MicroRNAs: key players of taxane resistance and their therapeutic potential in human cancers
Abstract
The successful long-term use of taxane for cancer therapy is often prevented by the development of drug resistance in clinic. Thus, exploring the mechanisms involved is a first step towards rational strategies to overcome taxane resistance. Taxane resistance-related microRNA (miRNAs) are under investigation and miRNAs could induce the taxane resistance of tumour cells by regulating cell cycle distribution, survival and/or apoptosis pathways, drug transports, epithelial–mesenchymal transition and cancer stem cell. This article summarizes current research involving miRNAs as regulators of key target genes for tanxanxe chemoresistance and discusses the complex regulatory networks of miRNAs. Also, the authors will envisage future developments towards the potential use of targeting miRNAs as a novel strategy for improving response of tumour patients to taxane. miRNAs play critical roles in taxane chemoresistance and the miRNA-based therapies will be helpful for overcoming drug resistance and developing more effective personalized anti-cancer treatment strategies. Further research studies should be performed to promote therapeutic–clinical use of taxane resistance-related miRNAs in cancer patients, especially in those patients with taxane-resistant cancers.
| ● Introduction |
| – miRNAs involved in taxane resistance |
| – miRNAs involved in regulation of taxane targets |
| – miRNAs involved in cell cycle control of taxanes |
| – miRNAs involved in taxane-induced survival and/or apoptosis pathways |
| – miRNAs involved in regulation of drug transports |
| – miRNA involved in regulation of EMT and cancer stem cell |
| • Discussion |
| • Conclusion |
| • Expert commentary |
| • Five-year view |
Introduction
The taxanes (paclitaxel and docetaxel) are microtubule-stabilizing agents that function primarily by interfering with spindle microtubule dynamics and causing cell cycle arrest and apoptosis. Currently, taxanes have been used in clinics for the treatment of several kinds of tumours such as ovarian, breast, head and neck, lung, and prostate cancers 1. However, drug resistance represents a major obstacle to improve the long-term effectiveness of taxanes in cancer chemotherapy. The mechanisms underlying the taxane resistance have not yet been fully elucidated.
As an integrated part of the multilayered regulatory networks of gene expression, miRNAs have raised great interest for their unique and powerful regulatory effects. They are short (20–23-nucleotide), endogenous, single-stranded RNA molecules, which negatively regulate the gene expression by base-pairing interactions between the ‘seed’ region (positions 2–8 from the 5′ end) of the miRNA and the partially complementary region (usually in the 3′ untranslated region) of the target miRNA. An estimated 60% of the miRNAs involved in distinct cellular processes have one or more predicted binding sites to interact with miRNAs. Although the repression effect of a single miRNA on specific miRNA is relatively modest compared with the transcriptional factor, its capacity to modulate tens to hundreds of target genes makes it have profound influence in normal and malignant cell homoeostasis. The aberrance of miRNAs can finally lead to disease and tumourgenesis 2.
Abnormal expression of miRNAs has been observed in diverse haematological and solid tumours 3, as well as in chemoresistant cancer cells 4. MicroRNAs can contribute to carcinogenesis through regulating multiple key cellular processes by functioning as oncogenes or tumour suppressors, depending upon the nature of their target gene (s). Those genes whose products play a role in the response to anti-cancer treatments are also likely to be regulated by miRNAs. As far as we know, there are a number of mechanisms involved in drug resistance of cancer cell, including alteration of drug target, altered regulation of the cell cycle and apoptosis, increased DNA damage repair and ejection of the drug from the cell by drug efflux pumps 5. Indeed, recent evidence indicates that miRNAs can influence the effect of anti-cancer agents, including taxanes, through all the above mechanisms (Fig. 1), suggesting that they can play a pivotal role in drug efficacy and have potential clinical implications for overcoming taxane resistance. Therefore, this review aims to discuss the role of miRNAs in the molecular mechanisms of taxane resistance and the potential use of miRNAs in overcoming taxane resistance.
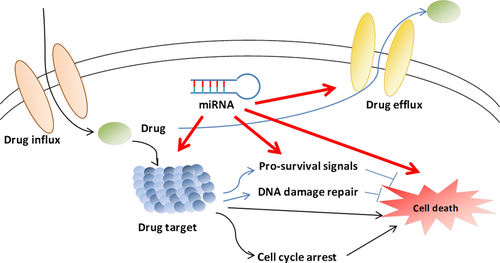
MiRNAs involved in taxanes resistance
MiRNAs involved in regulation of taxanes targets
The cellular target for paclitaxel has been identified as β-subunit of tubulin in microtubules. Docetaxel shares the same binding site as paclitaxel, although with greater affinity 6. They both inhibit the process of cell division, causing a ‘frozen mitosis’ by stabilizing microtubules and thus preventing disassembly 7. Mechanisms underlying taxane resistance that occur directly at the microtubule include mutations, alternations of tubulin isotype or regulatory proteins 8-10.
Mutations in β-tubulin can disrupt microtubule assembly, conferring taxane resistance 11. There are seven isotypes of β-tubulin in humans. β I and β IV-tubulin isotypes are constitutively expressed, while the other isotypes are tissue-specific 12. Overexpression of β III-tubulin has been found to be associated with paclitaxel resistance in ovarian cancer 13, breast cancer 14, non small-cell lung cancer 15 and pancreatic cancer 16. The possible mechanism was associated with reduced effects of paclitaxel on microtubule dynamic instability 17. β III-tubulin is one of the direct targets of miR-200c, and restoration of miR-200c enhanced sensitivity to paclitaxel in endometrial, breast and ovarian cancer cell lines 18. In addition to miR-200c, the other members of miR-200 family (miR-141, miR-200a, miR-200b, and miR-429) could down-regulate the expression of β III -tubulin in ovarian tumour, and might play a role as prognostic factors and markers of the response to paclitaxel-based chemotherapy in ovarian carcinoma 19. On the other hand, β-tubulin miRNAs of classes I, IIA, IIB and V were proved to be regulated by miR-100 in MCF-7 breast cancer cells 20. Microtubule-associated protein tau (MAPT) functions primarily by enabling tubulin assembly and microtubule stabilization 21. Dysregulation of MAPT by miR-34c-5p was critical in the chemosensitivity of gastric cancer to paclitaxel 22 (Fig. 2).
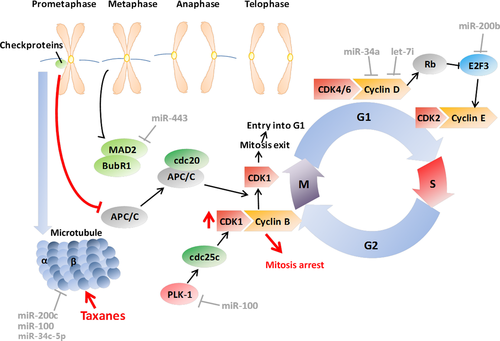
MiRNAs involved in cell cycle control of taxanes
Taxanes mainly induce mitotic arrest (G2/M arrest) and apoptosis by binding to the β–tubulin subunit of microtubules and suppressing dynamics of mitotic spindle 23. The mitotic spindle assembly checkpoint (SAC) is activated when the attachment of the microtubules to the kinetochores is interrupted by taxanes 24. Checkpoint proteins are then recruited, monitoring accurate microtubule attachment. The anaphase-promoting complex (APC/C) is suppressed by the mitotic checkpoint complex (MCC) formed by some checkpoint proteins, such as mitotic arrest deficiency protein 2 (MAD2), BUBR1 and Cdc20 until they leave the kinetochores when all microtubules attach to the kinetochores 25. Activated by cdc20, the APC/C ubiquitin ligase ubiquitinates and degrades cyclin B1 and thereby inactivates cyclin-dependent kinase 1 (CDK1) in anaphase, allowing exit from mitosis to occur 26. The sustained activation of checkpoint proteins by taxanes increases activity of cyclin B1-CDK1 through inhibiting APC/C, leading to cell cycle arrest at the M phase, which plays a key role in taxane function 27.
The MAD2 is a key component of the checkpoint proteins. Evidence shows that paclitaxel-induced activation of the SAC and apoptotic cell death requires MAD2 activity independent of MAD1 28. Reduced MAD2 expression was associated with increased resistance to paclitaxel in epithelial ovarian cancer (EOC) cells and poor outcome of high-grade serous EOC patients, and MAD2 was identified as a target of miR-433 29. Additionally, polo-like kinase 1 (PLK1) promotes mitotic progression by phosphorylating CDC25C, which in turn activates CDK1-Cyclin B1 complexes 30. Inhibition of PLK1 by LFM-A13 could enhance the effects of paclitaxel 31. Furthermore, PLK1-specific siRNAs and paclitaxel could exert synergistic effects in breast cancer by inducing inhibition of cell proliferation and increased apoptosis 32. Overexpression of PLK1 has been found in a number of human tumours and PLK1 miRNA is proposed to be the target of miR-100 33-35. In our previous report, miR-100 has been shown to be a chemosensitivity restorer to docetaxel in human lung adenocarcinoma cells by targeting PLK1 36.
Although taxanes mainly function in G2/M phase, aberrant changes occurring in G1/S phase also influence the taxane effects. Cyclin D1 binds to CDK4 and CDK6 to form a complex, supporting G1–S transition 37. Kastl et al. reported that reduced cyclin D1 expression modulated by miR-34a could induce G1 phase arrest, where docetaxel exerts little cytotoxicity, in turn leading to the resistance of docetaxel in breast cancer cells 38. Cyclin D1 is also a target of let-7i, and a chimera that combines Mucin 1 (MUC1) aptamer and let-7i miRNA was proved to reverse paclitaxel resistance in EOC cells through down-regulation of cyclin D1, cyclin D2, Dicer 1, and PGRMC1 expressions 39. E2F family members play an important role in the control of gene expression in several phases of the cell cycle and in multiple checkpoints. Their targets are diverse and cover genes controlling DNA replication and G1/S transitions, such as Cyclin A/E, Cdc6, and Mcms, as well as products involved in DNA repair and G2/M transitions, such as Cdc25a, Cdk1, Aurora-A and Survivin 40. In our previous study, E2F3 is also found to be a direct target of miR-200b, and inhibition of miR-200b, which led to E2F3 overexpression, contributed to resistance of lung adenocarcinoma cells to docetaxel 41 (Fig. 2).
Adenomatous polyposis coligene (APC) is a multi-functional tumour suppressor well known for its regulation of WNT signalling 42, and recent studies have identified its additional role of regulating cell adhesion and migration, microtubule networks, spindle formation and chromosome segregation 43. APC deficiency could compromise the response to paclitaxel in vivo because it could lead to destabilized microtubules, independent of its effect on WNT signalling 44. MiR-135a was associated with reduced APC in paclitaxel-resistant NSCLC cell lines and in vivo models, probably through interfering with the mitotic spindle checkpoint 45.
MiRNAs involved in taxane-induced survival and/or apoptosis pathways
The cytotoxic effect on tumour cells of paclitaxel is demonstrated to depend on drug concentration, cell type and exposure time 46. At low concentrations, paclitaxel induces mitotic arrest or an aberrant mitotic exit into a G1-like ‘multinucleate state’ ending up in apoptosis. Higher concentrations of paclitaxel can lead to extensive microtubule damage 7. Independent of cell cycle arrest, paclitaxel induces apoptosis through multiple mechanisms, including activation of mitogen-activated protein kinases (MAPK) 47, Raf-1 48, 49, and c-Jun N-terminal kinase (JNK) 50 and regulation of the expression of apoptosis-related proteins like Bcl-2, Bad, Bcl-xL, p21 WAF-1/CIP-1, and the tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) receptors, DR4 and DR5 48, 51, 52.
A number of miRNAs have been reported to participate in the regulation of taxane-induced apoptosis (Fig. 3). MiR-512-3p facilitated paclitaxel-induced apoptosis mediated by death receptor (DR) through directly targeting cellular FLICE-like inhibitory protein (c-FLIP) in hepatocellular carcinoma cells 53. By inhibiting casapase-8, c-FLIP plays an anti-apoptotic role in DR signalling 54. Additionally, miR-34c-induced apoptosis is commonly found in several cancer cell lines 55. However, as it might confer resistance to caspase-8- and paclitaxel-induced apoptosis in NSCLC cells, miR-34c also showed an oncogenic potential 56.
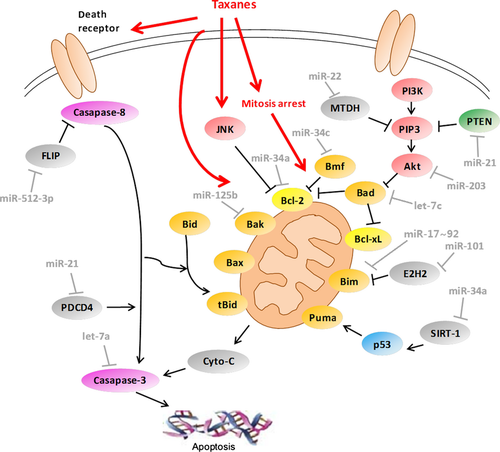
The Bcl-2 family plays a pivotal role in the mitochondrial apoptosis pathway and is considered associated with taxane-induced apoptosis. The development of drug resistance in various cancer cells has also been linked to abnormalities in the expression of Bcl-2 family proteins. The Bcl-2 family consists of two sets of proteins exerting opposite functions. The pro-apoptotic subfamily includes Bax, Bak and the BH3-only proteins includes Bid, Bim, Bad and PUMA, while the anti-apoptotic subfamily includes Bcl-2, Bcl-xL and McL-1 57. Bcl-2 has been demonstrated to be a target of miR-34a 58. Interestingly, down-regulation of miR-34a was observed in paclitaxel-resistant prostate cancer, and introduction of miR-34a precursor attenuated resistance to paclitaxel 59. In contrast, increased miR-34a expression was found in MCF-7 docetaxel-resistant breast cancer cells 38, suggesting that the role of miR-34a is ambiguous in the regulation of taxane resistance. Some reports have shown that increased Bcl-2 expression is beneficial for breast cancer treatment 60. On the other hand, miR-34a also has abundance of other targeted miRNAs besides Bcl-2, including SIRT-1, Cyclin D1, Cyclin E2, CDK4, CDK6, E2F3, MET and notch-1 55, which add to the complexity of the role of miR-34a in human cancers. Bcl-2 antagonist killer 1, Bak1, is a pro-apoptotic member of Bcl-2 family. Bak1 is also a direct target of miR-125b, and restoring Bak1 expression in breast cancer cells could recover paclitaxel sensitivity, overcoming miR-125b-mediated paclitaxel resistance 61. Bim is a member of the BH3-only family of pro-apoptotic proteins. In the ovarian cancer cells, miR-17–92 could induce paclitaxel resistance through targeting Bim 62. Also, miR-101 overexpression in NSCLC cells promoted paclitaxel-induced apoptosis through down-regulating Enhancer of zeste homologue 2 (EZH2) expression, which has been shown to regulate apoptosis through epigenetically modulating Bim expression 63.
Despite being activated by different stimuli, both the intrinsic and the extrinsic apoptosis pathways result in the activation of casapase-3, the ultimate effector casapase, which can be regulated by specific miRNAs. In particular, by targeting caspase-3, let-7a directly regulated paclitaxel-induced apoptosis in HCC and human squamous carcinoma 64. Accumulated evidence has demonstrated that increased activation of the phosphatidylinositol 3-kinase (PI3K)/Akt signalling pathway is responsible for resistance to taxanes. Once activated, Akt phosphorylates and inhibits several pro-apoptotic proteins such as Bim, Bad and caspase-9 65. Recent research showed that overexpression of miR-203 significantly sensitized the cytotoxicity of paclitaxel in the p53-mutated colon cancer cells by negatively regulating Akt2 expression 66. Phosphatase and tensin homologue (PTEN), another important tumour suppressor gene, inhibits Akt by dephosphorylating phosphoinositide 3-phosphatase (PIP3) and acts as a negative regulator of PI3K/Akt signalling. Loss of PTEN activity leads to increased cell growth and overactivation of the PI3K/Akt pathway 65. Overexpression of miR-22 enhanced the anti-cancer effect of paclitaxel in the p53-mutated cells through increasing cell apoptosis and reducing cell proliferation and survival, mediated by activation of PTEN signalling 67.
MiR-21 has been described as an oncogenic miRNA, and increased level of miR-21 expression has been observed in different cancer types, including breast cancer, prostate cancer, NSCLC, glioblastoma and hepatocellular carcinoma 68. MiR-21 functions mainly through repression of two targets. One is programmed cell death 4 (PDCD4), a novel suppressor of tumourigenesis, tumour progression and invasion 69. A recent study has demonstrated that PDCD4 negatively regulates YB-1 expression via its interaction with Twist1 and is involved in cancer cell growth and resistance to cisplatin and paclitaxel 70. MiR-21 contributed to the resistance of prostate cancer cells to docetaxel by targeting PDCD4 71. The second is the tumour suppressor PTEN. MiR-21 modulated chemosensitivity by targeting PTEN in NSCLC 72 and breast cancer 73. However, depletion of miR-21 neither resulted in change in downstream targets like PDCD4 and PTEN, nor affected the response to paclitaxel in prostate cancer cells 74. These data suggested that the oncogenic properties of miR-21 could be cell- and tissue-dependent. In another report, miR-21 was shown to participate in enhancing sensitivity of human glioblastoma cells to paclitaxel by inhibiting STAT3 expression, independent of PTEN status 75.
Mitogen-activated protein kinase pathway comprises several key signalling components, which play a role in tumourigenesis and chemoresistance 76, 77. Oncogene KRAS is a key molecule of EGFR/RAS/MAPK pathway, and miR-143 can restore chemosensitivity to docetaxel in prostate cancer cells by down-regulating KRAS 78. On the other hand, overexpression of miR-148a induced paclitaxel resistance in prostate cancer cells by targeting MSK-1(Mitogen- and stress-activated kinase 1), a down-stream target of ERK or p38 MAPK 79.
MiRNAs involved in regulation of drug transports
Multidrug resistance (MDR) is a phenomenon that resistance to one drug can result in cross-resistance to other structurally unrelated drugs. A key mechanism underlying multidrug resistance relates to the expression of the ATP-dependent transporter family known as the ATP-binding cassette (ABC) family. The human genome contains 48 genes that encode ABC transporters, which have been divided into seven subfamilies labelled A–G 80. The ABC transporters involved in taxane resistance include P-gp (ABCB1), ABCC2, ABCC10 and ABCB11 81.
P-glycoprotein (P-gp) is the best known and well-studied multidrug transporter, encoded by the MDR1 gene. Overexpression of P-gp confers cancer cell resistance to a broad spectrum of drugs, including colchicine, doxorubicin, etoposide, vinblastine and paclitaxel 82. The level of MDR1 expression is correlated with resistance to paclitaxel in some cancers, such as lung cancer and breast cancer 83, 84. MiR-451, miR-27, miR-326 and miR-331-5p have been shown to regulate the expression of the MDR1 gene 85-88. In particular, miR-27a was found to be involved in the development of paclitaxel resistance, partly by targeting Homoeodomain-interacting protein kinase-2 (HIPK2), which can lead to suppression of MDR1 gene by inhibiting hypoxia inducible factor-1α (HIF-1α) 89. Also, let-7 g was shown to affect the sensitivity of a paclitaxel-resistant ovarian cancer cell by targeting IGF-II miRNA-binding protein 1(IMP-1), which stabilized and protected MDR1 miRNA as a RNA binding protein 90.
MiRNA involved in regulation of EMT and cancer stem cell
Epithelial–mesenchymal transition is an essential developmental process by which cells get increased migratory behaviour by the loss of epithelial characteristics and the acquisition of a mesenchymal phenotype. Emerging evidence suggests that EMT may be responsible for cancer cell progression, invasion, metastasis and possibly, treatment resistance 91. Phenotypic and molecular changes consistent with EMT have been observed in paclitaxel-resistant epithelial ovarian carcinoma, breast cancer and docetaxel-resistant prostate cancer 92. However, the mechanisms by which the EMT regulates resistance to chemotherapeutic agents are not very well elucidated. Recently, miRNAs have been found to be involved in the acquisition and maintenance of EMT-type cells, which may play important roles in drug resistance and metastasis 93. The docetaxel-resistant lung adenocarcinoma cell lines (SPC-A1/DTX and H1299/DTX) previously established by our laboratorydisplayed chemoradioresistance and mesenchymal features with enhanced invasiveness and motility, and reintroduction of let-7c could reverse their chemoradioresistance and EMT phenotypes 94. Low or absent miR-200c was found in paclitaxel-resistant breast cancer, and the loss of the miR-200c was critical for the acquisition of EMT characteristics mediated by Zinc-finger enhancer binding (ZEB) transcription factors (ZEB1 and ZEB2) 18, which could induce EMT phenotype by suppressing the expression of many epithelial genes including E-cadherin, and form a feedback loop with miR-200 family 95.
Recent evidence suggests that EMT cells have cancer stem cell-like features and cancer stem cells (CSCs) exhibit a mesenchymal-like phenotype, highlighting a link between EMT and the CSCs. Increased stem cell-like properties and invasion potential could be seen in the docetaxel-resistant prostate cancer cells, and the E-cadherin loss in these cells was partially owing to the decreased expression of miR-200c and miR-205 96. Up-regulation of miR-125b mediated by the overexpression of Snail could enhance the resistance to paclitaxel and increase the CSC population (CD24−CD44+), through repressing Bak1 97. Lin28, a marker of CSCs, might attenuate the sensitivity to paclitaxel treatment of breast cancer cells by affecting the let-7 processing 98, but the mechanisms are still poorly understood.
Apart from these mentioned above, miRNAs can also regulate taxane response through other mechanisms. The amplification of HGF receptor tyrosine kinase MET has been shown to mediate the resistance to EGFR-TKI in NSCLC. Recent research has found that miR-31-dependent regulation of MET was responsible for the resistance to paclitaxel of ovarian cancer 99. A distinct miRNA profiling was shown in the paclitaxel-resistant ovarian cancer, and particularly down-regulation of miR-130a was associated with the translational activation of the macrophage colony-stimulating factor (M-CSF) gene, a known resistance factor for ovarian cancer 100, possibly owing to the role of matrix metalloproteinases (MMPs) in the CSF-1-mediated effect on tumour progression 101.
Discussion
The expression signature of many miRNAs is tissue-, tumour- or even pathology-specific, and related to treatment response or clinical outcome. These features make miRNA appealing as a diagnostic and prognostic tool. MicroRNA profiling has been performed to explore its relationship with the taxane treatment response in NSCLC, ovarian cancer, breast cancer, and head and neck squamous cell carcinoma 102, 103, 100, 104-106. For example, low expression of let-7a was significantly linked to better survival and response to the addition of paclitaxel to platinum-based chemotherapy of EOC patients 107. In particular, tumour-derived miRNAs in circulation have raised great interest. Recent research showed that malignant effusion supernatant had a different profile of miRNAs compared with benign samples and the expression levels of cell-free miR-152 might discriminate docetaxel-sensitive samples from resistant ones 108. Additionally, serum miR-21 levels were elevated in hormone-refractory prostate cancer patients, especially in those resistant to docetaxel-based chemotherapy 109. Many efforts have been made in promoting miRNA-based therapies and there are already some encouraging results. However, a great number of challenges still remain to be overcome, including effective targeted delivery strategies, as well as off-target effects and long-term safety. So far, the miRNA-based treatments of reversing taxane resistance have not been reported.
Conclusion
MicroRNAs have been demonstrated to regulate almost all the known processes and their dysregulation is involved in the mechanisms of carcinogenesis and ever more, drug resistance. In this review, we discuss recent studies which demonstrate that miRNAs could play a critical role in the regulation of taxane resistance (Table 1) and targeting miRNAs is a novel strategy for improving response to taxane.
| microRNA | Target gene | Function | Drug | Tissue type | Mechanism | Refs. |
|---|---|---|---|---|---|---|
| miR-200c | TUBB3 (β III-tubulin) | Tumour suppressor | Paclitaxel | Ovarian cancer | Microtubule system | 19 |
| miR-100 | TUBB2A (β 2A-tubulin), TUBB3 (β III-tubulin) | Tumour suppressor | Paclitaxel | Breast cancer | Microtubule system | 20 |
| miR-34c-5p | MAPT (Microtubule-associated protein tau) | Tumour suppressor | Paclitaxel | Gastric cancer | Microtubule system | 22 |
| miR-433 | MAD2 (mitotic arrest deficiency protein 2) | Oncogene | Paclitaxel | Ovarian cancer | Cell cycle | 29 |
| let-7i | Cyclin D1 and D2 etc. | Tumour suppressor | Paclitaxel | Ovarian cancer | Cell cycle | 39 |
| miR-100 | plk-1 (polo-like kinase 1) | Tumour suppressor | Docetaxel | NSCLC | Cell cycle | 36 |
| miR-200b | E2F3 | Tumour suppressor | Docetaxel | NSCLC | Cell cycle | 41 |
| miR-135a | APC (Adenomatous polyposis coligene) | Oncogene | Paclitaxel | NSCLC | Cell cycle | 45 |
| miR-34a | Cyclin D1Bcl-2 | Oncogene | Docetaxel | Breast cancer | Cell cycleapoptosis | 38 |
| SIRT1 (Silent mating type information regulation 2homologue 1) | Tumour suppressor | Paclitaxel | Prostate cancer | Apoptosis | 59 | |
| miR-34c | Bmf, c-myc | Oncogene | Paclitaxel | NSCLC | Apoptosis | 56 |
| miR-125b | Bak-1 (Bcl-2 antagonist killer 1) | Oncogene | Paclitaxel | Breast cancer | Apoptosis | 61 |
| miR-17–92 | Bim | Oncogene | Paclitaxel | Ovarian cancer | Apoptosis | 62 |
| miR-101 | EZH2 (Enhancer of zeste homologue 2) | Tumour suppressor | Paclitaxel | NSCLC | Apoptosis | 63 |
| Let-7a | Caspase-3 | Oncogene | Paclitaxel | Squamous carcinoma, hepatocellular carcinoma | Apoptosis | 64 |
| miR-203 | Akt-2 | Tumour suppressor | Paclitaxel | Colon cancer | Apoptosis | 66 |
| miR-22 | MTDH (Metadherin) | Tumour suppressor | Paclitaxel | Colon cancer | Apoptosis | 67 |
| miR-21 | PDCD4 (Programmed cell death 4) | Oncogene | Docetaxel | Prostate cancer | Apoptosis | 71 |
| PTEN (Phosphatase and tensin homologue) | Oncogene | Paclitaxel | Prostate cancer | Apoptosis | 74 | |
| STAT3 | Oncogene | Paclitaxel | Glioblastoma multiforme | Apoptosis | 75 | |
| miR-143 | KRAS | Tumour suppressor | Docetaxel | Prostate cancer | Proliferation | 78 |
| miR-148a | MSK-1 (Mitogen- and stress-activated kinase 1) | Tumour suppressor | Paclitaxel | Prostate cancer | Apoptosis | 79 |
| miR-27a | HIPK2 (Homoeodomain-interacting protein kinase-2) | Tumour suppressor | Paclitaxel | Ovarian cancer | MDR | 89 |
| let-7g | IMP-1 (IGF-II miRNA-binding protein 1) | Tumour suppressor | Paclitaxel | Ovarian cancer | MDR | 90 |
| let-7c | Bcl-xL | Tumour suppressor | Docetaxel | NSCLC | EMT | 94 |
| miR-200c and miR-205 | ZEB1 (Zinc-finger enhancer binding) | Tumour suppressor | Paclitaxel | Prostate cancer | EMT | 96 |
| miR-125b | Bak-1 ((Bcl-2 antagonist killer 1)) | Oncogene | Paclitaxel | EMT, CSCs | 97 | |
| miR-200c | ZEB1, ZEB2, TUBB3 | Tumour suppressor | Paclitaxel | Endometrial, breast and ovarian cancer | EMT, microtubule system | 18 |
| miR-31 | MET | Tumour suppressor | Paclitaxl | Ovarian cancer | Other | 99 |
| miR-130 | MCSF (Macrophage colony-stimulating factor) | Tumour suppressor | Paclitaxel | Ovarian cancer | Other | 100 |
Expert commentary
Chemoresistance poses an obstacle to improving the survival rates of cancer cells. It is still difficult to find effective ways to overcome resistance before we can elucidate the complex and multilayered mechanisms. MicroRNAs have raised much interest owing to their unique and powerful regulatory capacity. For example, recent research studies have discovered a great number of miRNAs that can predict or regulate the response to taxanes of cancers. In addition, the research of miRNAs is rapidly advancing towards in vivo delivery for therapeutic purposes, ultimately building a new class of targeted molecular therapies. Over the past decade, strategies and techniques of miRNA-based therapy have developed greatly. Blocking oncogenic miRNAs can be achieved by the use of antisense oligonucleotides, miRNA sponges, miR-mask and small RNA inhibitors, whereas restoring the tumour suppressor miRNAs expression could be achieved by using synthetic miRNAs (miRNA mimics) or re-introducing genes coding for miRNAs by viral vectors 110. Therapeutic silencing of miR-122 with a locked nucleic acid (LNA)-modified oligonucleotide in treatment of chronic hepatitis C virus has advanced into phase 2 clinical trials 111. However, many questions remain unanswered, such as which miRNA is the best and most reliable to predict taxane response in cancer patients, and then, which target gene(s) is (are) the most critical to regulate the taxane response, and moreover, how to accurately deliver the miRNA to the tumour lesions via an effective and safe drug transport system.
Five-year view
In the next 5 years, it is with great anticipation that miRNAs will have more profound clinical applications. MicroRNAs involved in specific networks, such as the apoptosis, cell cycle, or EMT-related signalling pathways, can influence the effects of anti-cancer treatments including taxanes, which highlights their potential values as biomarkers or modulators for anti-cancer drug response. We will see trials that explore the use of miRNAs or miRNA-based therapies for restoring chemosensitivity to some anti-cancer agents including taxanes. However, identification and verification of critical miRNA targets and lack of safe and specific delivery system still remain major difficulties to overcome. Based on a deeper understanding of the complex regulatory networks of miRNAs, the miRNA-based therapies hold great promise for overcoming drug resistance and developing more effective personalized anti-cancer treatment strategies.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 30901440, 81172335, 81071806 and 81172106). Thanks to every one of the Department of Medical Oncology for their sincere help.
Conflicts of interest
The authors do not have any financial interests to publish this article.




