A 78-year-old woman with diffuse white matter infiltration and predominant involvement of bilateral temporo-parieto-occipital regions
Abstract
Diffuse paediatric-type high-grade glioma, H3-wildtype and IDH-wildtype (H3/IDH-wt-pHGG) is a newly defined entity amongst brain tumours, primarily reported in children. It is a rare, ill-defined type of tumour and the only method to diagnose it is DNA methylation profiling. The case we report here carries new knowledge about this tumour which may, in fact, occur in elderly patients, be devoid of evocative genomic abnormalities reported in children and harbour a misleading mutation.
1 CLINICAL HISTORY
A 78-year-old woman was admitted to the hospital with the chief complaint of language and behaviour issues for 3 weeks. Brain MRI showed a diffuse infiltration with predominant involvement of bilateral temporo-parieto-occipital regions in T2-weighted sequence and foci of patchy enhancement after gadolinium injection (Figure 1A,B). Stereotactic biopsies were performed 10 days after the admission. The patient underwent treatment with temozolomide alone. Three months later, the evolution of the tumour was characterized by an increase in volume and an occurrence of necrotic and contrast-enhanced areas on MRI (Figure 1C–F), as well as a clinical aggravation rapidly leading to the death of the patient.
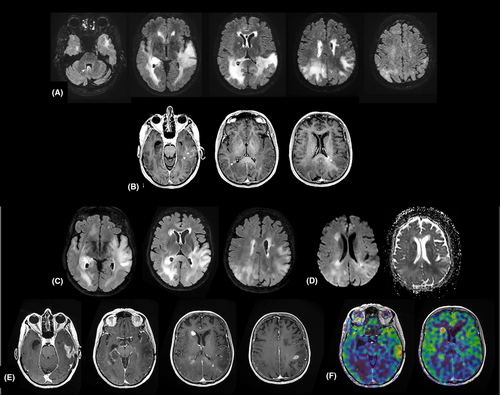
2 FINDINGS
Histological examination showed features of a moderately cellular tumour with some perivascular pseudorosettes, spongioblastic patterns and some microcalcifications without microvascular proliferation and necrosis (Figure 2A,B). It was composed of monotonous cells with delicate processes and an oligo-like appearance. Vessels were small-branched capillaries. After immunohistochemistry, tumour cells were positive for glial markers GFAP and Olig2 but negative for CD34, p53, synaptophysin, IDH1 R132H and FGFR3 (Figure 2C–E). ATRX was maintained. Proliferation index Ki67 was less than 1%. NGS and CGH/SNP array did not find any contributive molecular anomaly. Notably, there was no gain of Chromosome 7, no loss of Chromosome 10, no mutation was found in TERT promoter, IDH1/IDH2, BRAF, H3F3A and HIST1H3B genes (Figure 2F). In addition, no fusion was detected by RNA-Seq (ArcherDx FusionPlex panel). Only a tandem duplication of FGFR1 was detected by droplet PCR. By DNA methylation profiling, the methylation class in the brain classifier v12.8 of DKFZ was ‘Diffuse paediatric type high-grade glioma, H3 wildtype and IDH wildtype’, ‘RTK1 subtype’ with scores at 0.90 and 0.83, respectively.
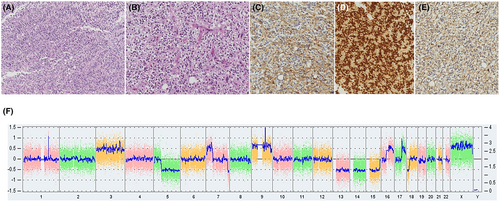
3 DIAGNOSIS
Diffuse paediatric-type high-grade glioma, H3 wildtype and IDH wildtype, World Health Organization (WHO) grade 4.
4 DISCUSSION
Diffuse paediatric-type high-grade glioma, H3-wildtype and IDH-wildtype (H3/IDH-wt-pHGG) has recently been introduced in the 2021 WHO Classification of Tumours of The Central Nervous System. This entity is a diffuse glioma with malignant features, mostly affecting children and adolescents. It is wildtype for the histone H3 genes as well as for IDH1 and IDH2.1 DNA methylation profiling of this group defines three distinct subtypes termed pHGG RTK1, pHGG RTK2 and pHGG MYCN, which are enriched in PDGFRA amplifications, EGFR amplification/TERT promoter mutations and MYCN amplifications, respectively, whereas the chromosomal pattern typical of adult-type glioblastoma Chromosome 7 gain (+7)/Chromosome 10 loss (−10)/EGFRvIII variant is absent. So far, DNA methylation profiling is the only method able to diagnose this new entity. As a rare and recently recognized tumour, H3/IDH-wt-pHGG remains to be further characterized in a clinicopathological point of view. Notably, its occurrence in the adult population remains unknown, as well as its imaging characteristics. Until the characterization by DNA methylation, the tumour in our case remained difficult to classify and the eventuality of a low-grade glioma was not ruled out. Furthermore, the detection of a tandem duplication of FGFR1, an abnormality frequently associated with low-grade gliomas, rendered the classification more confusing in this glioma of low-grade morphology. Despite a high score, the result of DNA methylation profiling was initially doubtful due to discordance with the low-grade morphology of the tumour and the context. However, this result ended up being consistent with the evolution of the tumour 3 months later evocative of an HGG. This observation is informative on an epidemiological and clinicopathological point of view. It shows that H3/IDH-wt-pHGG may occur, and are probably underestimated, in elderly patients, as recently observed by Bender et al.2 Moreover, H3/IDH-wt-pHGG may present as a diffuse glioma without frank enhancement on MRI. The bilateral involvement, present in our case, and described in children,3 could be a common presentation. Lastly, H3/IDH-wt-pHGG may present as a low-grade glioma upon histological examination and may be associated with a potentially misleading FGFR1 tandem duplication rather than the more evocative anomalies reported in this entity.
AUTHOR CONTRIBUTIONS
Charlotte Paoli: Writing – original draft (equal). Anne Mc Leer: Formal analysis (equal); writing – review and editing (equal). Julien Boyer: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Lydiane Mondot: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Bérengère Dadone-Montaudié: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Catherine Godfraind: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Fanny Burel-Vandenbos: Conceptualization (lead); supervision (lead); writing – review and editing (lead).
ACKNOWLEDGEMENTS
The authors thank Ms. Jessica Zufelt for her help with the English language.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
PATIENT CONSENT
Each patient whose case is reviewed in the national Network RENOCLIP-LOC is informed and gives consent.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




