Submucosal microbiome of peri-implant sites: A cross-sectional study
Angeliki Polymeri and Joyce van der Horst contributed equally to this work.
Abstract
Aim
To study the peri-implant submucosal microbiome in relation to implant disease status, dentition status, smoking habit, gender, implant location, implant system, time of functional loading, probing pocket depth (PPD), and presence of bleeding on probing.
Materials and Methods
Biofilm samples were collected from the deepest peri-implant site of 41 patients with paper points, and analysed using 16S rRNA gene pyrosequencing.
Results
We observed differences in microbial profiles by PPD, implant disease status, and dentition status. Microbiota in deep pockets included higher proportions of the genera Fusobacterium, Prevotella, and Anaeroglobus compared with shallow pockets that harboured more Rothia, Neisseria, Haemophilus, and Streptococcus. Peri-implantitis (PI) sites were dominated by Fusobacterium and Treponema compared with healthy implants and peri-implant mucositis, which were mostly colonized by Rothia and Streptococcus. Partially edentulous (PE) individuals presented more Fusobacterium, Prevotella, and Rothia, whereas fully edentulous individuals presented more Veillonella and Streptococcus.
Conclusions
PPD, implant disease status, and dentition status may affect the submucosal ecology leading to variation in composition of the microbiome. Deep pockets, PI, and PE individuals were dominated by Gram-negative anaerobic taxa.
Clinical Relevance
Scientific rationale for study: This study describes the microbiome of peri-implant sites in relation to patient- or implant-related parameters.
Principal findings: Deep pockets, peri-implantitis, and partial edentulism were mostly associated with Gram-negative anaerobic taxa, similar to those detected in periodontitis.
Practical implications: Better understanding of the peri-implant microbiome associated with probing pocket depth, disease status, and dentition status can contribute to establish effective and customized preventive, diagnostic, and treatment strategies for peri-implant diseases.
1 INTRODUCTION
Dental implants are a popular treatment for the prosthetic rehabilitation of partially dentate and fully edentulous patients (Elani et al., 2018). Nevertheless, peri-implant diseases, encompassing peri-implant mucositis (PM) and peri-implantitis (PI), are a growing concern in the dental community (Dreyer & Grischke, 2018). PM is characterized by inflammation confined to the soft tissues surrounding the implant, whereas the inflammatory process in PI leads to progressive loss of supporting bone and eventually loss of the implant (Berglundh et al., 2018). A recent study concluded that approximately one out of three patients and one out of five implants experienced PI (Kordbacheh Changi et al., 2019). The lack of effective and predictable treatments makes the management of peri-implant diseases even more challenging (Renvert & Polyzois, 2018).
The importance of biofilms in the aetiology of peri-implant diseases has been extensively studied (Charalampakis & Belibasakis, 2015; Lafaurie et al., 2017). We assume that dysbiotic biofilms may lead to inflammation, which in turn alters the ecology and favours further growth of dysbiotic communities, leading to a vicious cycle, similar to periodontitis (Hajishengallis et al., 2020; Loos & Van Dyke, 2020). Microorganisms colonize the peri-implant sulcus within 30 min after the surgical procedure, and a complex submucosal microbiota, similar to the microbiota around natural teeth, is established within 2 weeks (Quirynen et al., 2006; Furst et al., 2007). Teeth and mucosal surfaces act as microbial reservoirs for the colonization of implants in partially edentulous (PE) and fully edentulous (FE) individuals, respectively (Apse et al., 1989). If the biofilm is left undisturbed, clinical signs of inflammation in the peri-implant soft tissues start to appear, demonstrating a cause-and-effect relationship between biofilm and PM, similar to gingivitis on natural teeth (Pontoriero et al., 1994; Schwarz, Becker, et al., 2018). Untreated PM can at some point derail and progress to PI; the interactions between bacteria and the host immune system may trigger peri-implant bone loss in susceptible individuals and therefore affect the long-term stability of the implant (Schwarz, Derks, et al., 2018). While bone loss progresses, a deep pocket is formed, and this new anaerobic environment favours Gram-negative anaerobic bacteria (Mombelli & Decaillet, 2011; Kroger et al., 2018). The hypothesis that bacteria translocate from periodontally involved teeth to implant sites led to the conclusion that the composition of the peri-implant microbiota resembles the subgingival flora of periodontitis to a great extent (Mombelli & Decaillet, 2011; Lafaurie et al., 2017). However, the body of evidence supporting such perceived similarities is based on older targeted approaches, such as culture and DNA-checkerboard (Mombelli et al., 1995; Lafaurie et al., 2017). More recent evidence based on open-ended 16S rRNA gene sequencing and transcriptome sequencing methods has shown that the periodontal and peri-implant microbiomes have distinct features, which appear to be driven by substrate characteristics and environmental factors (Dabdoub et al., 2013; Becker et al., 2014; Shiba et al., 2016; D. M. Daubert & Weinstein, 2019).
Since the role of microorganisms in peri-implant diseases as an initial trigger for inflammatory reactions is well-established, the treatment approaches proposed for their management focus on the elimination of biofilm from the implant surface. The current protocols for the treatment of PI are based on the evidence available from studies related to the treatment of periodontitis (Renvert & Polyzois, 2018). Although most periodontitis cases respond favourably to treatment and maintain long-term periodontal stability, this does not hold true for peri-implantitis (Lindhe & Nyman, 1984). Existing therapeutic strategies are unpredictable in arresting peri-implant tissue inflammation and current evidence does not support a gold-standard protocol to treat peri-implant diseases (Garaicoa-Pazmino et al., 2019).
Therefore, a clear understanding of the microbial profiles of the peri-implant sulcus/pocket is of great importance to understand the sequelae of ecological changes and to establish effective preventive, diagnostic, and treatment strategies of peri-implant diseases. The aim of the present cross-sectional study is to describe the peri-implant microbiome using 16S rDNA amplicon sequencing and explore possible associations of the microbial composition with several patient- and implant-related parameters.
2 MATERIALS AND METHODS
2.1 Study design, ethical approval, and patient recruitment
The study was designed in 2010 as a descriptive, split-mouth cross-sectional study and was approved by the ethical committee of the VU Medical Centre, Amsterdam (#2011/370). The study was conducted in accordance with the guidelines of the World Medical Association Declaration of Helsinki. The study participants were recruited consecutively from patients visiting the Academic Centre for Dentistry Amsterdam (ACTA) for regular maintenance of their dental implants. To be included in the study, patients had to be older than 18 years and systemically healthy with at least one functional dental implant. Exclusion criteria included the use of systemic antibiotics within the past 6 months, any chronic medical disease or condition, pregnancy or lactation, and presence of implant mobility. Each participant was informed about the aims, the potential risks and benefits of the study, and provided written informed consent.
2.2 Clinical examination
The following parameters were recorded: age, gender, dentition status, smoking habit, implant location, implant system, time of functional loading, probing pocket depth (PPD), and presence of bleeding on probing (BoP). The clinical parameters were recorded at six sites per implant (mesiobuccal, buccal, distobuccal, mesiolingual, lingual, and distolingual). Intra-oral peri-apical radiographs were obtained with the parallel technique, and peri-implant bone levels were evaluated. A diagnosis of implant health and disease was made according to the definitions presented at the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions (Berglundh et al., 2018). Briefly, a healthy implant (HI) was diagnosed when the peri-implant crevice demonstrated no bleeding or suppuration on probing and absence of bone loss beyond the initial crestal bone remodelling. Implants with reduced bone support, which presented with absence of clinical signs of inflammation, were also considered healthy (Renvert et al., 2018). PM was defined by the presence of clinical signs of inflammation and absence of radiographic bone loss, whereas PI was diagnosed on the basis of clinical inflammation, PPD ≥6 mm and radiographic bone loss of ≥3 mm from the implant shoulder (Berglundh et al., 2018). For data analysis, we considered shallow pockets with PPD <5 mm and deep pockets with PPD ≥5 mm.
2.3 Peri-implant biofilm sample harvesting and DNA extraction
Two implants per patient were originally sampled. In patients with >2 implants, two implants were randomly chosen using a randomization tool (http://www.randomization.com/). Submucosal biofilm samples were obtained from the deepest submucosal site of the selected implants using one sterile paper point per implant (Absorbent Points # 504; Henry Schein Inc, Melville, NY). The sampling sites were isolated using cotton rolls and the supra-mucosal plaque was removed. After drying with air, a paper point was introduced into the bottom of the deepest submucosal site and removed after 10 s, then placed in an empty sterile Eppendorf tube and stored at −80°C until further analysis. DNA was extracted with the AGOWA mag Mini DNA Isolation Kit (LGC Genomics), as described previously (Bizzarro et al., 2016).
2.4 Quantitative PCR, amplicon preparation, and sequencing
Real-time qPCR, amplicon preparation, and sequencing were carried out as described previously (Kraneveld et al., 2012). Real-time qPCR was performed using a LC480-II light cycler (Rocher Diagnostics, Basel, Switzerland) according to the manufacturer's instructions. Barcoded amplicon libraries of the 16S rRNA gene hypervariable region V5–V7 were generated, pooled, and sequenced with the 454 GS-FLX + Titanium system (Roche Molecular Diagnostics, Branford, CT) (Kraneveld et al., 2012).
2.5 Sequencing data analysis
The sequencing data were processed using the Quantitative Insights Into Microbial Ecology (QIIME) version 1.8.0 (Caporaso et al., 2010). The data were demultiplexed (split_libraries.py) and barcodes, forward primers (one mismatch allowed), and reverse primers (two mismatches allowed) removed. In addition, no ambiguous base calls (N) were allowed and the sequences were quality-filtered using a sliding window size of 50 nucleotides with an average Phred quality score of 30, and otherwise default parameters.
Next, the reads were denoised (Reeder & Knight, 2010) and scanned again for the presence of reverse primers, allowing two mismatches, and were filtered for chimeric sequences with UCHIME (Edgar et al., 2011) as implemented in USEARCH version 6.1 (Edgar, 2010) using “identify_chimeric_seqs.py” (chimera retention set to “intersection”). Thereafter, the reads were clustered into operational taxonomic units (OTUs) at a minimal sequence similarity of 97% and taxonomy was assigned using the naïve Bayesian classifier provided by the Ribosomal Database Project (RDP) (Wang et al., 2007), with a minimum confidence of 0.8, retained on the Human Oral Microbiome Database 16S rDNA sequences (HOMD v.14.51) (Chen et al., 2010). With the use of the RDP classifier, as indicated above, many representative sequences had a species-level identification. In addition, all representative sequences were assigned a taxonomy using BLAST (Altschul et al., 1997) on the HOMD website (www.homd.org) using default parameters and database HOMD 16S rRNA RefSeq Version 14.51 (Starts at position 28). Next, the 20 resulting hits were parsed and species names were assigned to the top hit only if the alignment had ≥98% coverage and ≥98.5 similarity. The taxonomies of tied hits were combined.
Earlier, we showed that the sequencing profiles of the samples could be dominated by sequences from non-oral microorganisms. The source of this “foreign” bacterial DNA was attributed to the paper points used for sample collection (van der Horst et al., 2013). Therefore, we removed the OTUs detected in the unused sterile paper points. To allow for comparisons among different samples and to avoid the effect of variable sample sequencing depth on the diversity analyses, all samples were analysed by rarefaction and the OTU table was subsampled to an equal depth of 1200 reads per sample.
2.6 Data analysis
The demographic and clinical characteristics of the study population were expressed as mean (standard deviation [SD]) or percentages (%). The microbiological data were analysed at OTU level. To compare the microbial composition between samples by disease status, dentition status, smoking habit, gender, implant location, implant system, time of functional loading, PPD, and BoP, beta-diversity measurements were performed with the principal component analysis (PCA) and one-way permutational multivariate analysis of variance (PERMANOVA) in PAST version 3.23 (Hammer et al., 2001). The data were log2-transformed for PCA analysis to normalize the distributions of OTUs. PERMANOVA was performed using the Bray–Curtis similarity index and 9999 permutations to evaluate the compositional differences between groups (with Bonferroni correction when applying to more than two groups). p Values with a false discovery rate (FDR) of 5% or less were considered significant. Furthermore, we performed general linear model-based multivariate statistical analyses of patients' peri-implant microbiome to identify parameters associated with the microbial composition (MaAsLin, version 1.0.1, https://huttenhower.sph.harvard.edu/galaxy/). Covariates including age, gender, smoking, implant disease status, PPD, BoP, implant location, dentition status, time of functional loading, and implant system were entered into the model. False discovery correction was used with a threshold of q < 0.25.
Analysis of the relative abundance of the microbial communities between groups with significant differences was performed with linear discriminant analysis (LDA) Effect Size (LEfSe) in order to determine the OTUs that most likely explain the differences between the groups (Goecks et al., 2010; Segata et al., 2011). LEfSe was performed online via the Galaxy framework, using a size-effect threshold of 4.0 on the logarithmic LDA score. OTUs, which were identified differentially abundant between the groups in LEfSe, were tested for differences in relative abundance with Mann–Whitney U test or Kruskal–Wallis test in case of more than two groups, in SPSS (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, version 24.0; IBM Corp., Armonk, NY). The alpha diversity indices Shannon, Chao1, and observed OTUs were calculated using QIME. The level of statistical significance was set at 5%.
3 RESULTS
3.1 Demographic and clinical characteristics of the study population
The study was conceived and designed in 2010 as a split-mouth cross-sectional study, aiming to compare within the same individual diseased implants (either PI or PM) to HI. Forty-eight patients contributing two implants each were initially enrolled from December 2011 until June 2012 (Figure 1). Two samples from one patient were lost at DNA isolation stage due to insufficient high-quality DNA. After subsampling at 1200 reads and removing the contaminants originating from the paper points (van der Horst et al., 2013), 27 additional samples and six patients were excluded resulting in 41 patients contributing 67 peri-implant samples. At that point, only 26 out of 41 patients had paired samples, of which 19 participants presented with the same disease status (3 with HI, 14 with PM, and 2 with PI). On this premise, only one sample per patient was selected based on the higher amount of isolated DNA. Therefore, 41 implant sites in total were included in the analysis (Figure 1). Since the criteria for a split-mouth study design could not be satisfied, the current study was considered a descriptive cross-sectional study.
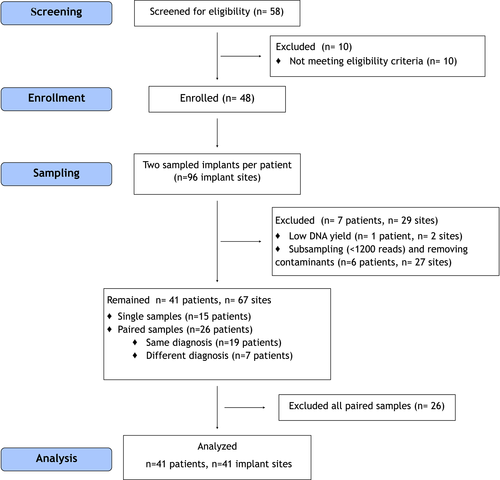
The demographic characteristics of the participants and the clinical features of the included implants are summarized in Table 1. The patients were on average 65.6 (8.8) years old (range: 49–83). The patient cohort comprised 13 males and 28 females, 17 PE, 24 FE individuals, and 5 smokers. The implants were in function for on average 7.2 (5.5) years (range: 1–18 years). Thirty out of 41 implants (73%) were located in the mandible. The mean PPD at the sampled sites was 4.2 mm (1.1), and 15 out of 41 sites (37%) presented with PPD ≥5 mm. Thirty out of 41 sites (73%) had signs of bleeding. Eleven implants (27%) were HI, 24 implants (58%) were diagnosed with PM, and 6 implants (15%) were diagnosed with PI. The implants belonged to seven different implant systems (Table 1). The characteristics of the excluded implants (n = 26) are summarized in Table S1.
| Demographic and implant characteristics | |
|---|---|
| Age (SD) | 65.6 (8.8) |
| Gender: Female/male | 28 (68%)/13 (32%) |
| Smoking status: Non-smoker/smoker | 36 (88%)/5 (12%) |
| Dentition status: FE/PE | 24 (59%)/17 (41%) |
| Functional loading, years (SD) | 7.2 (5.5) |
| Individuals with: | |
| Implant in function <5 years | 20 (49%) |
| Implant in function ≥5 years | 21 (51%) |
| Implant disease status: | |
| HI | 11 (27%) |
| PM | 24 (58%) |
| PI | 6 (15%) |
| PPD at sampled site, mm (SD) | 4.2 (1.1) |
| Individuals with: | |
| PPD <5 mm at implant | 26 (63%) |
| PPD ≥5 mm at implant | 15 (37%) |
| BoP at sampled site: No/yes | 11 (27%)/30 (73%) |
| Implant site | |
| Maxilla, Anterior | 3 (7%) |
| Maxilla, Posterior | 8 (20%) |
| Mandible, Anterior | 18 (44%) |
| Mandible, Posterior | 12 (29%) |
| Implant types | |
| Straumann | 12 (29%) |
| Nobel/branemark | 9 (22%) |
| 3i | 12 (29%) |
| Astra | 5 (12%) |
| Other | 3 (7%) |
- Note:Values represent mean (SD) or frequencies (%).
- Abbreviations: BoP, bleeding on probing at sampled site; FE, fully edentulous; HI, peri-implant health; PE, partially edentulous; PI, peri-implantitis; PM, Peri-implant mucositis; PPD, probing pocket depth; SD, standard deviation.
3.2 Sequencing results
After quality filtering, denoising, chimera removal, and removal of contaminants, 178,239 reads from 41 samples were clustered into OTUs (mean: 4347 reads per sample, SD: 2326, range: 1243–10,829). The subsampled OTU table of the 41 samples (1200 reads/sample) contained 489 OTUs, with an average of 53 (SD: 22, range: 14–114) OTUs per sample. The reads were classified using HOMD into 10 phyla: Firmicutes (41.2%), Proteobacteria (16.9%), Bacteroidetes (15.9%), Actinobacteria (13.4%), Fusobacteria (10.5%), Spirochaetes (0.9%), Synergistetes (0.3%), Saccharibacteria TM7 (0.3%), Chloroflexi (0.01%), and Gracilibacteria GN02 (0.03%). Some OTUs (0.5% of reads) could only be classified as bacteria. The OTUs were further classified into 23 classes, 39 orders, 68 families, and 124 genera.
3.3 Microbial profile analyses
PCA followed by PERMANOVA revealed significant differences in microbial profiles by PPD (F = 3.931, p = .0001), disease status (F = 1.716, p = .017), dentition status (F = 1.941, p = .020), and implant location (F = 1.927, p = .020) (Table 2, Figure 2). After FDR correction at 5%, all the aforementioned parameters remained statistically significant (Table 2). BoP, implant type, time of functional loading, and gender were not significantly associated with the composition of the peri-implant microbiome (p > .05) (Table 2). Since only 5 out of 41 patients were smokers, analysis on smoking habit was not performed. MaAsLin did not detect any associations of a specific microbial community member with clinical metadata. The same held true, when MaAs Lin was repeated using only four covariates (PPD, dentition status, implant disease status, and implant location).
| Parameter | Test value, p value | FDR corrected p value |
|---|---|---|
| Dentition status (PE vs. FE) | F = 1.941, p = .020 | 0.045 |
| Implant disease status (HI vs. PM vs PI) | F = 1.716, p = .017 | 0.045 |
| PPD (<5 mm vs. ≥5 mm) | F = 3.931, p = .0001 | .004 |
| BoP (presence vs. absence) | F = 1.260, p = .183 | .235 |
| Implant location (maxilla vs. mandible) | F = 1.927, p = .020 | .045 |
| Implant system (Straumann vs. Nobel vs. 3i vs. Astra vs. other) | F = 1.107, p = .245 | .245 |
| Functional loading time (<5 vs. ≥5 years) | F = 1.223, p = .208 | .235 |
| Gender (male vs. female) | F = 1.471, p = .095 | .142 |
- Abbreviations: BoP, bleeding on probing; FDR, false discovery rate; FE, fully edentulous; HI, healthy implant; PE, partially edentulous; PI, peri-implantitis; PM, peri-implant mucositis; PPD, probing pocket depth.
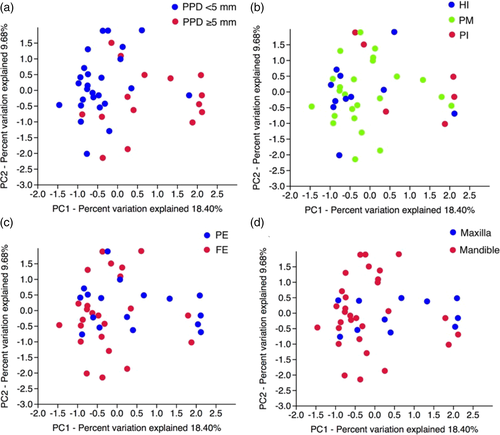
The four variables that were identified as significant from PERMANOVA were tested for possible associations between each other using the Chi-square test. Disease status was significantly associated with PPD (p = .009), and implant location was significantly associated with dentition status (p < .001), PPD (p = .008), and implant disease status (p = .026). Therefore, any possible impact of implant location on the composition of the peri-implant microbiome would be masked by the aforementioned associations. On this premise, implant location was excluded from further analyses.
LEfSe was used to determine which OTUs most likely explain the observed differences between groups by PPD, implant disease status, and dentition status. From all OTUs, nine OTUs significantly discriminated between shallow and deep pockets, four OTUs significantly discriminated between HI and PI, and five OTUs significantly discriminated between PE and FE individuals (p < .05, LDA > 4.0 for all parameters). Figure 3 illustrates the output of the LEfSe analyses. Figure 4 depicts the relative abundance of OTUs, which differed significantly by PPD, disease status, and dentition status, based on the LEfSE LDA scores and the Mann–Whitney U test or Kruskal–Wallis test.
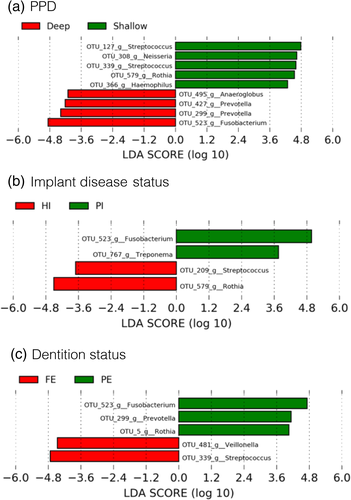
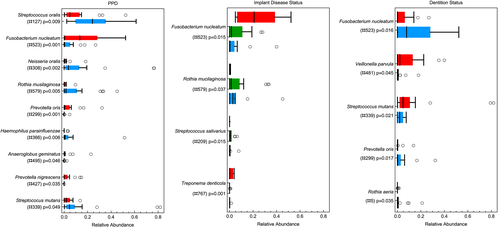
Deep pockets harboured significantly higher proportions of OTUs: #523 (Fusobacterium nucleatum) (p = .001), #299 (Prevotella oris) (p = .001), #427 (Prevotella nigrescens) (p = .035), and #495 (Anaeroglobus geminatus) (p = .046) compared with shallow pockets that harboured more OTUs#579 (Rothia mucilaginosa) (p = .005), #308 (Neisseria oralis) (p = .002), #366 (Haemophilus parainfluenzae) (p = .006), #127 (Streptococcus oralis) (p = .009), and #339 (Streptococcus mutans) (p = .049) (Figure 4a). Implants diagnosed with PI were colonized by higher proportions of OTUs #523 (Fusobacterium nucleatum) (p = .015) and #767 (Treponema denticola) (p = .001) compared with HI and PM, which were colonized by higher proportions of#579 (Rothia mucilaginosa) (p = .037) and #209 (Streptococcus salivarius) (p = .015) (Figure 4b). Interestingly, the microbial profiles of HI and PM did not differ significantly (p > .05 for all four OTUs, data not shown). PE individuals showed significantly more OTUs #523 (Fusobacterium nucleatum) (p = .016), #299 (Prevotella oris) (p = .017), and #5 (Rothia aeria) (p = .035), whereas FE individuals presented more OTUs #481 (Veillonella parvula) (p = .045) and #339 (Streptococcus mutans) (p = .021) (Figure 4c). The alpha diversity indices Shannon, Chao1, and observed OTUs did not present any statistically significant differences for PPD and dentition status. No statistical tests were performed to compare the alpha diversity indices among HI, PM, and PI, because the patients were unevenly distributed among the health/disease categories, which did not allow to draw any meaningful conclusions (Table 3).
| Sample source | Shannon index | Chao1 index | No. of OTUs |
|---|---|---|---|
| PPD | |||
| <5 mm (n = 26) | 3.1 (1.1–4.5) | 78.5 (19.0–177.7) | 49.5 (14–114) |
| ≥5 mm (n = 15) | 3.7 (2.2–4.2) | 68.0 (30.2–184.7) | 49 (29–105) |
| p = .201 | p = .445 | p = .947 | |
| Disease status | |||
| HI (n = 11) | 3.6 (1.8–4.5) | 91 (38–154.2) | 59 (23–88) |
| PM (n = 24) | 3.1 (1.1–4.5) | 63.6 (19–177.7) | 44 (14–114) |
| PI (n = 6) | 4.0 (3.2–4.2) | 70.6 (53.5–184.7) | 58 (49–105) |
| N/Aa | N/Aa | N/Aa | |
| Dentition status | |||
| PE (n = 17) | 3.3 (1.7–4.5) | 79.0 (52.3–184.7) | 55 (37–114) |
| FE (n = 24) | 3.3 (1.1–4.5) | 64.0 (19.0–127.2) | 45.5 (14–94) |
| p = .634 | p = .153 | p = .080 |
- Note: Data are presented as median (range). Between-group differences at alpha diversity indices were assessed using Mann–Whitney U test.
- Abbreviations: FE, fully edentulous; HI, healthy implant; OTUs, operational taxonomic units; PE, partially edentulous; PI, peri-implantitis; PM, peri-implant mucositis; PPD, probing pocket depth; SD, standard deviation.
- a No statistical tests were performed to compare the alpha diversity among HI, PM, and PI, becausethe PI group had very small sample size relative to HI and PM, which did not allow to draw any meaningful conclusions.
4 DISCUSSION
Ecological changes in the peri-implant submucosal sites may lead to shifts in the microbiome, providing favourable conditions for the overgrowth of potential pathogenic bacteria (dysbiosis), thus increasing the host's odds to develop peri-implantitis (Kroger et al., 2018). This paradigm has been proposed for periodontitis (Hajishengallis et al., 2020; Loos & Van Dyke, 2020). To the best of our knowledge, this is the first study investigating the association of parameters such as implant disease status, dentition status, gender, implant location, implant system, time of functional loading, PPD, and BoP on the peri-implant microbiome using next-generation sequencing. In the present study, we found significant associations of the bacterial communities with the following factors: PPD, implant disease status, and dentition status.
Deep peri-implant pockets had a higher relative abundance compared with shallow pockets of anaerobic Gram-negative bacteria of the following genera: Fusobacterium, Prevotella, and Anaeroglobus. Fusobacterium and Prevotella are pathogens, which have been associated with periodontitis and increased pocket depths (Wolff et al., 1993; van Winkelhoff et al., 2002; Socransky & Haffajee, 2005). In addition to the classical periodontopathogens, other microorganisms including Anaeroglobus have been associated with periodontitis (Fernandez et al., 2017). Furthermore, the presence of Anaeroglobus in the oral cavity has been associated with symptomatic atherosclerosis and new-onset rheumatoid arthritis (Scher et al., 2012; Fåk et al., 2015). In contrast, pockets <5 mm were mostly inhabited by aerobic and facultative anaerobic bacteria belonging to the genera Rothia, Neisseria, Haemophilus, and Streptococcus. A recent study that characterized the submucosal microbiome of PI at different severity levels reported that increased PPD is associated with a shift in submucosal microbiome favouring the growth of anaerobes, which outcompeted the health-associated genera Rothia, Neisseria, and Streptococcus (Kroger et al., 2018).
The biofilm of PI sites presented a different microbial composition compared with HI or PM. We observed that the microbial characteristics of PM were more similar to HI than PI. PI sites presented significantly higher proportions of Fusobacterium and Treponema (Socransky & Haffajee, 2005). HI and PM sites presented higher proportions of the genera Rothia and Streptococcus when compared with PI sites. These results are in line with other studies that compared the microbiome of healthy and diseased implants using pyrosequencing and reported that species of the genus Streptococcus were mostly associated with peri-implant health (Kumar et al., 2012; Dabdoub et al., 2013; D. Daubert et al., 2018), whereas Fusobacterium andTreponema were more abundant in disease (Dabdoub et al., 2013; Zheng et al., 2015). Similar studies that used open-ended techniques other than pyrosequencing corroborate these findings (Koyanagi et al., 2010; Koyanagi et al., 2013) and further report on Rothia, which was mostly associated with health (Sanz-Martin et al., 2017). It is worth noting that the aforementioned studies detected more genera that showed statistically significant differences between healthy and diseased implants, such as Porphyromonas, Filifactor, Veillonella, Fretibacterium, Tannerella, Campylobacter, Eubacterium, Chlorofexi, Tenericutes, Synergisetes, Desulfobulbus, Dialister, and Mitsukella, which were mostly present in PI and Neisseria, Veillonella, Haemophilus, Actinomyces, Atopobium, Gemella, Kingella, Leptotrichia, Propionibacter, and Capnocytophaga, which were mostly associated with health (Koyanagi et al., 2010; Kumar et al., 2012; da Silva et al., 2014; Zheng et al., 2015; Belibasakis et al., 2016; Sanz-Martin et al., 2017; Al-Ahmad et al., 2018; D. Daubert et al., 2018). The fact that we identified only a few genera associated with implant disease status could possibly be attributed to the fact that the aforementioned studies had higher subsampling depth, and more even sample distribution by disease status, as compared with the present study, which included only six PI patients. Furthermore, in the present study, we used a more stringent LDA threshold of 4.0 for the LEfSe analysis.
PE patients harboured significantly higher proportions of Fusobacterium, Prevotella, and Rothia compared with FE patients. Fusobacterium and Prevotella are also detected in PI, whereas Rothia has been associated with health (Socransky & Haffajee, 2005; da Silva et al., 2014). The genera Veillonella and Streptococcus, which have been associated with health (da Silva et al., 2014; Al-Ahmad et al., 2018), were detected in higher proportions in FE patients. In agreement with our results, the microbial colonization of dental implants in FE patients has been characterized by lower proportions of microorganisms and less pathogenic microbiota compared with dentate patients (Apse et al., 1989; Kocar et al., 2010; Quirynen & Van Assche, 2012; Siddiqi et al., 2016). Other studies, however, did not find any differences in the peri-implant microbiota between PE and FE (Hultin et al., 1998).
The factors BoP, implant system, time of functional loading, and gender did not seem to be associated with the composition of the peri-implant biofilms. To the best of our knowledge, there are no studies on the relationship between inflammation and the submucosal peri-implant microbiota using next-generation sequencing techniques. Two studies however, examined the relationship between clinical inflammation (presence or absence of BoP) and the subgingival microbiota in chronic periodontitis using pyrosequencing of 16S rRNA gene and reported conflicting results (Abusleme et al., 2013; Camelo-Castillo et al., 2015). Abusleme et al. reported that inflammation was not associated with a distinct microbiome, whereas Camelo-Castillo et al. showed that increased inflammation was associated with more diverse microbiota and higher abundance of Desulfobulbus, Eubacterium, Filifactor, Streptococcus, Tannerella, and Treponema (Abusleme et al., 2013; Camelo-Castillo et al., 2015). A third study, which examined the subgingival microbiome of restored and unrestored teeth, reported differences in the microbial profiles between bleeding and non-bleeding restored sites; Prevotella and Treponema were detected in higher abundance in bleeding sites, whereas Enterococcus was associated with non-bleeding sites (Rademacher et al., 2019). In accordance with our results, the implant system is not associated with the composition of the submucosal microbiome of peri-implant sites (Sanz-Martin et al., 2017). Regarding the time of functional loading of the implant, it has been reported that the microbial complexity increased with longer loading times, but a history of periodontitis had a greater impact on the peri-implant microbiota than loading time (Lee et al., 1999). As far as gender is concerned, it has been shown that female sex hormones affect the microbial profiles in many sites of the body, especially the gut (Neuman et al., 2015). Nevertheless, regarding oral microbiota, a review by Kumar (Kumar, 2013) concluded that there is no definitive evidence to indicate gender-specific differences in the subgingival microbiome. Furthermore, most data on the impact of gender on the composition of oral microbiome are based on females of reproductive age (Kumar, 2013). Here, the female patients were between 49 and 83 years of age (mean: 66.5 years); therefore, presenting most probably reduced levels of sex hormones.
In the present study, the microbial communities did not differ significantly in alpha diversity by PPD. These results are in contrast with a study that examined the submucosal microbiome of PI lesions at different severity levels, and reported that the alpha diversity was significantly decreased in samples with deeper pockets as compared with shallow pockets (Kroger et al., 2018). The aforementioned study, however, included only PI cases and defined shallow pockets as ≤7 mm and deep pockets as >7 mm, whereas in the present study the majority of participants was diagnosed with HI or PM and a PPD of 5 mm was used to distinguish between shallow and deep pockets (Kroger et al., 2018). Furthermore, the three alpha diversity indices did not differ by dentition status. To the best of the authors' knowledge, no other studies have compared the peri-implant microbiome between PE and FE, using open-ended techniques. Regarding the implant disease status, the sample distribution in this study was too skewed to draw any meaningful conclusions. Nevertheless, previous studies, which compared the microbiome of healthy and diseased peri-implant sites, reported that diseased sites presented higher alpha diversity (Zheng et al., 2015; Sanz-Martin et al., 2017) or lower alpha diversity compared with healthy sites (Apatzidou et al., 2017; D. Daubert et al., 2018). Yet, a study by Dabdoub et al. (Dabdoub et al., 2013) did not find any difference in Shannon diversity index between healthy and diseased implants. The aforementioned study, however, included in diseased implants both PM and PI. Therefore, differences between studies in microbiome characterization could be attributed to differences in disease definition, the presence of confounding factors such as smoking, differences in sampling technique, different microenvironment, subject to subject variation, or even geographical variations.
Even though the sample size of the present study seems adequate compared with similar studies (Koyanagi et al., 2010; da Silva et al., 2014; Zheng et al., 2015; Apatzidou et al., 2017; Al-Ahmad et al., 2018; D. Daubert et al., 2018; Kroger et al., 2018), some factors such as smoking habit or implants diagnosed with PI are not evenly distributed among the study participants, which makes comparisons difficult. Furthermore, information on history of periodontal disease was not available for all patients; therefore, this parameter could not be evaluated in association with peri-implant microbiome. The sampling technique employed in the present study was based on the use of sterile paperpoints. While these samples were taken and stored, we discovered later that the paperpoints can harbour exogenous DNA of non-oral microorganisms and we therefore recommended the use of sterile curettes when using DNA-based techniques (van der Horst et al., 2013). Although in the present study we subtracted the contaminants originating from the paperpoints, we can still not preclude the effects of foreign DNA on microbial profiling results (Salter et al., 2014). Another limitation of the present study is the low-depth coverage, which precludes the detection of rare members of the microbial community, which might be highly virulent (Charalampakis & Belibasakis, 2015). It has been reported that the accuracy of species-level identification on regions of 16S rRNA gene is limited; therefore, the species names assigned to the representative OTU sequences may not be accurate (Edgar, 2018). This study focuses on bacterial taxonomy in relation to several patient- and implant-related parameters, and no metagenome predictions tools (e.g., PICRUSt) of the functional profiles of the microbial communities were applied. Finally, although we acknowledge that multivariate analysis by linear models such as MaAsLin can be a useful tool to find associations between microbial profiles and clinical metadata, its use in the present study did not yield significant results. This could be due to the relation between the size of the study population and the number of variables. We would therefore recommend for future research a more extensive study including a few hundred patients (Morgan et al., 2015; Swarte & Douwes, 2020) and using a multivariate analysis, such as MaAsLin, to further confirm and strengthen our findings.
In conclusion, we report differences in the composition of peri-implant microbiota based on PPD, implant disease status, and dentition status. Well-recognized periodontal pathogens such as Fusobacterium, Prevotella, and Treponema were present in higher proportions in deep peri-implant pockets, PI, and PE individuals. Our results add to the knowledge that the microbiome of peri-implant sites shares common features with the periodontal microbiome.
ACKNOWLEDGEMENTS
We thank Evangelos Sfakianakis for his support in constructing the graphs of this study.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest in this study. This study was partly supported by the German Society of Periodontology (DGP) and Biomet 3i from the Periimplantitis Forschungsfonds 2011.
ETHICS STATEMENT
The study was approved by the ethical committee of the VU Medical Centre, Amsterdam (#2011/370). The study was conducted in accordance with the guidelines of the World Medical Association Declaration of Helsinki.
AUTHOR CONTRIBUTIONS
Angeliki Polymeri: Analysed the data; drafted the manuscript; critically revised the manuscript and gave final approval of the submitted version. Joyce van der Horst: Performed the clinical examination; collected the biofilm samples; critically revised the manuscript and gave final approval of the submitted version; critically revised the manuscript and gave final approval of the submitted version. Mark J. Buijs: Performed DNA extraction and sequencing; critically revised the manuscript and gave final approval of the submitted version. Egija Zaura: Conceived the idea; designed the study; achieved financial support; analysed the data; critically revised the manuscript and gave final approval of the submitted version. Daniel Wismeijer: Conceived the idea; designed the study; achieved financial support; critically revised the manuscript and gave final approval of the submitted version. Wim Crielaard: Conceived the idea; designed the study; achieved financial support; critically revised the manuscript and gave final approval of the submitted version. Bruno G. Loos: Conceived the idea; designed the study; achieved financial support; critically revised the manuscript and gave final approval of the submitted version. Marja L. Laine: Conceived the idea; designed the study; achieved financial support; analysed the data; critically revised the manuscript and gave final approval of the submitted version. Bernd W. Brandt: Analysed the data; critically revised the manuscript and gave final approval of the submitted version.
Open Research
DATA AVAILABILITY STATEMENT
The raw sequence data and metadata that support the findings of this study are openly available in the NCBI BioProject database under accession number PRJNA694635 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA694635/).




