RETRACTED: Celastrol inhibit the proliferation, invasion and migration of human cervical HeLa cancer cells through down-regulation of MMP-2 and MMP-9
Jing Zhang and Ranran Wang contributed to this work equally
Funding information
None.
Abstract
The present study evaluated the anticancer potential of celastrol through down-regulation of matrix metalloproteinase-2 (MMP-2) and MMP-9. HeLa cells were incubated with different concentrations of celastrol (1, 10 and 100 µM) for 48h. Doxorubicin was used as a reference drug. Cancer cell migration, apoptosis, cell viability and mitochondrial fragmentation were evaluated following celastrol treatment. In addition, the expression level of MMP-2, MMP-9 and caspase-3 was evaluated following celastrol treatment. HeLa cell viability was 94.1 ± 7, 53.4 ± 4 and 36.3 ± 2% at 1-100 µM of celastrol, respectively. Apoptotic cell numbers were increased, and inhibition of larger wounds in cancer cells was observed following celastrol treatment. Celastrol-treated cells showed condensed nuclei and clumped mitochondria. Reduced expression of MMP-2 and MMP-9 and increased expression of caspase-3 were observed following celastrol treatment. Based on the experimental results, we are concluding that the celastrol was effective against HeLa cervical cancer cells.
1 INTRODUCTION
Cervical cancer is the frequently occurring cancer type in female population worldwide.1 Few plant-derived drugs with a good side-effects profile are available for treating cervical cancer.2 Researchers have reported that the cervical cancer develops due to cultural and lifestyle factors, lack of access to health care and exposure to human papillomaviruses.3 Researchers have reported that the plant-derived natural agents induce apoptosis and exert anticancer effects.4 Celastrol is a quinine methide triterpene isolated from Tripterygium wilfordii Hook F with anticancer potential.5 Zhou et al6 reported that the therapeutic effect of celastrol is exerted through various cellular and biological pathways. Qi et al7 reported that the cytotoxic effect of celastrol was achieved in human colon cancer cells through the suppression of tryptophan metabolism. Thus, the new therapeutic drug is required to inhibit growth and proliferation of cervical cancer. Our study analysed the celastrol mediated inhibition of proliferation, invasion and migration of human cervical HeLa cancer cells through down-regulation of matrix metalloproteinase-2 (MMP-2) and MMP-9.
2 MATERIALS AND METHODS
2.1 Materials
Celastrol (C0869 Shangai, China), foetal bovine serum (FBS), antibiotics and RPMI medium were obtained from Sigma-Aldrich (Shanghai, China). Primary antibodies of MMP-2 (ab97779), MMP-9 (ab38898) and caspase-3 (a190437) were purchased from Abcam, Cambridge, UK. Acridine orange (AO, A9231) and ethidium bromide (EB, E8751) were obtained from Sigma-Aldrich (Shanghai, China).
2.2 Cell culture
Human HeLa cervical cancer and primary cervical epithelial cells (PCS-480-011) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were supplemented with RPMI medium, FBS (10%) and antibiotics (1%) and maintained in a CO2 incubator at 37˚C. cervical cancer and epithelial cells were cultured for 24 h, and then treated with celastrol (1, 10 and 100 µM) for 48 h. Doxorubicin (1 µM) was used as a reference drug.
The growth-inhibiting effects of celastrol on human HeLa cervical cancer cells and primary cervical epithelial cells were investigated by Sulphorhodamine B assay.7 Wound healing and migration assays were assessed using a previously reported method.7AO/EB staining is commonly used for evaluating apoptosis.7 Mitochondrial fragmentation of human cervical cells was assessed using a previously reported method.8 The mRNA expression was determined according to a previously reported method.7 The specific primers used for the amplification of MMP-2, MMP-9 and caspase-3 are shown in Table S1. Western blot analysis was used to evaluate the protein levels in the cancer cells according to a previously described method.7 Immunofluorescence was performed according to previously reported method.7
2.3 Statistical analyses
Experimental values are given as the means with standard error of the mean (SEM). The differences between the control and celastrol-treated groups were evaluated using Student's t test and analysis of variance (ANOVA). P <.05 was taken as significant.
3 RESULTS
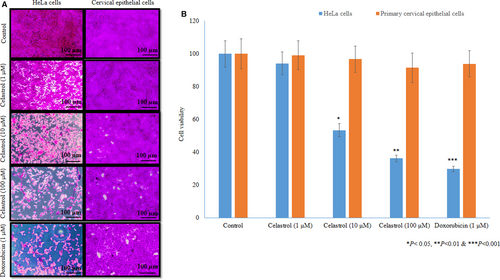
Cells were treated with different concentrations of celastrol for 48 h, and the cell viability was 94.1 ± 7, 53.4 ± 4 and 36.3 ± 2%, respectively (P <.05, Figure 1A & B), which shows indicates the cytotoxic effect of celastrol on cervical cancer cells. Epithelial cell viability was 99.1 ± 8.7, 96.7 ± 8 and 91.5 ± 9% at 1, 10 and 100 µM of celastrol, respectively (P <.05, Figure 1A & B), which shows no cytotoxic potential of celastrol against primary cervical epithelial cell proliferation.
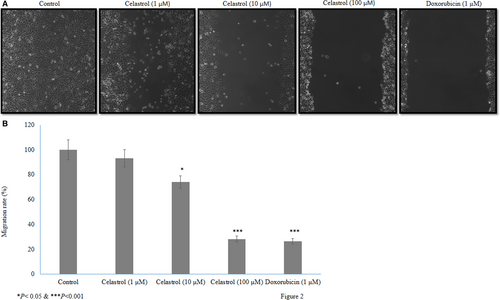
Celastrol inhibited human cervical cancer cell migration. Higher concentrations of celastrol reduced the larger wounds formation (Figure 2A). The 10 and 100 µM of celastrol reduced cancer cell motility, by 25.9% and 71.7%, respectively (P <.05, Figure 2B). Celastrol treatment increased early apoptotic and apoptotic human cervical cancer cell expression more (P <.05, Figure S1, Table S2). MitoTracker Red and Hoechst 33 258 stains were used to analyse the effect of celastrol on mitochondrial fragmentation. Control cancer cells showed normal morphology, whereas celastrol-treated cells showed condensed nuclei and clumped mitochondria (Figure S2). Celastrol increased the mRNA and protein expression of caspase-3 and reduced the mRNA and protein expression of MMP-2 and MMP-9 in a dose-dependent manner (P <.05, Figure S3A-C). In addition, immunohistochemical analysis, celastrol increased the protein expression of caspase-3 and reduced MMP-2 and MMP-9 in a dose-dependent manner P <.05, Figures S4, S5),
4 DISCUSSION
Cervical cancer is the frequently occurring cancer type in female population worldwide.1 Few plant-derived drugs with good side-effect profiles are available for treating cervical cancer.2 Researchers have reported that cervical cancer develops due to cultural and lifestyle factors, lack of access to health care and exposure to human papillomaviruses.3 Researchers have reported that the plant-derived natural agents induce apoptosis and exert anticancer effects.4 Natural products such as celastrol were effective against ovarian cancers.9 Bufu et al10 reported that the anti-proliferative effect of celastrol against colorectal cancer was exerted through inhibition of MMP-3 and MMP-7, and migration. In this study, we evaluated the anticancer potential of celastrol through down-regulation of matrix metalloproteinase-2 (MMP-2) and MMP-9. Our results showed that the celastrol decreased cervical cancer cell proliferation, and the expression of MMP-2 and MMP-9, indicating reduced cell migration. Our results agreed with Hu et al,5 who reported that celastrol inhibited cervical cancer cell proliferation and migration.
Celastrol inhibited cervical cancer cell viability than doxorubicin. Epithelial cell viability was not altered significantly, which indicates that celastrol does not exert cytotoxic effects on primary cervical epithelial cells. However, 1 mM of celastrol reduced cervical epithelial cell viability. HeLa cells treated with celastrol increased apoptosis, and apoptosis is a programmed death cancerous cells.11 Celastrol exhibits anticancer activity through inhibition of cell proliferation and induction of apoptosis.9 Bioactive compounds mediated inhibition of MMP-2 and MMP-9 expression leads to inhibition of cancer cell metastasis.12 Furthermore, Hu et al5 reported that celastrol inhibited HeLa cell metastasis by inhibiting proliferation and migration.
Mitochondrial fragmentation is an important pathological marker in cancer research and therapeutics. MitoTracker Red and Hoechst 33 258 were used to analyse the effect of celastrol on mitochondrial fragmentation. Control cancer cells showed normal morphology, whereas celastrol-treated cells showed condensed nuclei and clumped mitochondria. Anticancer effects of drugs have been reported through the induction of mitochondrial fragmentation in cancer cells.13 Caspase-3 is an important regulator of the apoptotic response during tumorigenesis.14 Anticancer effects of compounds have been reported through the up-regulation of caspase-3 in cancer cells.15 In this study of celastrol, caspase-3 expression was increased in HeLa cells, which was degree comparable to doxorubicin. Researchers have reported the celastrol inhibited the expression of MMP-2 and MMP-9, and which leads anticancer activity in ovarian cancer cells. Celastrol exhibits anticancer activity by inducing cytotoxicity, apoptosis, mitochondrial fragmentation and the expression of caspase-3. Celastrol inhibits cervical cancer cell migration and MMP-2/9 expression. Based on the experimental results, we are concluding that the celastrol was effective against HeLa cervical cancer cells.
ACKNOWLEDGEMENT
None.
CONFLICT OF INTEREST
All authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTION
Jing Zhang: Formal analysis (equal); Funding acquisition (equal); Investigation (equal). Ranran Wang : Conceptualization (equal); Data curation (equal); Validation (equal). Li Cheng : Funding acquisition (equal); Project administration (equal); Visualization (equal). Haisheng Xu: Supervision (equal); Validation (equal); Writing-original draft (equal); Writing-review & editing (equal).
Open Research
DATA AVAILABILITY STATEMENT
The data and material during the current study are available from the corresponding author on reasonable request.




