): Reported in 10 articles (10.87%). Primary outcome in nine articles (9.78%).
- Implant success rate: Reported in nine articles (9.78%). Specifically, the Smith and Zarb criteria (Smith & Zarb, 1989) were used in three articles (3.26%), the Albretksson et al. criteria (Albrektsson et al., 1986) in two articles (2.17%), the Buser et al. criteria (Buser et al., 1990) in two articles (2.17%), the van Steenberghe criteria (van Steenberghe, 1997) in one article (1.09%), and the Albretksson and Zarb criteria (Albrektsson & Zarb, 1998) in one article (1.09%).
- Clinical attachment level using a periodontal probe: Reported in eight articles (8.70%).
- Sulcus bleeding index (Muhlemann & Son, 1971) using a periodontal probe: Reported in five articles (5.43%).
- Surgical time: Reported in five articles (5.43%).
- Full-mouth bleeding score using a periodontal probe: Reported in four articles (4.35%).
- Full-mouth plaque score using a periodontal probe: Reported in four articles (4.35%).
- Facial mucosa phenotype using a periodontal probe (transparency method): Reported in three articles (3.26%).
- Implant stability using a technological device: Reported in three articles (3.26%).
- Modified bleeding index (Mombelli et al., 1987) using a periodontal probe: Reported in three articles (3.26%).
- Facial gingival recession depth on teeth adjacent to implant site: Reported in two articles (2.17%).
- Facial PMMD (recession) width using a periodontal probe or a precision gauge: Reported in two articles (2.17%).
- Direct visual assessment of soft tissue colour and texture (mucosal surface structure): Reported in two articles (2.17%).
- Direct visual assessment of soft tissue healing: Reported in two articles (2.17%).
- Direct visual assessment of wound healing index: Reported in two articles (2.17%).
- Indirect visual assessment of alveolar process deficiency according to Pink Aesthetic Score (PES) criterion using stone casts: Reported in two articles (2.17%).
- Vestibular depth using a periodontal probe: Reported in two articles (2.17%).
- Facial defect concavity depth and width using a periodontal probe: Reported in one article (1.09%).
- Crestal KMW using a periodontal probe: Reported in one article (1.09%).
- Direct visual assessment of facial contour deficiency or concavity (improved/worsened/no change): Reported in one article (1.09%).
- Direct visual assessment of the convexity of the facial mucosal profile: Reported in one article (1.09%).
- Graft shrinkage area using a periodontal probe: Reported in one article (1.09%).
- Indirect visual assessment of soft tissue colour: Reported in one article (1.09%).
- Mesio-distal length of keratinized tissue in grafted area using a periodontal probe: Reported in one article (1.09%).
- Lingual KMW using a periodontal probe: Reported in one article (1.09%).
- Lingual MT via transmucosal bone sounding (e.g., endodontic file, periodontal probe, or anaesthesia needle) or using a calliper: Reported in one article (1.09%).
- Need for additional bone and soft tissue augmentation procedures at the time of implant placement: Reported in one article (1.09%).
- Percentage of complete correction of PMMDs: Reported in one article (1.09%).
- Percentage of mucosal graft shrinkage using intra-oral photographs: Reported in one article (1.09%).
- Proportion of implants exhibiting peri-implant health, peri-implant mucositis, and peri-implantitis: Reported in one article (1.09%).
- Suppuration using a periodontal probe: Reported in one article (1.09%).
Digital imaging outcomes
- Marginal bone loss measured using periapical radiographs: Reported in 36 articles (39.13%). Primary outcome in one article (1.09%).
- Facial MT: Reported in 23 articles (25.00%). Specifically, using Standard Tessellation Language (STL) files in 11 articles (11.96%), using DICOM files in 5 articles (5.43%), using an ultrasound device in 6 articles (6.52%), and using superimposed STL and DICOM files in 1 article (1.09%). Primary outcome in 11 articles (11.96%).
- Facial peri-implant soft tissue volume: Reported in 12 studies (13.04%). Specifically, using STL files in 11 articles (11.96%) and using superimposed STL and DICOM files in 1 article (1.09%). Primary outcome in nine articles (9.78%).
- Facial BT using DICOM files: Reported in seven articles (7.61%).
- Facial mucosal margin position/recession: Reported in three articles (3.26%). Specifically, using STL files in two articles (2.17%), and using superimposed STL and DICOM files in one article (1.09%). Primary outcome in one article (1.09%).
- Facial vertical bone loss using DICOM files: Reported in two articles (2.17%).
- Facial bone plate integrity using DICOM files: Reported in one article (1.09%).
- Facial mucosa profile assessment using DICOM files: Reported in one article (1.09%). Primary outcome in one article (1.09%).
- Graft surface area using STL files: Reported in one article (1.09%). Primary outcome in one article (1.09%).
- Facial KMW using STL files: Reported in one article (1.09%). Primary outcome in one article (1.09%).
- Facial mucosal margin position/recession using DICOM files: Reported in one article (1.09%).
- Papilla height changes using STL files: Reported in one article (1.09%).
- Proximal bone level on the teeth adjacent to the implant site using periapical radiographs: Reported in one article (1.09%).
Aesthetic outcomes
- PES assessment (Furhauser et al., 2005): Reported in 23 articles (25.00%). Primary outcome in two articles (2.17%).
- Papilla Index Score assessment (Jemt, 1997): Reported in eight articles (8.70%).
- White Aesthetic Score assessment (Belser et al., 2009): Reported in seven articles (7.61%).
- Direct visual assessment of facial mucosa colour: Reported in two articles (2.17%).
- Direct visual assessment of colour, texture, contour of facial mucosa compared to a contra-lateral or adjacent site: Reported in two articles (2.17%).
- Mucosa Scarring Index assessment (Wessels et al., 2019): Reported in two articles (2.17%). Primary outcome in one article (1.09%).
- Complex Aesthetic Index (Juodzbalys & Wang, 2010): Reported in one article (1.09%).
- Copenhagen Index Score assessment (Dueled et al., 2009): Reported in one article (1.09%).
- Direct visual assessment of grey show-through on the facial mucosa (yes/no): Reported in one article (1.09%).
- Implant Crown Aesthetic Index (Meijer et al., 2005): Reported in one article. Primary outcome in one article (1.09%).
- Overall aesthetic assessment using a visual analog scale (VAS): Reported in one article (1.09%).
- Spectrophotometric assessment of facial peri-implant mucosa colour: Reported in one article (1.09%).
Histological outcomes
- Descriptive histological analysis of peri-implant soft tissue samples: Reported in five articles (5.43%).
- Histomorphometric analysis (e.g., height and width, presence of residual graft material and elastic fibres, and inflammatory infiltrate) of peri-implant soft tissue samples: Reported in two articles (2.17%).
- Immunohistochemical analysis (i.e., cytokeratin 5/6, 13, and 14) of peri-implant soft tissue samples: Reported in one article (1.09%).
Biomarker outcomes
- Interleukin-1β concentration: Reported in one article (1.09%).
Safety outcomes
Defined as assessments to monitor the occurrence of complications and adverse events. Out of the 92 selected articles, only in 20 articles (21.7%) safety outcomes were not reported. For specific details, see data collection form in Supporting Information. It must be noted that in most articles, information pertaining to safety outcomes was reported in Section 3, with no previous mention in Section 2.
3.4.2 Patient-reported outcome measures
- Perceived post-operative pain/discomfort using a VAS: Reported in 17 articles (18.48%).
- Aesthetic satisfaction using a VAS: Reported in 10 articles (10.87%).
- Overall satisfaction using a VAS: Reported in eight articles (8.70%).
- Quality of life questionnaire (OHIP, OHIP-14, or OHIP-G14): Reported in seven articles (7.61%).
- Amount of post-operative inflammatory medication taken: Reported in three articles (3.26%).
- Willingness to undergo the same treatment again (yes/no): Reported in two articles (2.17%).
- Oedema and haematoma using a VAS: Reported in one article (1.09%).
- Masticatory function using a VAS: Reported in one article (1.09%).
- Modified version of the complex aesthetic index (Juodzbalys & Wang, 2010): Reported in one article (1.09%).
The top 20 most frequently reported outcome measures and all primary outcome measures reported in the selected literature are displayed in Tables 2 and 3, respectively.
| Outcome measure | Percentage of reporting | Type of outcome measure |
|---|---|---|
| Facial keratinized mucosa width | 48.91 | Clinical |
| Plaque index | 48.91 | Clinical |
| Probing depth | 48.91 | Clinical |
| Marginal bone loss | 39.13 | Digital imaging |
| Facial mucosal margin position | 35.87 | Clinical |
| Bleeding on probing | 31.52 | Clinical |
| Facial mucosal thickness | 29.35 | Clinical |
| Facial mucosal thickness | 25.00 | Digital imaging |
| Pink Aesthetic Score | 25.00 | Aesthetic |
| Perceived post-operative pain | 18.48 | Patient-reported |
| Implant survival rate | 17.39 | Clinical |
| Inter-proximal papilla height | 13.04 | Clinical |
| Facial peri-implant soft tissue volume | 13.04 | Digital imaging |
| Gingival index | 11.96 | Clinical |
| Supracrestal tissue height | 10.87 | Clinical |
| Aesthetic satisfaction | 10.87 | Patient-reported |
| Implant success rate | 9.78 | Clinical |
| Clinical attachment level | 8.70 | Clinical |
| Papilla Index Score | 8.70 | Aesthetic |
| Overall satisfaction | 8.70 | Patient-reported |
| Outcome measure | Percentage of reporting as primary outcome | Type of outcome measure |
|---|---|---|
| Facial mucosal thickness | 22.83 | Clinical |
| Facial keratinized mucosa width | 19.57 | Clinical |
| Facial mucosal margin position | 18.48 | Clinical |
| Facial mucosal thickness | 11.96 | Digital imaging |
| Gingival index | 9.78 | Clinical |
| Facial peri-implant soft tissue volume | 9.78 | Digital imaging |
| Pink aesthetic score | 2.17 | Aesthetic |
| Marginal bone loss | 1.09 | Digital imaging |
| Facial mucosal margin position | 1.09 | Digital imaging |
| Facial mucosa profile | 1.09 | Digital imaging |
| Graft surface area | 1.09 | Digital imaging |
| Facial keratinized mucosa width | 1.09 | Digital imaging |
| Mucosa Scarring Index | 1.09 | Aesthetic |
| Implant Crown Aesthetic Index | 1.09 | Aesthetic |
- Note: Some articles included more than one primary outcome.
4 DISCUSSION
The present systematic review mainly revealed the following: (1) An increasing number of publications reported the findings of clinical studies on the topic of soft tissue augmentation in the context of implant therapy over the past 10 years. (2) The most common study type was RCT performed at a university setting. (3) Clinical outcome measures were the most frequently reported outcomes overall. (4) The most frequently reported primary outcomes were facial mucosa thickness and facial KMW, followed by facial mucosal margin position (recession) and STH changes.
The increase in the number of investigations in peri-implant soft tissue augmentation in the past decade is likely due to two main reasons: (1) the recognition of the critical role of the peri-implant soft tissues in the maintenance of peri-implant health and the enhancement of aesthetic outcomes in implant therapy, and (2) the emergence of soft tissue graft substitutes as an alternative to autogenous grafts.
In several classic periodontal investigations on the topic of soft tissue augmentation conducted in the late 1960s and early 1970s, the focus was on understanding the healing process after autogenous oral soft tissue transplantation as well as the development and optimization of surgical interventions (Oliver et al., 1968; Karring, Cumming, et al., 1975; Karring, Lang, & Loe, 1975). In the 2000s, after the consolidation of the fields of mucogingival surgery and implant dentistry, further clinical models were introduced to assess the efficacy of novel soft tissue graft substitutes (as alternatives to autogenous soft tissue grafts that are commonly associated with an increased patient morbidity) for periodontal and peri-implant soft tissue augmentation purposes (Del Pizzo et al., 2002; Griffin et al., 2006; McGuire & Scheyer, 2010; Jung et al., 2011; Thoma et al., 2012). Several clinical studies have demonstrated the suitability of various soft tissue graft substitutes as plausible alternatives to autogenous soft tissue transplants for specific indications (Thoma et al., 2018; Cairo et al., 2019; Fickl et al., 2021; Tavelli et al., 2021).
Assessment and interpretation of outcomes are essential components of clinical research. Broadly, there are two types of clinical research outcomes: primary and secondary. The primary outcome is the most relevant variable to answer the main research question. Depending on their design, number of hypotheses, and objectives, some studies may have more than one primary outcome, as was the case in some of the studies selected in this systematic review. The primary outcome(s) should be used a priori to determine the minimum number of participants required to achieve statistical power and a posteriori to either reject or accept the study hypothesis. Secondary outcomes are supplementary outcomes monitored to help interpret the results of the primary outcome or increase the amount of information obtained through a study (Ferreira & Patino, 2017). Interestingly, clinical studies assessing the performance of soft tissue graft substitutes in peri-implant soft tissue augmentation in the past 10 years have predominantly considered gain of MT or volume and KMW as the primary outcome. Management of peri-implant soft tissue dehiscences and understanding the significance of the STH around dental implants, originally referred to as “peri-implant biologic width” (Berglundh et al., 1991; Berglundh & Lindhe, 1996), have become a relevant topic of research interest in recent years. This would explain why changes in facial mucosal margin position (recession) and STH were also frequently reported as primary outcomes in the selected literature.
Data analysis with a focus on primary outcome measures and methods of assessments revealed that clinical methods (e.g., use of periodontal probes, callipers, and endodontic files) were most often employed in the past 10 years. This may be associated with the fact that these traditional methods are part of daily clinical practice and have been used for decades in the field of mucogingival surgeries as part of the conventional clinical examination (Friedman, 1962; Diedrich et al., 1972; Edel, 1974; Bachmann & Bernimoulin, 1980; de Trey & Bernimoulin, 1980). Consequently, clinical assessments served as primary outcome in nearly 60% of the articles selected in the present systematic review. However, while these methods are well established, have been validated, and are associated with low cost and simple logistics, they also are limited to some extent as they do not allow capturing the entire extent of the therapeutic effect of some surgical interventions. This is particularly critical for interventions primarily aimed at modifying the peri-implant soft tissue contour, as clinical measurements based on the use of analogue instruments do not permit a reliable three-dimensional (3D) assessment of the outcomes (e.g., volume changes).
Technological advancements have derived into the implementation of digital assessment methods, such as linear and 3D analyses of STL files obtained from digitization of casts or intra-oral surface scanning (Windisch et al., 2007; Bienz et al., 2017; Pirc et al., 2021). Such analyses accounted for nearly 10% of the methods of choice to assess the primary outcome in the selected articles. The main benefits of digital assessment methods based on STL file analyses are a reduction in measurement errors, higher reproducibility and reliability, and their non-invasiveness (Schneider et al., 2014; Couso-Queiruga et al., 2021). A progressive shift from clinical towards digital imaging methods for assessment of outcomes can be expected in future years in this area of research.
Although PROMs were reported in 30 articles, nearly one-third of the total, in none of the selected studies were they designated as primary outcomes, and in many articles, minimal information about the methodology applied to assess PROMs was provided. Furthermore, PROMs specifically related to patient morbidity (e.g., post-operative pain and discomfort) were inconsistently investigated in studies that involved the use of a soft tissue graft substitute.
It must be noted that frequency of reporting of a specific method and outcome measure does not necessarily correlate with its significance in contemporary clinical research. Therefore, a highly reported clinical outcome per the findings of this systematic review (e.g., PD or PI) should not be automatically considered a core outcome in future research reports related to peri-implant soft tissue augmentation. By the same token, some under-reported outcomes, such as advanced digital imaging analyses and PROMs, should be considered as core outcomes given their methodological advantages and relevance in clinical practice, respectively.
Considering the result of the RoB analyses conducted in this systematic review, no distinguishable patterns of association between specific types or quality of clinical studies and the choice of primary outcomes were observed. It might be speculated that properly designed studies associated with a high level of evidence (e.g., RCTs) would include a more consistent and exhaustive selection of outcomes of interest.
However, this was not observed in the selected literature. This may be attributed to individual preferences by the investigators depending on the study goal, as well as the continuous development and refinement of research methods over time.
Finally, this is the first systematic review focused on comprehensively identifying and reporting outcome measures and methods of assessment on soft tissue augmentation interventions performed in the context of dental implant therapy. Therefore, it is not possible to compare the methods and findings reported here with other similar publications.
5 CONCLUSIONS
Clinical research on soft tissue augmentation in the context of implant therapy has progressively increased over the last decade. Although clinical outcome measures were the most frequently reported outcomes, the continuous development and refinement of assessment methods based on advanced digital imaging, as well as their high reliability and reproducibility, will likely result in an increasing number of studies incorporating the use of such tools in coming years. Moreover, the routine incorporation of PROMs should be recommended in future clinical investigations on this topic, particularly in clinical trials involving the use of a soft tissue graft substitute.
AUTHOR CONTRIBUTIONS
Gustavo Avila-Ortiz and Daniel S. Thoma conceived the idea and initial structure of the systematic review; Leandro Chambrone performed the search; Emilio Couso-Queiruga and Miha Pirc screened the initial entries, selected the articles, and collected the data; Gustavo Avila-Ortiz verified the validity and standardization of collected data; Emilio Couso-Queiruga and Miha Pirc assessed the risk of bias; Leandro Chambrone contributed to the design of the final manuscript and data analysis; Gustavo Avila-Ortiz and Daniel S. Thoma led the writing; Emilio Couso-Queiruga, Miha Pirc, and Leandro Chambrone critically revised the manuscript.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report pertaining to the conduction of this systematic review.
Open Research
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request made to the corresponding author.
REFERENCES
- 1 Note that some articles included more than one primary outcome.




