Structural phenotyping in atrial fibrillation with combined cardiac CT and atrial MRI: Identifying and differentiating individual structural remodelling types in AF
Disclosures: None.
Abstract
Introduction
Atrial remodelling (AR) is the persistent change in atrial structure and/or function and contributes to the initiation, maintenance and progression of atrial fibrillation (AF) in a reciprocal self-perpetuating relationship. Left atrial (LA) size, geometry, fibrosis, wall thickness (LAWT) and ejection fraction (LAEF) have all been shown to vary with pathological atrial remodelling. The association of these global remodelling markers with each other for differentiating structural phenotypes in AF is not well investigated.
Method
Patients referred for first-time AF ablation and controls without AF were prospectively recruited to undergo cardiac computed tomographic angiography (CCTA) and magnetic resonance imaging (MRI) with 3D atrial late-gadolinium enhanced (LGE) sequences. LAWT, atrial myocardial mass, LA volume and sphericity were calculated from CT. Biplane LA EF and LA fibrosis burden were derived from atrial MRI. Results were compared between patients with AF and controls.
Results
Forty two AF patients (64.3% male, age 64.6 ± 10.2 years, CHA2DS2-VASc 2.48 ± 1.5, 69.0% paroxysmal AF, 31% persistent AF, LVEF 57.9 ± 10.5%) and 37 controls (64.9% male, age 56.6 ± 7.2, CHA2DS2-VASc 1.54 ± 1.1, LVEF 60.4 ± 4.9%) were recruited. Patients with AF had a significantly higher LAWT (1.45 ± 0.52 mm vs 1.12 ± 0.42 mm, p = 0.003), tissue mass (15.81 ± 6.53 g vs. 12.18 ± 5.01 g, p = 0.011), fibrosis burden (9.33 ± 8.35% vs 2.41 ± 3.60%, p = 0.013), left atrial size/volume (95.68 ± 26.63 mL vs 81.22 ± 20.64 mL, p = 0.011) and lower LAEF (50.3 ± 15.3% vs 65.2 ± 8.6%, p < 0.001) compared to controls. There was no significant correlation between % fibrosis with LAWT (p = 0.29), mass (p = 0.89), volume (p = 0.49) or sphericity (p = 0.79). LAWT had a statistically significant weak positive correlation with LA volume (r = 0.25, p = .041), but not with sphericity (p = 0.86). LAEF had a statistically significant but weak negative correlation with fibrosis (r = −0.33, p = 0.008) and LAWT (r = −0.24, p = 0.07).
Conclusion
AF is associated with significant quantifiable structural changes that are evident in LA size, tissue thickness, total LA tissue mass and fibrosis. These individual remodelling markers do not or only weakly correlate with each other suggesting different remodelling subtypes exist (e.g. fibrotic vs hypertrophic vs dilated). If confirmed, such a detailed understanding of the structural changes observed has the potential to inform clinical management strategies targeting individual mechanisms underlying the disease process.
1 INTRODUCTION
Atrial remodelling (AR) describes a persistent change in atrial structure and/or function and contributes to the initiation, maintenance, and progression of AF by increasing the likelihood of ectopic or re-entrant activity.1 The interaction between atrial fibrillation and structural remodelling is commonly thought of as a self-perpetuating cycle with the progression of each maintaining and driving the progression of the other, respectively. Yet, in contrast to data from disease processes affecting the ventricle, differentiation of different morphological patterns of remodelling are less well investigated in the atria. This is in part because the resolution of noninvasive imaging has previously limited the reliable assessment of some structural features in the thin-walled atria. Recent years have seen substantial progress in cardiac CT and MRI technology which has facilitated noninvasive assessment of left atrial (LA) size, geometry, fibrosis, wall thickness (LAWT) and ejection fraction (LAEF).2-5 These parameters have all been associated with pathological atrial remodelling, however their association with each other still remains poorly investigated.
This study aimed to compare global parameters of atrial remodelling between AF patients and a control group without history of AF to identify and different structural phenotypes observed in AF and assess the correlation between different parameters of atrial remodelling. This may have therapeutic and prognostic implications for patient management.
2 METHODS
This is a prospective single centre case-control study. The study was conducted according to the principles outlined in the 1964 Declaration of Helsinki and its amendments. The study received a favourable opinion from a national Research Ethics Committee (REC, ref 15/LO/1803). Written informed consent was obtained from all individual participants enrolled in the study.
2.1 Study population
The study recruited two groups. The “invasive” cohort comprised patients with paroxysmal or persistent AF referred and scheduled for first time AF ablation. The “control” cohort comprised patients without AF but with clinical indication for cardiac computed tomographic angiography (CCTA) identified from Rapid Access Chest Pain outpatient clinics. Patients were recruited between November 2016 and June 2019. Enrolled patients in both groups underwent cardiac MRI and CCTA imaging, before ablation in the case of the invasive cohort.
Exclusion Criteria to participate in the study include general CMR contraindications (incompatible metal implants, claustrophobia, body habitus, allergy to gadolinium based contrast agents) or CCTA (chronic or acute kidney impairment with eGFR <50 mL/min, allergy to iodine based contrast medium), as well as patients with moderate to severe valvular stenosis or regurgitation, hypertension not adequately controlled or pre-existing for >10 year, LV hypertrophy >15 mm and/or LVEF < 40% regardless of aetiology.
2.2 Cardiac CT acquisition and processing protocol
All CT scans were performed on a clinical CT scanner (Siemens SOMATOM Force dual source scanner). If required, beta-blockade with intravenous metoprolol was used to achieve a heart rate of <65 beats/min for those patients in sinus rhythm and <100 BPM for patients in atrial fibrillation. Patients undergoing coronary assessment received sublingual GTN. Following adequate heart rate control, intravenous contrast (Omnipaque; GE Healthcare) was power-injected using a triphasic protocol. Descending aorta contrast-triggered (120 HU), ECG-gated scanning was performed in a single inspiratory breath hold with patient in supine positioned with axial slice thickness of 0.6 mm. Typical scanning parameters included a heart rate dependent pitch (0.2–0.45), gantry rotation time of 270 ms, tube voltage of 100 or 120 kVp, depending on the patient's BMI and a tube current of 125–300 mAs depending on the thoracic circumference measurement. For postprocessing the best diastolic sequence was identified and left atrial wall thickness, derived left atrial mass, left atrial volume and sphericity were calculated as below.
2.2.1 Left atrial wall thickness and mass estimation
Left atrial tissue thickness was identified from segmented CCTA employing a semi-automated imaging processing pipeline and LAWT-calculation based on an eikonal approach as previously reported.6 A detailed description of LAWT estimation workflow is provided in Supplement SI. Left atrial mass was estimated from the total atrial myocardial volume multiplying by myocardial tissue density as previously reported.7
2.2.2 Left atrial volume and sphericity
Left atrial volume, left atrial appendage volume and sphericity were calculated from CCTA following automated anatomical segmentation in Adas3D (Galgo Medical). Pulmonary veins, left atrial appendage and mitral annulus were manually cropped and LA volume obtained for the LA body and separate for the LAA (reported in mL). LAA volume was reported separately as it may more sensitively gauge pressure changes than the LA body given that the wall stress is thought to be amplified due to its geometry and therefore may be a more sensitive marker for remodelling driven by elevated left atrial pressure.8 LA Sphericity describes the difference of the actual LA shape and a perfect sphere and is reported in %. Left atrial sphericity was calculated using Adas3D software which offers a sphericity estimation as previously described9, 10: Following anatomical segmentation, the radius of the sphere, which best fits the LA, is calculated as the mean of distances between all points of the LA wall and the centre of mass (average radius, AR). The coefficient of variation of the sphere (CVS = AR standard deviation/AR) is obtained to define left atrial sphericity (LASP).
2.3 Cardiac MRI acquisition and processing protocol
A standard cardiac MRI protocol on a 1.5 T clinical MRI system involving left ventricular functional assessment, balanced Steady-State Free Precession (b-SSFP) whole heart and 3D late gadolinium enhanced T1 weighted sequences (ECG-triggered, respiratory navigated, spoiled gradient-echo acquisition) was acquired. Specific sequences for atrial wall evaluation included high-resolution 3D LGE images acquisition using ECG- and respiratory gated phase sensitive inversion recovery sequence applied at 20–25 min after contrast injection (typical LGE acquisition parameters were repetition time (TR) 4.0 ms, Echo time (TE) 2.0 ms, Flip angle 20 ͦ).
2.3.1 Atrial fibrosis estimation
LA fibrosis quantification was performed using the commercially available ADAS3D LA imaging post processing software (Galgo Medical). Segmentation of the CMR left atrium was performed directly on the 3D LGE images on multiple axial slices by manually contouring the LA body and pulmonary veins. Automated interpolation between contours was applied followed by further manual adjustment as needed. Automated 3D reconstruction of the LA was generated and pulmonary veins and appendage manually cropped. The mitral valve annulus was removed to exclude the enhanced valve area to not affect final fibrosis quantification. For fibrosis estimation, the image intensity ratio (IIR) method was used, which involves normalization of signal intensity of the LA wall to the mean blood pool signal intensity.11 A threshold of 1.2 was employed to indicate fibrosis and 1.32 as dense fibrosis as previously reported.12
2.3.2 Biplane left atrial ejection fraction
LA EF was calculated using the biplane area length method13, 14 for all patient in sinus rhythm at the time of the scan. Patients in AF during MRI were excluded from this analysis. Two and four chamber cardiac MRI SSFP cine series were used to contour the LA endocardial boundaries at atrial end systole and end diastole (using Horos V3.3.6, Osiri XTM). Pulmonary veins, left atrial appendage were excluded from the LA contours. La volumes were calculated based on the formula recommended in the ASE guidelines for echocardiographic chamber quantifications (LA volume = (0.848 * area4chamber * area2chamber)/(length2chamber + length4chamber)/2)15
2.4 Statistical analysis
Continuous variables are expressed as mean ± SD if normally distributed or median±IQR if non normally distributed and compared using students t-test or Mann–Whitney U test as appropriate. Categorical variables are displayed as frequency counts and percentage and compared using the Fisher exact test or chi square analysis as appropriate. Correlation analysis employed Pearson correlation or Spearman based on normality assumptions and variable types. Results were compared for the total population and separately for AF and control cohorts. To consider the contribution of clinical baseline demographics to atrial structural remodelling features, a simple linear regression analysis was run for left atrial volume (total and indexed to body surface area), wall thickness, fibrosis burden and left atrial function with selected baseline characteristics respectively (age, gender, BMI, presence of structural heart disease, hypertension, diabetes mellitus, OSAS and kidney function, time from AF diagnosis to recruitment). Structural heart disease in this cohort was defined as presence of myocardial scar in cardiac MRI and/or abnormal myocardial wall thickness or mass and/or ventricular size/volume and/or left ventricular ejection fraction below the lower limit of normal (but not <40% at time of recruitment), Statistical significance was set at α 0.05. Statistical analysis was performed using IBM SPSS Statistics 27.
3 RESULTS
Forty two AF patients and 37 controls were recruited. Detailed baseline demographics are listed in Table 1. Patients in the AF cohort were on average 8 years older than controls (AF 64.6 ± 10.2 vs. control 56.6 ± 7.2 years, p < 0 .001) and had a higher CHA2DS2-VASC score by on average 1 point (AF 2.5 ± 1.5 vs control 1.5 ± 1.1, p = 0.002). Amongst the AF patients 69% (N = 29) were diagnosed with paroxysmal AF and 31% (N = 13) with persistent AF at time of recruitment. Time from AF diagnosis to recruitment in paroxysmal AF patients was on average 24 ± 71 months (range 4 to 192) and in persistent AF 45 ± 39 (range 14–162 months). Within the PAF group 14 (48%) were on class IC or III antiarrhythmic drug and 7 (24%) had undergone direct current cardioversion (DCCV) before their ablation. Within the persistent AF group 7 (53.8%) were treated with class IC or III anti-arrhythmics and 8 (61.5%) had a previous DCCV. None of the control patients had a history of AF or atrial flutter. A total of 76 contrast enhanced CTs (37 Control, 39 AF) were acquired. 3 patients with atrial fibrillation were excluded due to high calcium score and no contrast medium application, two CT scans had to be excluded due to insufficient slice numbers following data loss during the storage process, four patients had moderate step artefact at the level of the left atrium. 72 cardiac MRIs were acquired, two without gadolinium enhancement due to patient preference. 70 cardiac MRIs (35 AF, 35 control) were included in the final analysis.
| AF (N = 42) | Control (N = 37) | p-valuea | |
|---|---|---|---|
| Age (years) | 64.6 ± 10.2 | 56.6 ± 7.2 | <0.001 |
| CHA2DS2-VASc | 2.47 ± 1.52 | 1.54 ± 1.1 | 0.002 |
| Female | 15 (35.7%) | 13 (35.1%) | 0.921 |
| Hypertension | 22 (52%) | 13 (35%) | 0.124 |
| BMI (kg/m2) | 28.3 ± 4.1 | 27.8 ± 5.1 | 0.614 |
| BSA (m2) | 2.01 ± 0.19 | 1.95 ± 0.25 | 0.268 |
| Diabetes mellitus | 6 (14%) | 5 (13.5%) | 0.921 |
| CAD | 20 (47.6%) | 10 (27%) | 0.008 |
|
6 (14%) | 2 (5%) | |
| OSAS | 4 (9.5%) | 1 (2.7%) | 0.214 |
| PulM. disease | 10 (23.8%) | 9 (24.3%) | 0.427 |
| GFR (mL/min) | 80.6 ± 18.2 | 80.4 ± 16.2 | 0.972 |
| Previous stroke | 4 (9.5%) | 3 (8.1%) | 0.825 |
| LVEF % | 57.9 ± 10.5 | 60.4 ± 4.9 | 0.191 |
| Ventricular scar | 8 (19%) | 3 (8.1%) | 0.262 |
| Valvular disease | 5 (11.9%) | 0 (0%) | 0.095 |
| Anticoagulation | 35 (83%) | 0 (0%) | <0.001 |
| Antiplatelets | 5 (11.9%) | 11 (29.7%) | 0.49 |
| Beta blocker | 34 (80.9%) | 8 (21.6%) | <0.001 |
| AF pattern | |||
|
29 (69%) | n/a | |
|
13 (31%) | ||
| AF Durationb | |||
|
24 ± 71 | n/a | |
|
45 ± 39 | ||
| AAR Ic or III | 21 (50%) | 0 (0%) | <0.001 |
|
4 (9.5%) | ||
|
2 (4.7%) | ||
|
15 (35.7%) | ||
| DCCV | 15 (35.7%) | ||
|
7 (24%)c | n/a | |
|
8 (61.5%)c |
- Abbreviations: PAF, paroxysmal, PsAF, persistent.
- a Statistical significance set at α 0.05. Continuous variables reported as mean ± SD, categorical variables in absolute numbers and %.
- b Duration in months from first documented diagnosis to recruitment (irrelevant of total time/burden of AF).
- c % refers to patients within the respective subgroup of AF pattern.
3.1 Structural remodelling markers in AF compared to control
Patients with AF had increased left atrial wall thickness compared to controls (1.45 ± 0.52 mm vs 1.12 ± 0.42 mm, p = 0.003), left atrial myocardial mass (15.81 ± 6.53 g vs. 12.18 ± 5.01 g, p = 0.011) and absolute and indexed left atrial volume (LA volume 95.68 ± 26.63 mL vs 81.22 ± 20.64 mL, p = 0.011, BSA indexed volume 47.98 ± 13.2 vs 41.72 mL/m2, p = 0.021), respectively. Also, left atrial fibrosis burden as a percentage of total LA surface (9.33 ± 8.35% vs 2.41 ± 3.60%, p = 0.013) and absolute area fibrosis (17.42 ± 14.46 vs. 4.59 ± 7.06cm2, p = 0.002) was higher in AF patients compared to controls. Excluding patients in AF at the time of the scan, left atrial function as assessed by ejection fraction was lower in AF patients compared to controls (50.3 ± 15.3 vs 65.2 ± 8.6%, p < 0.001). Results for structural remodelling markers in AF compared to control are summarized in Table 2 and exemplary illustration provided in Figure 1.
| AF STATUS | |||||
|---|---|---|---|---|---|
| Total | AF | Control | p-value* | ||
| CT & CMR REMODELLING MARKERsa | LA wall thickness (mm) | 1.30 ± 0.50 | 1.46 ± 0.52 | 1.12 ± 0.42 | 0.003 |
| LA mass (g) | 14.07 ± 6.07 | 15.81 ± 6.53 | 12.18 ± 5.01 | 0.011 | |
| Atrial fibrosis (% area of LA surface) | 5.87 ± 7.28 | 9.33 ± 8.35 | 2.41 ± 3.60 | 0.013 | |
| Sphericity (CT) | 82.25 ± 6.72 | 83.40 ± 8.60 | 81.12 ± 3.40 | 0.146 | |
| LA volume (mL) | 88.45 ± 24.75 | 95.68 ± 26.63 | 81.22 ± 20.64 | 0.011 | |
| LAA Vvolume (mL) | 10.41 ± 4.21 | 12.59 ± 4.02 | 8.22 ± 3.18 | <0.001 | |
| Biplane LAEF (%) | 58.30 ± 14.20 | 50.27 ± 15.18b | 65.18 ± 8.59 | <0.001 | |
- Abbreviations: AF, atrial fibrillation; LA, left atrial.
- a all results reported as mean +/- standard deviation.
- b Patients in AF at time of CMR scan excluded (N = 6).
- * Significance level α = 0.05.
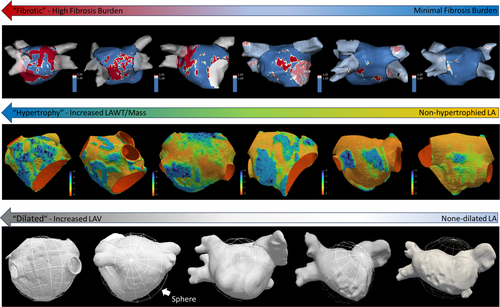
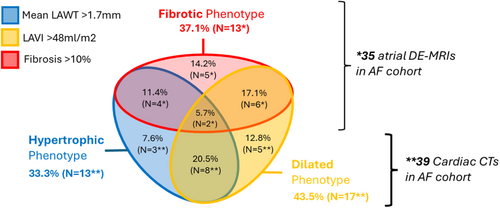
Within the AF group, 13 (37.1%) presented with a significant fibrosis burden defined as >10% of total LA surface, 11 (31.4%) with a fibrosis burden of 5%–10%, and 12 (34%) with <5% burden. Using a cut-off of LAVI 34 mL/m2, 89% of the AF cohort presented with a dilated LA and 43.5% with a severely dilated LA (LAVI > 48 mL/m2). Using the 90th percentile of the mean LAWT of the control cohort as cut-off (1.70 mm), 13 (33.3%) of the AF patients in the presented cohort may be classified as having an abnormally increased LAWT. Only 4 AF patients were found to have both significant fibrosis (>10%) and increased mean LAWT (>1.7 mm) and 6 AF patients had significant fibrosis and severely dilated left atria. Lastly, 8 patients with severely dilated LA also had abnormally increased LAWT (see Figure 2 for Venn diagram of different atrial phenotypes in the AF cohort).
3.2 Correlation of global remodelling markers derived from cardiac CT and atrial MRI
Including both arms, there was no significant correlation between % fibrosis with LAWT (r = 0.13, p = 0.29), mass (r = 0.02, p = 0.89), volume (r = 0.18, p = 0.13) or sphericity (r = 0.03, p = 0.79). LAEF had a statistically significant but weak negative correlation with fibrosis (r = −0.33, p = 0.008), LAWT (r = −0.24, p = 0.07) and LA volume (r = −0.29, p = 0.02), but not with sphericity or LA mass. Lastly, LAWT had a statistically significant weak positive correlation with LA volume (r = 0.25, p = 0.041), but not with sphericity (r = 0.25, p = 0.86).
Within the AF cohort, left atrial fibrosis was not statistically significantly correlated with left atrial wall thickness, mass, volume, sphericity or ejection fraction. Left atrial wall thickness in atrial fibrillation had a strong significant correlation with left atrial mass, but not with fibrosis, volume or ejection fraction. Left atrial volume had a significant weak to moderate correlation with left atrial mass and a moderate negative correlation with ejection fraction. A detailed summary of correlation analysis for the total cohort and sub-analysis for atrial fibrillation cohort is shown in Table 3.
| LAWT | LA Mass | Atrial fibrosis | Sphericity | LA volume | LAEF^ | |
|---|---|---|---|---|---|---|
| LAWT | - | 0.76 (p < 0.001) | 0.13 (p = 0.29) | 0.21 (p = 0.86) | 0.25 (p = 0.041) | −0.24 (p = 0.07) |
| LA Mass | 0.83 (p < 0.001) | - | 0.02 (p = 0.89) | 0.14 (p = 0.24) | 0.49 (p = 0.001) | −0.26 (p = 0.054) |
| Atrial Fibrosis | 0.02 (p = 0.89) | −0.06 (p = 0.764) | - | 0.03 (p = 0.79) | 0.18 (p = 0.13) | −0.33 (p = 0.008) |
| Sphericity | 0.16 (p = 0.35) | 0.15 (p = 0.392) | −0.07 (p = 0.71) | - | 0.23 (p = 0.054) | −0.17 (p = 0.171) |
| LA Volume | 0.17 (p = .0.33) | 0.41 (p = 0.013) | 0.15 (p = 0.42) | 0.22 (p = 0.18) | - | −0.29 (p = 0.02) |
| LAEF^ | −0.22 (p = 0.27) | −0.28 (p = 0.16) | −0.16 (p = 0.40) | −0.29 (p = 0.14) | −0.51 (p = 0.008) | - |
- Note: Correlation coefficient r with p value in brackets. Blue shading -correlation results for total cohort. Green shading – correlation analysis for AF cohort (including paroxysmal and persistent) only. ^6 patients in AF at time of CMR scan excluded.
- Abbreviation: LA, left atrial.
3.3 Impact of baseline demographics on structural phenotype
The results of the regression analysis to probe for a relationship between baseline demographics and cardiovascular risk factors with structural remodelling markers of CT and CMR are reported in Table 4.
| Age | Gender | BMI | SHD | HT | Diabetes | OSAS | eGFR | AFDurb | |
|---|---|---|---|---|---|---|---|---|---|
| LA Volume | r2 = 0.112 p = 0.004 |
r2 = 0.66 p = 0.027 |
r2 = 0.17 p = 0.275 |
r2 = 0.55 p = 0.044 |
r2 = 0.043 p = 0.075 |
r2 = 0.001 p = 0.765 |
r2 = 0.013 p = 0.335 |
r2 = 0.005 p = 0.565 |
r2 = 0.05 p = 0.205 |
| LAWT | r2 = 0.057 p = 0.638 |
r2 = 0.00 p = 0.871 |
r2 = 0.006 p = 0.536 |
r2 = 0.28 p = 0.164 |
r2 = 0.004 p = 0.614 |
r2 = 0.00 p = 0.954 |
r2 = 0.019 p = 0.252 |
r2 = 0.009 p = 0.428 |
r2 = 0.039 p = 0.265 |
| LA Fibrosis | r2 = 0.056 p = 0.048 |
r2 = 0.01 p = 0.399 |
r2 = 0.011 p = 0.398 |
r2 = 0.006 p = 0.538 |
r2 = 0.008 p = 0.467 |
r2 = 0.056 p = 0.048 |
r2 = 0.00 p = 0.981 |
r2 = 0.019 p = 0.256 |
r2 = 0.024 p = 0402 |
| LAEFa | r2 = 0.148 p < 0.001 |
r2 = 0.001 p = 0.827 |
r = 0.008 p = 0.489 |
r2 = 0.129 p = 0.003 |
r2 = 0.028 p = 0.180 |
r2 = 0.02 p = 0.695 |
r2 = 0.011 p = 0.409 |
r2 = 0.079 p = 0.024 |
r2 = 0.019 p = 0.451 |
- Abbreviations: HT, Hypertension; LA, left atrial; OSAS, obstructive sleep apnoe syndrome; SHD, Structural heart disease.
- a patients in atrial fibrillation at time of CMR scan excluded, bold = statistically significant results.
- b AFDur – Time from atrial fibrillation diagnosis to recruitment in months.
Left atrial volume was found to have a statistically significant relationship with age, gender and structural heart disease. In turn, BMI, Hypertension, Diabetes mellitus, OSAS and kidney function did not predict LAV. In this cohort, there was no statistically significant relationship between left atrial wall thickness with any of the selected baseline demographics including age, gender, BMI, presence of structural heart disease, hypertension, diabetes mellitus, OSAS, and kidney function. Left atrial fibrosis burden had a statistically significant relationship with age and diabetes mellitus, but not gender, BMI, structural heart disease, hypertension, OSAS or kidney function. Left atrial function had a statistically significant relationship with age, structural heart disease and kidney function but not gender, BMI, hypertension, diabetes mellitus or OSAS.
4 DISCUSSION
-
Left atrial wall thickness, mass, size, and fibrosis burden increased and left atrial ejection fraction decreased in presence of atrial fibrillation.
-
Individual remodelling features were not or only weakly associated with each other.
-
Specific demographics and risk factors for the development of atrial fibrillation (including advancing age, structural heart disease and diabetes mellitus) were associated with different patterns of atrial remodelling
Based on the results of this study we hypothesize that different structural remodelling patterns with preferential dilatation, myocardial hypertrophy or fibrosis may exist in patients with atrial fibrillation. If confirmed, detailed structural phenotyping may help to elucidate the underlying mechanism and allow individualized management strategies based on the identified pattern of structural remodelling. Assessment in a larger cohort may allow to derive thresholds for further sub-classification into “mild,” “moderate,” and “severe” fibrotic, hypertrophic and dilated phenotypes and refine definitions respectively diagnostic criteria for these phenotypes. Yet, all remodelling markers present in vivo as a continuum and categorization is always to a degree artificial and does not reflect the true nature of the disease process. Thus classifications should ideally be linked to prognostic outcomes and/or thresholds for treatment interventions. This requires prospective trials to be evaluated.
The extent of adverse atrial structural changes characterised by left atrial enlargement, atrial fibrosis and atrial function increased with age. The progression of these changes with increasing age is likely to reflect structural changes that contribute to the increasing prevalence of AF with age.16 The presence of structural heart disease, which is associated with an increased risk of AF, was associated with left atrial dilatation and decreased atrial function, while not shown to be associated with a greater degree of fibrotic remodelling. In turn, diabetes mellitus was associated with an increased degree of atrial fibrosis, while not associated with increased LA volume or function, which may reflect a particular pattern of atrial remodelling associated with DM. These observations suggest that comorbidities associated with AF promote particular patterns of remodelling that representing distinct structural AF phenotypes. This may depend on the specific processes responsible for remodelling and whether they represent primarily pro-fibrotic states or promote dilatation and reduced LAEF.
In this cohort, increased tissue thickness in the left atrium was associated with AF, which may reflect myocyte hypertrophy under loading conditions that promote AF. However, importantly, there was no association with conditions which may have presented plausible mechanisms by which atrial myocyte hypertrophy is promoted, specifically hypertension. Therefore, while increased tissue thickness is identified in AF patients - in this group of limited sample size at least - no significant association was found with baseline demographic features.
Overall, our results are in agreement with previously reported association of atrial fibrillation with abnormal changes in left atrial size,17 atrial wall fibrosis (assessed by CMR)18, 19 and thickness20 although we were unable to replicate findings of other studies in regard to the importance of geometrical assessments like sphericity21, 22 in atrial fibrillation. Bisbal et al.21 used CMR to estimate sphericity (compared to CT in our cohort) and reported spherical LA geometries to be more prevalent in persistent AF patients, of which our cohort included a comparatively low number. This may have contributed to the discrepancies in the results. An additional marker not assessed in our study, is epicardial fat infiltration,23 which may be of additional diagnostic value and could be derived from the available CT data.
Our data provides further evidence that the structural changes that occur in patients with AF are complex and heterogenous requiring detailed individualised assessment and treatment strategies to effectively reduce arrhythmia burden, prevent complications and manage symptoms. This has already been reflected in the most recent ESC guidelines24 recommending a structured characterisation of atrial fibrillation to address specific domains (Stroke Risk, Symptom Severity, AF burden Severity, Substrate Severity) to inform and tailor multidisciplinary management strategies. The importance and implications of atrial remodelling has been recognised also beyond the field of atrial fibrillation and the concepts of atrial cardiomyopathies proposed.25
With the significant technological improvements in noninvasive imaging modalities26, 27 assessment and quantification of severity of atrial structural alterations as employed in our study has now become feasible.
5 LIMITATIONS
Most importantly, patients in the AF cohort were significantly older and, related to this, also had a higher CHA2DS2 VASc score than controls. Given the important impact of age on remodelling markers this may have affected the comparison to the controls independent of AF.
No detailed evaluation of blood pressure control and sleep apnoea index was undertaken as part of this study, although patients diagnosed with hypertension or sleep apnoea were on active treatment which might have mitigated the effect on structural atrial changes. Also, none of the participates had significant valvular heart disease. Further evidence derived from a larger cohort is required to better characterise and quantify the impact of clinical baseline demographics on atrial structural remodelling phenotypes.
The number of CT and CMR scans included in the final analysis was relatively low, however this was a hypothesis generating study and facilitated consistent data collection across the cohort. The study exclusively employed noninvasive assessments of structural alterations therefore is presented without histological validation to define the ground truth. This is a necessity for clinical studies in which tissue analysis is not available. Also, for each of the assessed structural parameters, different calculation and estimation methods have been reported and there is often no consensus on the preferred methodology. In each case the method chosen was a previously reported approach that was felt to represent a reasonable strategy, but it is acknowledged that other methods are available in each case and the use of alternative methods might impact the results. Also, analysis as performed in our study requires access to dedicated processing software, some of which were commercial (Adas3DTM), and may not be available at all institutions and thus limit wide-spread use of this advanced atrial imaging methods.
Lastly, this study was not designed for establishing a predictive model of atrial structural remodelling. Association of clinical baseline demographics and structural remodelling markers are hypothesis-generating only and the nature of the study prevents inferring a causative relationship.
Association of time from AF diagnosis to remodelling markers is a suboptimal surrogate for AF burden, which is arguably the more relevant driver of structural remodelling in AF. Yet detailed information about the exact individual burden was not available in our cohort and high proportion of patients had undergone successful active rhythm control strategies (pharmacological or by DCCV) ahead of their referral for AF ablation possible further contributing to the lack of association between time from AF diagnosis and remodelling markers.
6 CONCLUSION
AF is associated with significant quantifiable structural changes that are evident in LA size, tissue thickness, total LA tissue mass and fibrosis. These individual remodelling markers do not or only weakly correlate with each other suggesting different atrial remodelling subtypes may exist (e.g. fibrotic vs hypertrophic vs dilated). Different patterns of structural remodelling were associated with different conditions known to be associated with AF. Detailed structural phenotyping may identify the prevalent remodelling processes within individual patients which may form the basis for identifying specific groups who benefit from individualised therapy directed at the predominant pattern of remodelling associated with that condition. In addition, focused structural assessment may be a valuable tool to assess reversibility and reverse remodelling following interventions targeting atrial fibrillation and associated conditions predisposing to AF.
ACKNOWLEDGMENTS
This study was supported by the Medical Research Council for research costs and Clinical Research Training Fellowship for JW (CRTF grant reference MR/N001877/1).
ETHICS STATEMENT
The study received a favourable opinion from a national Research Ethics Committee (REC, ref 15/LO/1803). All enrolled patients gave written informed consent for the procedure and the publication.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.