Hyperuricemia and high blood pressure at rest and during exercise: Guilty or innocent? The jury is still out
Uric acid (UA) is generated as part of the normal turnover of nucleic acid. Purine bases and their nucleotides are captured by the liver and converted into xanthines, metabolized by UA, or recycled by salvage pathways or de novo synthesis.1, 2 UA formation by xanthine occurs via hypoxanthine by the action of xanthine oxidase (XO), an enzyme present in the peroxisomes of most cells, which is an important source of reactive oxygen species involved in various forms of ischemic and other types of vascular injuries and chronic heart failure.2, 3 In humans, higher primates, and in a particular species of dog (Dalmatians), UA is the final product of the purine catabolism. In most other mammals, the enzyme uricase further oxidizes UA to allantoin, reducing serum uric acid (SUA).1, 2
UA circulates in plasma predominantly in the form of a monovalent sodium salt (urate).1 Hyperuricemia (HU) is commonly observed in hypertensive patients, especially in those with metabolic syndrome (MetS), where it may be a marker of insulin resistance as well as of renal dysfunction and diuretic use.2-4 Moreover, a significant association between SUA and the intrarenal resistance index measured by duplex ultrasound has been described,5 suggesting that HU in patients with essential hypertension (EH) reflected intrarenal hemodynamic alterations.
The concept that UA may be involved in hypertension is not a new one. In fact, in the paper published in 1879 that originally described EH, Frederick Mohamed noted that people with high blood pressure (BP) had a greater chance of coming from “gouty” families or themselves suffering from gout.6 This lead him to suggest that UA might be one of the causes of hypertension. Picking up on this and based on his own observations a decade later, Haig proposed low purine diets as a means to prevent hypertension and vascular disease.3 Nathan Smith Davis said whilst delivering the presidential address to the American Medical Association in 1897, “High arterial tension in gout is due in part to uric acid or other toxic substances in the blood.3”
In spite of these anecdotal initial observations, the field lay dormant for many years. HU was simply associated with gout and nephrolithiasis and UA regarded as an inert molecule, which serum levels were in part increased as the consequence of the metabolic and hemodynamic abnormalities having as a common feature insulin resistance (Figure 1). However, in the past 2 decades a growing body of evidence changes this perspective suggesting that, rather than being an “innocent bystander,” UA per se may be a “guilty party,” being causally related to cardiometabolic and renal disorders, with the focus of many studies being hypertension.2, 3, 7-12
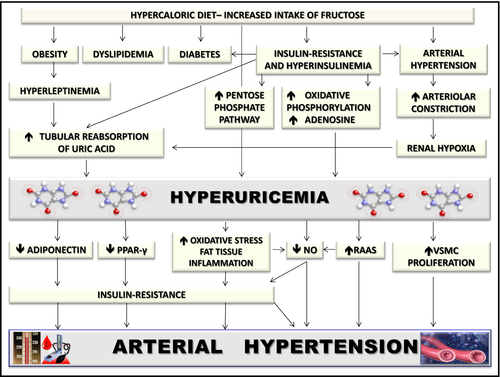
The first direct evidence that high UA may lead to hypertension was provided by the development of an experimental model of mild HU, in which rats were made unable to metabolize UA to allantoin by means of oxonic acid (OA), a uricase inhibitor that reproduces the enzyme deficiency that the human species developed during evolution.2, 3, 7-12 In these rats, hypertension is caused by progressive renal injury via crystalline-independent mechanisms, including induction of oxidative stress, inhibition of nitric oxide and activation of the renin angiotensin system (RAS), and development of microvascular renal disease with histology that is similar to arteriolosclerosis, the classic lesion of EH.3, 7 Finally, UA promotes proliferation of vascular smooth muscle cells and stimulates synthesis by the same cells of the monocyte chemoattractant protein-1, which is known to play a key role in promoting macrophage infiltration in atherosclerotic vessels.2, 8
In this animal model, hypertension developed in 2 phases.3, 7, 9 Initially, the hypertension could be directly reversed by reducing UA levels with either XO inhibitors or uricosuric agents. Hypertension during this phase was chiefly mediated by uric acid-dependent activation of the RAS and could be observed even in the presence of a low-salt diet.3, 7, 9 However, over time the rats developed significant renal microvascular disease and tubulointerstitial inflammation and the hypertension became salt sensitive and no longer renin and UA dependent. It persisted despite allowing UA levels to return to baseline levels.3, 7, 9
This biphasic relationship between UA and high BP might help to explain the results of some recent studies that fail to detect an independent association between plasma renin activity and UA in hypertensive adults,13 as well as an intrarenal activation of the RAS.14 Other supporting arguments for the theory that high UA may lead to hypertension are based on epidemiological surveys showing an association between SUA and the development of hypertension.10, 11 The more recent meta-analysis addressing this issue included 25 studies with 97 824 participants and indicates that hyperuricemia was associated with a higher risk of incident hypertension, independently of traditional risk factors, and regardless of whether the data were categorical or continuous (adjusted: RR = 1.48, 95% CI: 1.33-1.65 for categorical data, RR = 1.15, 95% CI: 1.06-1.26 for a 1 mg/dL increase).11 It has been also observed that the relationship between SUA levels and BP was more robust in young populations than it was in elderly individuals.10
Children and adolescents have relatively fewer confounding factors such as diabetes, CV diseases, age-related illness, and renal dysfunction than adults. Thus, they are the ideal population to study the early pathophysiological steps that initiate EH.15 It is generally accepted the concept that the roots of primary hypertension are present in childhood and that a family history of hypertension is an excellent predictor of high BP in adults. It this regard may be interesting the findings of two recent reports showing greater SUA levels in normotensive children and adolescents with a positive parental history of hypertension than in those with a negative one.16, 17 Moreover, it was observed that lowering SUA with allopurinol or probenecid resulted in a marked BP reduction in adolescents with newly diagnosed hypertension.11, 15
In contrast to the above described clinical and experimental data, in a double-blind placebo-controlled trial, including 149 overweight or obese adults with SUA >5.0 mg/dL, lowering SUA with either probenecid or allopurinol did not change either kidney specific or systemic RAS activity.14 Moreover, BP measured using 24-hour ambulatory BP monitoring did not show any benefit from lowering UA.14 These results are consistent with other reports12, 18, 19 suggesting that observational association of elevated UA with high BP and with hypertensive target organ damage (TOD) may not be causal in adults.
In keeping with these findings, large Mendelian randomization studies have been able to link polymorphisms in urate transporters with HU and gout, but not with hypertension.20 Therefore, despite the evidences obtained in animal studies and in adolescents, the question regarding the exact role of uric acid in inducing hypertension and CV diseases remains unanswered.
Pertinent to this still debated question regarding the role of HU in hypertension, Jae and colleagues report in this issue of the Journal the results of a study aimed to assess, for the first time, the influence of SUA levels on the systolic BP (SBP) response to maximal exercise testing in 4640 men who were free of known CV disease, hypertension and type 2 diabetes.21 Multivariable logistic regression analyses showed that participants in the highest quartile of SUA distribution (>6.6 mg/dL) demonstrated a more than double chance of having an exaggerated SBP response (≥210 mm Hg) to maximal exercise, as compared with participants in the lowest quartile of SUA (<5.1 mg/dL) and after adjusting for confounding variables, including resting SBP and insulin resistance,21 which have been previously shown to predict an exaggerated SBP response to maximal exercise.
The extent of such response in some, but not all, studies may predict the development of hypertension at rest in normotensive subjects.22 Furthermore, an excessive rise of BP during exercise has been associated, albeit not unanimously, with an increased risk of future CV events.23 Therefore, the results of this investigation may have potential prognostic implications.
The findings of the paper of Jae and colleagues need to be interpreted in the context of its limitations. The first one is inherent to its the cross-sectional design that does not permit assessment of the temporality of the observed associations and thus determination of causality. An additional limitation is that the influence of relevant confounding factors, such as prevalence of gout, treatment with SUA lowering drugs, and renal function parameters was not taken into account into multivariate analyses because information about these data were not available.
Moreover, the authors employed the homeostatic model assessment for insulin resistance (HOMA-IR) to estimate insulin sensitivity. However, HOMA-IR is only a surrogate marker of insulin-resistance that may be accurately assessed only by more time-consuming and labor-intensive methods.24 Therefore, a residual confounding effect of “true” insulin resistance cannot be excluded.
Finally, the study population consists only of men. Further research will be needed before generalizing these findings to women and in order to better understand the relationships between uric acid and exercise BP.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.




