Proteolytic Shedding of Human Colony-Stimulating Factor 1 Receptor and its implication
Yue Wei, Menghui Ma, Sheng Lin contributed equally to this study.
Abstract
Both Colony-stimulating factor 1 receptor (CSF1R) and triggering receptor expressed on myeloid cells-2 (TREM2) are trans-membrane receptors and are expressed in the brain primarily by microglia. Mutations in these two microglia-expressed genes associated with neurodegenerative disease have recently been grouped under the term “microgliopathy”. Several literatures have indicated that CSF1R and TREM2 encounters a stepwise shedding and TREM2 variants impair or accelerate the processing. However, whether CSF1R variant affects the shedding of CSF1R remains elusive. Here, plasmids containing human CSF1R or TREM2 were transiently transfected into the human embryonic kidney (HEK) 293T cells. Using Western Blot and/or ELISA assay, we demonstrated that, similar to those of TREM2, an N-terminal fragment (NTF) shedding of CSF1R ectodomain and a subsequent C-terminal fragment (CTF) of CSF1R intra-membrane were generated by a disintegrin and metalloprotease (ADAM) family member and by γ-secretase, respectively. And the shedding was inhibited by treatment with Batimastat, an ADAM inhibitor, or DAPT or compound E, a γ-secretase inhibitor. Importantly, we show that the cleaved fragments, both extracellular domain and intracellular domain of a common disease associated I794T variant, were decreased significantly. Together, our studies demonstrate a stepwise approach of human CSF1R cleavage and contribute to understand the pathogenicity of CSF1R I794T variant in adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP). These studies also suggest that the cleaved ectodomain fragment released from CSF1R may be proposed as a diagnostic biomarker for ALSP.
1 INTRODUCTION
Colony-stimulating factor 1 receptor (CSF1R), a single trans-membrane receptor containing a ligand-binding domain in the extracellular region and a tyrosine kinase domain in the cytoplasm, is predominantly expressed in monocyte, macrophage and bone marrow cell precursors, as well as microglia in central nervous system (CNS).1, 2 Studies have revealed that people with CSF1R variants bear an adult-onset neurodegenerative disorder, named adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP).2, 3 Furthermore, genetic variants of triggering receptor expressed on myeloid cells 2 (TREM2), a trans-membrane receptor specifically expressed in microglia in the brain, increase the risk of developing neurodegenerative disorders including Alzheimer's disease (AD).4 Studies have shown that TREM2 undergoes a proteolytic processing.5 T66 M or Y38C mutation in the immunoglobulin (Ig)-like extracellular domain of TREM2 results in reduced shedding 6 whereas TREM2 H157Y variant leads to enhanced proteolysis.7 CSF1R has been reported to be involved in two separate cleavage events.8, 9 As a pathogenic variant for ALSP, I794T is the most common variant of CSF1R among the identified seventy more variants.2 However, whether CSF1R undergoes a proteolytic cleavage similar to that of TREM2 and whether CSF1R variant affects the proteolytic shedding of CSF1R fragments remain largely elusive.
This study demonstrates a two-step proteolytic processing of CSF1R and the decreased cleavage fragments of disease-associated CSF1R I794T variant, indicating that the extracellular fragment of CSF1R may be a potential biomarker for the early diagnosis of ALSP.
2 MATERIALS AND METHODS
2.1 Reagents and Antibodies
Batimastat and compound E were purchased from Millipore. DAPT was bought from Sigma-Aldrich. Cycloheximide (CHX) was from APExBIO and CSF1 was from R&D Systems. The following antibodies, GFP Tag antibody (RRID: AB_11182611) and HA Tag antibody (RRID: AB_1104232), were from Proteintech. Anti-MCSF receptor antibody (peptide selected from 955-972; RRID: AB_776253) was from Abcam and c-Fms/CSF-1R antibody (B-8) (peptide selected from 11-310; RRID: AB_627620) from Santa Cruz. Phospho-CSF1R (Tyr723) (49C10) rabbit mAb was bought from Cell Signaling Technology. Goat anti-mouse or anti-rabbit IgG (H + L) secondary antibodies were all from Thermo (RRID: AB_228307; RRID: AB_228341).
2.2 cDNA Constructs
The vectors used in these experiments are as follows: TREM2-GFP, pEGFP-N1, HA-CSF1R-EGFP, pCMV3.1-HA, CSF1R-NTF-HA, CSF1R-HA, CSF1R-I794T-HA (Figure 1A). The full-length CSF1R in HA-CSF1R-EGFP was obtained from the 972 amino acid of CSF1R in pCMV3-CSF1R-HA (Sino Biological, Beijing, China). Plasmid extraction was performed after sequencing verification.
2.3 Cell Culture and Treatments
HEK 293T cells were cultured in DMEM containing 10% FBS and were transfected with different plasmids using TurboFect transfection reagent (Thermo) according to the manufacturer's specifications. Transfected cells were incubated with or without 10 μM Batimastat, 500 nM compound E or 10 μM DAPT for 16 hours. Cells or medium were then collected for the following Western blotting and/or ELISA assay.
2.4 Western Blot
Equivalent protein samples or supernatant samples were tested by SDS-PAGE and transferred to a PVDF membrane. Proteins bound with primary (1:1000 CSF1R or 1:2000 GFP or 1:10 000 HA) and secondary antibodies were visualized by ECL and analysed by Image J software.
2.5 ELISA assay
The CSF1R-NTF in the conditioned medium was tested using a microplate reader (Thermo, USA) at 450 nm according to the instructions of Human CSF1R ELISA Kit (Sino Biological, Beijing).
2.6 Statistical analysis
Data were represented as mean ± SEM. Statistical significance was determined using two-way analysis of variance (ANOVA) test (GraphPad Prism 7.0) or two-tailed Student's t test. A p value less than 0.05 was considered significant.
3 RESULTS
3.1 Sequential Shedding of the CSF1R by ADAM family and γ-secretase
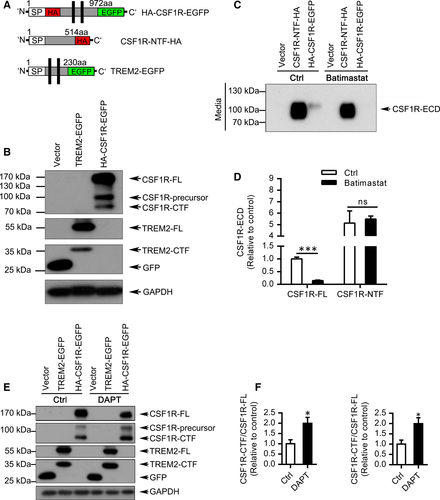
We first analysed whether CSF1R suffers proteolytic shedding at the cell surface. As a positive control, the shedding of TREM2-EGFP was detected simultaneously by Western blotting (Figure 1A-B). Robust levels of CSF1R-precursor, the full length of CSF1R (CSF1R-FL), and the C-terminal fragment of CSF1R (CSF1R-CTF) were detected (Figure 1B).
Given that members of the disintegrin and metalloprotease (ADAM) family are major proteases involved in the proteolytic processing of cell surface proteins, HEK293T cells transfected with CSF1R N-terminal fragment (CSF1R-NTF) or HA-CSF1R-EGFP were incubated with or without Batimastat, a known inhibitor of ADAM families.10 Interestingly, Batimastat treatment resulted in a dramatical decrease of CSF1R ectodomain (CSF1R-ECD) in the medium (Figure 1C-D) and the accumulation of CSF1R-FL (Figure S1A-D) in cell lysates, indicating that the ectodomain of CSF1R can be shed by ADAM and this cleaved CSF1R-ECD can be released into the medium. Previous studies suggested that C terminus of proteins usually selectively processed by γ-secretase.11 Intriguingly, treatment with DAPT, a γ-secretase inhibitor, resulted in a dramatic increase of CSF1R-CTF, which is consistent with the inhibition effects of γ-secretase on TREM2-CTF accumulation (Figure 1E-F). These findings were further confirmed by another γ-secretase inhibitor compound E in which both CSF1R-CTF and TREM2-CTF were notably accumulated (Figure S2A-C).
3.2 Decreased cleavage fragments of disease-associated CSF1R-I794T variant
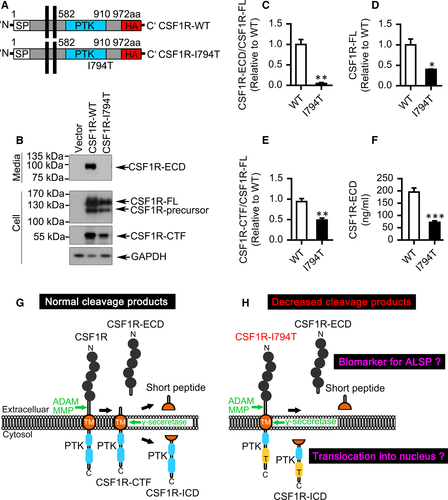
The common mutation CSF1R I794T affects the tyrosine–kinase activity of CSF1R causing ALSP.2 Compared with wild-type (WT) CSF1R, the level of CSF1R-ECD in the CSF1R I794T mutant culture medium was almost undetectable quantified by Western blotting and ELISA assay (Figure 2A-F). Moreover, the expression of CSF1R-ECD or CSF1R-CTF in CSF1R I794T mutant cells was all dramatically reduced, though the full length of CSF1R I794T was also significantly decreased (Figure 2A-F). Unexpectedly, we found that I794T variant did not affect the transcription (Figure S3A) or the stability of CSF1R (Figure S3B-D). Furthermore, the I794T variant cannot be phosphorylated (Y723) in response to CSF1, the ligand of CSF1R (Figure S4A-B). These results indicate the dysfunction of ALSP-associated I794T variant.
4 DISCUSSION
Together, we show that CSF1R undergoes a two-step proteolytic cleavage similar to that of TREM2. Extracellular of the CSF1R ectodomain can be shed by ADAM family and the intramembrane of the CSF1R-CTF can be subsequently cleaved and generated by γ-secretase. Notably, we can detect CSF1R-ECD in the wild-type CSF1R medium whereas CSF1R-ECD from CSF1R-I794T mutant was almost undetectable. Thus, the present data demonstrate a stepwise approach of human CSF1R cleavage and that CSF1R fragments may execute important physiological and pathophysiological functions (Figure 2G-H).
Given that the soluble form of CSF1R (sCSF1R) was detected in goldfish serum 12 and that soluble TREM2 can be detected in human cerebrospinal fluid,13 whether sCSF1R, namely CSF1R-ECD, can be determined in human cerebrospinal fluid or serum of ALSP patients as a biomarker needs to be explored. Whether sCSF1R plays a role in CNS, similar to the function of soluble TREM2 in microglial activation, remains elusive.14
It has been proposed that the cytoplasmic domain of CSF1R can translocate to the nucleus.8 Thus, it will be interesting to investigate whether the nuclear translocation of CSF1R-ICD regulates genes expression. Moreover, due to the dramatically reduced expression of CSF1R-CTF released from CSF1R I794T mutant, it will be interesting to further investigate whether this reduction is a result of the degradable nature of CSF1R I794T mutant or the abnormal shedding of the ALSP-associated I794T variant. Although previous studies showed that I794T and other CSF1R mutant proteins expressed in a factor-dependent cell line are exposed normally on the cell surface,3, 15 this dispute needs to be clarified. Of course, additional experiments, such as samples from CSF1R variant bearing human brain or mutant knock-in animal models, are needed to be performed to see how this variant contributes to the biology of ALSP.
ACKNOWLEDGEMENTS
This work was supported by grants (91949129 and 81771164 to HZ, 81771377 and U1705285 to Y-WZ, 92049202 to XX) from the National Natural Science Foundation of China. This work was also supported by the National Innovative Training Programme for College Students of Xiamen University (2018X0589 to YW and S201910384499 to Y. S.), and the Open Research Funds of State Key Laboratory of Cellular Stress Biology, Xiamen University (SKLCSB2019KF014 to HZ) and the National Key Research and Development Program of China (2018YFC2000400 and 2016YFC1305903 to Y-WZ). This work was also supported by grants from the Natural Science Foundation of Fujian Province of China (2018D0022 and 2020J01014 to HZ). The authors also thank Dr Li Zhong in our Institute of Neuroscience for providing TREM2 plasmids. The cell culture of this work was conducted in the Innovative Experiment Platform,Dept. of Basic Medical Sciences, School of Medicine, Xiamen University. The authors also sincerely thank Rui Huang and Fengguang Gao for cell culture suporting.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTION
Yue Wei: Data curation (equal); Methodology (equal); Software (equal); Writing-original draft (equal). Menghui Ma: Data curation (equal); Methodology (equal); Software (equal); Writing-review & editing (equal). Sheng Lin: Data curation (equal); Methodology (equal); Software (equal); Writing-review & editing (equal). Xin Li: Data curation (equal); Methodology (equal); Software (equal); Writing-review & editing (equal). Yue Shu: Data curation (supporting); Methodology (supporting); Resources (equal); Software (supporting); Validation (supporting); Visualization (supporting); Writing-review & editing (supporting). Ziwei Wang: Data curation (equal); Methodology (supporting); Software (supporting); Validation (supporting); Writing-review & editing (supporting). Yuhang Zhou: Data curation (supporting); Methodology (supporting); Validation (supporting); Writing-review & editing (supporting). Banglian Hu: Data curation (supporting); Methodology (supporting); Validation (supporting). Baoying Cheng: Resources (supporting); Visualization (supporting). Shengshun Duan: Methodology (supporting); Resources (supporting). Xiaohua Huang: Project administration (supporting). Huaxi Xu: Project administration (supporting); Supervision (supporting); Writing-review & editing (supporting). Yun-Wu Zhang: Funding acquisition (equal); Project administration (supporting); Supervision (supporting); Writing-review & editing (supporting). Honghua Zheng: Data curation (supporting); Funding acquisition (lead); Methodology (supporting); Project administration (lead); Software (supporting); Supervision (lead); Writing-original draft (lead); Writing-review & editing (lead).
Open Research
DATA AVAILABILITY STATEMENT
The authors are willing to provide the data related to this manuscript upon reasonable request.




