From Genes to Shapes: Exploring Local Adaptation in Carpathian Ox-Eye Daisies
Funding: This research was funded by the National Science Centre (Poland) for the project ‘Phylogeography and Hybridization Within Carpathian Oxeye Daisies (Leucanthemum, Compositae)’, grant no. 2016/23/D/NZ8/00935.
ABSTRACT
Aim
Historical processes have shaped the Carpathian biogeography, yet ongoing evolutionary forces continue to drive population differentiation. We aimed to test whether local adaptation in the Carpathian subendemic Leucanthemum rotundifolium correlates with genetic, morphological and environmental factors, and to assess how these patterns relate to established taxonomic concepts.
Location
The Carpathian Mountains, Central Europe.
Taxon
The genus Leucanthemum (Asteraceae), with a focus on L. rotundifolium.
Methods
We analysed multiple populations of L. rotundifolium across its Carpathian distribution. Each individual was characterised using a comprehensive set of measures encompassing: (1) genetic variation via SNP, Silico-DArT and chloroplast markers; (2) morphological traits, including detailed measurements and shape analysis of involucral bracts; and (3) environmental variables such as climate, soil characteristics, topography and indicators of human disturbance. We first evaluated how these data collectively distinguish different Leucanthemum species and align with current taxonomic concepts. Next, we examined how genetic, morphological and environmental factors interact within L. rotundifolium to differentiate individuals and populations.
Results
Taxonomically recognised species within Leucanthemum were supported by distinct genetic signatures (particularly SNPs), environmental preferences and key morphological traits (notably the measurements of the upper row of involucral bracts). At the intraspecific level, L. rotundifolium populations were primarily differentiated by genetic variation (across all markers) and by environmental factors. In contrast, morphological variability showed no strong spatial signal, though subtle tendencies toward differentiation emerged when morphological traits were interpreted in conjunction with consistent genetic and environmental clustering.
Main Conclusions
Our findings highlight that the taxonomy of Leucanthemum corresponds closely with genetic, environmental and certain morphological parameters. Within L. rotundifolium, local adaptation is strongly reflected in genetic and environmental divergence, while morphological traits appear more conservative. Nevertheless, subtle morphological shifts may still track underlying genetic and ecological differentiation, revealing a complex interplay of factors shaping the evolutionary trajectory of this Carpathian subendemic.
1 Introduction
How natural selection acts on a species whose range has been fragmented into several disjunct areas remains a fundamental question in evolution and biogeography. While natural selection is widely recognised as the principal force shaping genetic architecture in plant populations (Linhart and Grant 1996), initial isolation often arises through genetic drift, with local adaptation proceeding thereafter (Kawecki and Ebert 2004). Adaptation may become evident on a ‘visible’ level through morphological traits, whose variability and plasticity can sometimes facilitate responses to novel environmental conditions (e.g., rapid climatic changes; Fenollosa and Munné-Bosch 2019). Equally important are ‘invisible’ adaptations, such as enhanced physiological or metabolic tolerances (e.g., salt or drought stress), which can substantially affect fitness yet remain undetected by external morphology (Munns and Tester 2008). Indeed, intraspecific trait variation plays a pivotal role in ecological processes, influencing competition, resource use and community dynamics (Westerband et al. 2021). Understanding how these different facets of adaptation unfold in spatially isolated populations can thus shed light on both short-term evolutionary trajectories and longer-term speciation events.
We aim to link these concepts of local adaptation, population differentiation and biogeographic history to the specific context of the Carpathians, a region known for its complex orography and long-term refugial role for many taxa (Juřičková et al. 2014; Ronikier 2011). While multiple studies have addressed Carpathian biogeographic barriers, they typically focus on a single organism or a single data type, leaving gaps in our understanding of how various lines of evidence (e.g., genetic, morphological, environmental) intersect. In this work, we examine multiple Leucanthemum taxa and focus on one organism—round-leaved ox-eye daisy (Leucanthemum rotundifolium)—across several marker systems and trait datasets to investigate the spatial dimensions of population structure. Our goal is to identify robust evolutionarily significant units that exhibit coherent membership across genetic, morphological and environmental axes, thereby providing a solid framework to investigate local adaptation, colonisation history and conservation strategies in the Carpathians.
Floral characters are among the primary classification tools for many plant groups (Fu et al. 2023). Beyond their taxonomic importance, they often fulfill critical ecological functions. In Leucanthemum and other members of the Asteraceae, the inflorescence (i.e., capitulum) hosts numerous seeds, making it an attractive target for herbivores. Consequently, plants have evolved multiple traits to safeguard their reproductive investment, thus ensuring successful gene transmission. Here, we focus on one such adaptive feature typical of the Asteraceae family: the involucral bracts (phyllaries). A hallmark of the pseudanthium-type capitulum in Asteraceae (Hernández and Rosetti 2016; Zhang and Elomaa 2024), these bracts—together with the receptacle—form a protective barrier around the developing flowers, ovaries and seeds, shielding them from both biotic (e.g., pre-dispersal seed predators, herbivores, pathogens) and abiotic stressors (drought, UV radiation; Villagra et al. 2014).
While Leucanthemum species typically share a similar overall flower-head arrangement (orange disk florets surrounded by white ligulate florets; Andersson 2008; Cerca et al. 2019; Stuessy et al. 1986), the involucre exhibits comparatively greater variation. Such variation may hold adaptive potential. Not only does it serve as an ecological shield against external factors, but morphological traits of the bracts—such as coloration, shape and venation—are also commonly used in species delimitation (Oberprieler et al. 2022; Vogt 1991). The rib of the phyllaries is often dark (sometimes nearly black), and the bracts themselves may carry pigmented veins (brown or otherwise). These pigments can produce chemicals that either deter or intoxicate herbivores (Lusa et al. 2018; Pandey and Dhakal 2001), representing a subtle but critical defence strategy.
The ecological relevance of these defenses is evident in the considerable rates of pre-dispersal seed predation documented in Leucanthemum. Up to 26% of flower heads can be infested by seed-eating insects (Fenner et al. 2002), including flies (Diptera; Tephritis neesii, Ozirhincus longicollis), thrips (Thysanoptera; Haplothrips leucanthemi) and beetles (Coleoptera; Microplontus campestris). These insects bore into the receptacle, compromising both the flowers and seeds. Understanding how variation in involucral bracts influences these interactions may shed light on the evolutionary pressures that shape morphological differentiation and local adaptation within Leucanthemum and related taxa.
The Carpathians constitute a recognised biodiversity hotspot at the continental scale, hosting numerous endemic species and serving as a corridor for faunal and floral migrations (Bálint et al. 2011; Harl et al. 2014; Mráz and Ronikier 2016; Pawłowski 1970; Ronikier 2011). They are part of a broader mountain system connecting the Alps, Scandinavian Mountains, Balkan Mountains and additional ranges to the east (Janiczek et al. 2025; Mráz and Ronikier 2016; Schmitt 2007). However, today they function largely as isolated ‘mountain islands’ given the limited number of high-altitude peaks and their fragmented distribution (Favilli et al. 2015; Papp et al. 2022). Traditionally, the Carpathians are divided into the Western, Eastern and Southern Carpathians, along with the Apuseni Mountains. Yet, historical links with adjacent ranges are evident: climatic suitability models show that corridors emerged during colder phases of the Pleistocene, then disappeared in warmer periods (Jankovská and Pokorný 2008; Juřičková et al. 2019; Konowalik 2022). This cyclical connectivity helps explain why regions now separated—such as the Apuseni and Rakhiv Mountains, or the Western and Eastern Carpathians—were once joined by more continuous habitats. These fluctuations in habitat connectivity have played a crucial role in shaping the present-day biodiversity and endemism observed across the Carpathian arc (Hurdu et al. 2016; Mráz and Ronikier 2016).
Morphological variation frequently underpins species delimitation, yet the degree to which these visible traits mirror genetic divergence—whether assessed via haplotypes, SNPs or other markers—remains uncertain and needs to be assessed for a taxon in question (Cornuault et al. 2024; De Giorgi et al. 2022; Hightower et al. 2024; Hodač et al. 2023; Jacobs et al. 2020; Reinhart et al. 2024). Our dataset encompasses both allopatric populations and separate taxa, but it includes also cases in which distinct haplotypes show little or no morphological divergence. Consequently, we ask: how do morphological, genetic and environmental datasets converge—or diverge—in defining population groups and identifying barriers? By comparing multiple marker systems (morphometric data, genetic markers and environmental parameters), we aim to pinpoint robust clusters and evolutionarily significant units, shedding light on how historical processes, geographical isolation and local adaptation have shaped ox-eye daisies as an example of the Carpathian flora. Our findings ultimately tie into broader discussions of biodiversity conservation in this mountain region, illustrating how different data sources can either reinforce or challenge existing notions of species boundaries and biogeographic barriers in the Carpathians.
2 Materials and Methods
2.1 Study Species
This study focuses primarily on the Carpathian subendemic Leucanthemum rotundifolium (Zelený 1970), along with five additional species (L. ircutianum DC., L. gaudinii Dalla Torre, L. × pawlowskii Piękoś, L. vulgare Lam., and L. illyricum [Horvatić] Vogt and Greuter). We sampled populations from the entire Carpathian range of L. rotundifolium and included one disjunct population from the Dinaric Alps (Data S1). Although L. rotundifolium was historically documented at three sites in the Dinaric Alps (Horvat and Pawłowski 1938; Paczoski 1932), later confirmed by several botanists in the 20th century (KRA and KRAM herbarium records), we were able to relocate only one of these populations during fieldwork in 2017 and 2018. For each sampled plant, both flowering heads and leaves were placed together in a teabag and dried in silica gel inside an airtight container, ensuring that morphological and genetic data could be matched to the same individuals.
2.1.1 Choice of Morphological Characters
Inflorescence elements in Asteraceae typically follow a Fibonacci spiral arrangement (Zhang et al. 2021). The involucral bracts (phyllaries) of Leucanthemum exhibit a visual arrangement in three or more series (rows, Figure 1). We selected these bracts for measurement because they are taxonomically significant in Leucanthemum systematics (Figure 1a–h; Oberprieler et al. 2022; Piękoś 1971; Vogt 1991), robust enough to remain undamaged during silica-gel drying, and ecologically relevant, as they protect developing inflorescences from insect herbivory and environmental stresses (Figure 1i,j; Song et al. 2024). By contrast, rosette or stem leaves in Leucanthemum can be highly variable and more prone to weather-related damage, mechanical constraints, or inconsistent positional identity among different specimens (e.g., ‘middle’ leaves may not be directly comparable across plants; Zelený 1970). Given these considerations, involucral bracts represent a more consistent and potentially adaptive trait, making them well suited for comparative morphometric analyses.
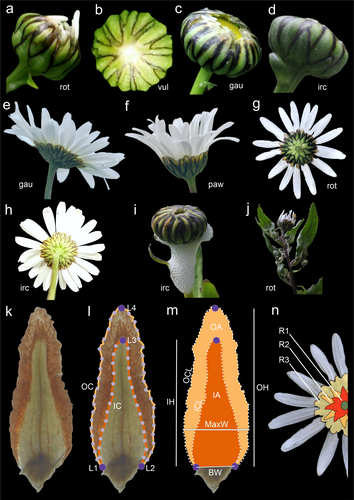
2.2 Wet-Lab Protocol
Dry flower heads were placed in Petri dishes and immersed in boiling water, then left overnight to soften. The following day, inflorescences were dissected in water, with special attention given to the involucral bracts (Figure 1k). From each flower head, fully developed, undamaged bracts were collected from three rows (Figure 1n)—the lowest (R1), middle (R2) and upper (R3)—and placed on a microscope slide. Observations were performed using a Nikon SMZ25 stereo microscope equipped with a 0.5× lens. Photographs were taken with a scale reference, using NIS-Elements software, and then imported into tpsDig2 ver. 2.32 (Rohlf 2005, 2015), where the scale was read for calibration.
Four landmarks were placed on each bract: L1 and L2 at the base, L3 at the inner curve apex, and L4 at the outer curve apex (Figure 1l). Two curves were then defined along the inner and outer margins of the bract—one encompassing the inner green part and the other the outer brown margin—each originating from the two base landmarks. The inner curve was subsequently resampled into 30 semilandmarks, and the outer curve into 40 semilandmarks, providing a detailed morphometric representation of bract shape (Figure 1l).
2.3 Morphometric Measurements
The landmark coordinates were imported from TPS files into R using the geomorph package (Adams et al. 2021) separately for each row of involucral bracts (R1, R2, R3). Procrustes alignment, without scaling or reflection, was performed to standardise the positions of landmarks size-related information, given the focus on absolute dimensions. Landmark coordinates were aligned using the Generalised Procrustes Analysis (procGPA function), ensuring comparability across specimens. Inner and outer curves were subsequently aligned by applying the Procrustes transformation matrices. For each specimen, key morphometric indices were computed. Measurements included inner bract height (IH), outer bract height (OH), inner curve length (ICL), outer curve length (OCL), base width (BW, defined as the distance between L1 and L2 landmarks) and maximum width (MaxW), calculated as the greatest horizontal distance between points on the outer curve and the mean x-coordinate of the base landmarks, representing the widest part of the bract perpendicular to its height, excluding the base width (Figure 1m). Shape areas were calculated for both the inner curve (IA) and the outer curve (OA) using polygon geometry, which were summed to determine the total bract area (BA = IA + OA). Additional indices, such as inner aspect ratio (IAR = IH/MaxW), outer aspect ratio (OAR = OH/MaxW), inner compactness (IC = IA/ICL2), outer compactness (OC = OA/OCL2), average inner curvature (average angle change between consecutive segments normalised by segment length, summed across the curve), average outer curvature and symmetry indices (based on left–right balance), were derived to quantify shape characteristics (Figure 1m, Data S2). Morphometric indices were used to compute a pairwise Euclidean distance matrix for all specimens. All analyses were conducted in R (v4.4.1 (R Core Team 2021)), employing the packages geomorph (Adams et al. 2021), shapes (Dryden 2023), ggplot2 (Wickham, Chang et al. 2023) and dplyr (Wickham, François et al. 2023).
2.4 Morphometric Shape Analaysis
Landmark-based morphometric data for three involucral bract rows (R1, R2, R3) were imported from TPS files using the geomorph package (readland.tps) with scaling enabled. Curves associated with semilandmarks were defined programmatically for inner (30 semilandmarks) and outer (40 semilandmarks) curves, with slider matrices specifying the start and end landmarks for each curve. Generalized Procrustes Analysis (gpagen) with Procrustes distance scaling (ProcD) was applied to align landmarks while optimizing semilandmark positions for geometric consistency. Pairwise Procrustes distances between specimens were calculated using iterative comparisons of aligned landmark configurations (procrustes), and results were stored in a distance matrix.
2.5 Genetic Data (SNP and SilicoDArT) Processing
The methods for DArTSeq and Single Nucleotide Polymorphism (SNP) data overlap with those used by Konowalik et al. (2025), but the datasets were generated anew from the raw data. Silico-DArT markers are dominant presence/absence markers analogous to those obtained through AFLP or microarray-based scoring methods. Files obtained from the sequencing company (Diversity Arrays Technology Pty Ltd., Australia) were imported using the dartR package (Gruber et al. 2023), and individual assignment files were created and applied to align samples across datasets. The datasets were filtered to retain loci with a call rate > 90% (gl.filter.callrate) and reproducibility > 95% (gl.filter.reproducibility) and to remove monomorphic loci (gl.filter.monomorphs) and loci with missing data (gl.filter.allna). Pairwise genetic distances were calculated using the Euclidean method for SNP data and the Jaccard method for SilicoDArT data (gl.dist.ind).
2.6 cpDNA Data Processing
Methods for chloroplast DNA (cpDNA) sequences (psbA-trnH, ndhC-retF, trnT-GUU) overlapped with those used by Konowalik (2022), with the addition of 60 individuals. Chromatograms were manually screened and aligned using BioEdit (Hall 1999) and mafft (Katoh and Standley 2013). Gaps were coded using the simple indel coding method of Simmons and Ochoterena (2000). Gaps that produced reticulate events in the haplotype network constructed using TCS (Clement et al. 2000) and popart (Leigh and Bryant 2015) were removed. The data were then imported into R and analysed using the ape package (Paradis et al. 2004). Pairwise distances were computed using the Tamura-Nei model (dist.dna) and exported as a matrix.
2.7 Environmental and Geographic Distance Analysis
Environmental variables were extracted from the CHELSA 2.1 dataset covering the period 1981–2010 (Karger et al. 2017, 2018). Raster layers representing non-categorical, uncorrelated bioclimatic variables were pre-screened for multicollinearity (r > 0.75) and stacked using the raster package (Hijmans et al. 2021). Environmental values were extracted at specimen occurrence points based on their geographic coordinates. The list of climatic layers is available in the supplementary file (Data S3). Extracted environmental variables were standardised using scale to ensure comparability.
Geographic distances were computed using the Haversine formula (geosphere::distHaversine) based on the latitude and longitude coordinates of specimen occurrences (Hijmans et al. 2024).
Soil variables were sourced from SoilGrids (Hengl et al. 2014), which provides globally consistent, high-resolution soil property data. Correlation among variables was assessed, and highly correlated features were excluded (r > 0.75). The retained variables are listed in Data S3. Soil variables were extracted at occurrence points based on their geographic coordinates.
Topographic variables were derived from the MERIT DEM (Yamazaki et al. 2017) a high-resolution digital elevation model. Metrics characterising topographic features were calculated using SAGA GIS (ver. 9.71, (Conrad et al. 2015)). These variables characterise the physical landscape and its potential influence on the occurrence of species. All derived topographic metrics are detailed in the Supporting Information (Data S3).
The Human Disturbance Index (HDI) was used to quantify the impact of human-made structures. HDI raster data were obtained from a dataset specifically developed for the Carpathians (Konowalik et al. 2025). Values were extracted at specimen occurrence points. For all datasets, pairwise distances between specimens were calculated using Euclidean distance (dist), separately for climatic, soil, topographic and HDI variables.
2.8 Combined Distance Analysis and Mantel Tests
To ensure comparability across different datasets, all distance matrices were standardised. A reordering function was applied to all matrices to align their row and column names with the reference order. We analysed two datasets separately: (1) containing all species, (2) containing only L. rotundifolium individuals. For each unique pair of matrices, a Mantel test was performed using the mantel function from the vegan package in R (Oksanen et al. 2024). Pearson's correlation method was used with 10,000 permutations to assess statistical significance. To account for multiple comparisons, p-values from the Mantel tests were adjusted using the Bonferroni correction method. To control for the potential confounding effect of geographic distance, Partial Mantel tests were performed. These tests assess the correlation between two distance matrices while controlling for the geographic distance matrix. Pairwise Partial Mantel tests were conducted for all combinations of matrices using function mantel.partial from the vegan package with Pearson's correlation method and 10,000 permutations. Adjusted p-values were calculated using the Bonferroni correction to account for multiple testing.
2.9 Clustering
Clustering was performed on each dataset represented as a distance matrix reflecting similarities or differences among individual samples. Two clustering approaches were employed: hierarchical clustering using the Unweighted Pair Group Method with Arithmetic Mean (UPGMA) and partitioning around medoids (PAM). To determine the optimal number of clusters for each distance matrix, a silhouette-based criterion was applied. Specifically, for each dataset, hierarchical clustering was performed over a range of possible cluster numbers (k = 2 to 10), and the configuration yielding the highest mean silhouette width was selected. The final number of clusters determined by this process ensured that each partitioning balanced internal cohesion and separation. Similar to UPGMA, the optimal number of clusters for PAM was chosen based on the silhouette criterion, iterating over a range of k values and selecting the one that maximised the average silhouette width. For each dataset and clustering method, final cluster memberships were extracted and consolidated into a single data frame, allowing direct comparison of cluster assignments across variables and methods (Data S4).
To quantify the consistency of cluster assignments across different datasets, we used the Adjusted Rand Index (ARI). The ARI measures the agreement between two cluster solutions while correcting for random chance, thereby providing a more robust assessment of consistency than simple overlap measures. Values range from −1 to 1, where 0 indicates an agreement no better than random expectation, and 1 signifies perfect correspondence between partitions. ARI values were computed for every pairwise combination of datasets separately for both UPGMA- and PAM-derived clusters, enabling a direct evaluation of clustering stability and comparability across multiple variables and methods.
To infer spatially consistent clusters across different datasets and designate evolutionarily significant units (ESUs), we utilised UPGMA-derived clusters for SNP, Silico-DArT, cpDNA and environmental data, as these datasets exhibited similar spatial structures. For each individual, a unique cluster key was created by combining their cluster assignments from each dataset. Individuals were then grouped based on this cluster key. Groups containing fewer than ten individuals were excluded. The final groups comprised individuals with consistent clustering across all datasets. Individuals assigned to more than one cluster were designated as ‘unassigned’. An exception was made for the ‘Southern’ group, where SNP analysis identified a few separate individual clusters that were merged.
Distance matrices were exported from the R environment as plain text files and imported into Barrier 2.2 software (Manni et al. 2004) to infer geographical barriers. In Barrier, the number of barriers was predetermined by setting it to the number of UPGMA clusters minus one, aligning with the hypothesis that each cluster represents a distinct group.
3 Results
3.1 Mantel and Partial Mantel Tests
The Mantel (rM) and Partial Mantel (rPM) tests revealed several significant relationships among genetic, environmental, morphometric and geographic variables in the multispecies dataset. In the multispecies dataset, morphometric shapes of involucral bracts exhibited moderate positive correlations (Figure 2a). Specifically, the 1st row of bracts was correlated with the 2nd row (rM = 0.16, p = 0.01) and with the 3rd row (rM = 0.17, p = 0.01). After controlling for geographic distance, these correlations remained significant (rPM = 0.15 and rPM = 0.17, respectively; p = 0.009), indicating that the relationships are not solely due to spatial proximity. Measurements of bract size showed strong associations, particularly between the 3rd row and the 1st row (rM = 0.85, p = 0.01), which remained significant after controlling for geography (rPM = 0.74, p = 0.009). Genetic markers displayed strong inter-correlations. SNP distances were strongly correlated with SilicoDArT distances (rM = 0.66, p = 0.01; rPM = 0.65, p = 0.009), reflecting congruence between nuclear genomic markers, independent of geography. SNP distances also showed moderate correlations with the morphometric shape of bracts in the 2nd row (rM = 0.17, p = 0.01; rPM = 0.15, p = 0.009) and the 3rd row (rM = 0.11, p = 0.01; rPM = 0.10, p = 0.009), suggesting a genetic influence on bract morphology beyond spatial effects. Environmental distance was strongly correlated with cpDNA distances (rM = 0.77, p = 0.01; rPM = 0.64, p = 0.009) and with SNP distances (rM = 0.52, p = 0.01; rPM = 0.41, p = 0.009), indicating that environmental factors are associated with genetic differentiation among species, even when accounting for geography. Geographic distance correlated significantly with genetic distances, including SNP (rM = 0.37, p = 0.01) and cpDNA (rM = 0.56, p = 0.01), suggesting isolation by distance effects. It also showed strong correlations with bract measurements, particularly for the 3rd row of bracts (rM = 0.73, p = 0.01), indicating spatial structuring of morphological traits. Taxonomy was highly correlated with genetic distances, notably SNP (rM = 0.71, p = 0.01; rPM = 0.66, p = 0.009) and cpDNA distances (rM = 0.63, p = 0.01; rPM = 0.47, p = 0.009), suggesting that genetic differentiation aligns with taxonomic classification independently of geography. Some correlations diminished after controlling for geographic distance. For instance, the correlation between measurements for the 1st row of bracts and cpDNA distances was significant in the Mantel test (rM = 0.22, p = 0.01) but became non-significant in the Partial Mantel test (rPM = −0.21, p = 1), indicating that the initial correlation was largely due to spatial effects.
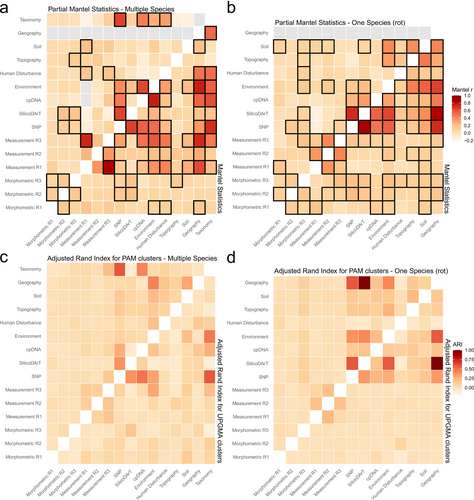
In the single-species dataset, SNP distances were highly correlated with SilicoDArT distances (rM = 0.88, p = 0.009; rPM = 0.73, p = 0.008), showing consistent genetic patterns within the species beyond geographic influence (Figure 2b). SNP distances also correlated strongly with geographic distance (rM = 0.72, p = 0.009), suggesting isolation by distance within the species. Environmental distance correlated significantly with SNP distances (rM = 0.56, p = 0.009) and remained significant after controlling for geography (rPM = 0.17, p = 0.008), indicating environmental influences on genetic variation partially independent of spatial proximity. Shapes in the 3rd row of bracts correlated with 2nd row (rM = 0.18, p = 0.009; rPM = 0.16, p = 0.008), suggesting consistency in bract shape within the species not solely due to geographic proximity. The correlation between the shape of bracts in the 3rd row and SNP distances was significant (rM = 0.20, p = 0.009) and remained so after controlling for geography (rPM = 0.13, p = 0.016), indicating a genetic influence on bract shape beyond spatial effects. Topography was significantly correlated with environmental distance (rM = 0.45, p = 0.009; rPM = 0.35, p = 0.008) and with soil properties (rM = 0.48, p = 0.009; rPM = 0.40, p = 0.008), suggesting that topographical variation influences environmental conditions and soil characteristics independently of geography.
3.2 Clustering Consistency Across Multiple Datasets (ARI)
For the multispecies dataset, comparing partitions derived from different sets of variables revealed distinct patterns of consistency (Figure 2c). Under the PAM algorithm, SNP-based clustering aligned relatively well with the taxonomic classification (ARI ~0.60), while cpDNA-based clusters also showed some agreement with taxonomy (ARI ~0.44). These findings indicate that genomic variation at both nuclear and chloroplast levels matches established taxonomic boundaries. Environmental distance-based clusters displayed moderate overlap with both cpDNA and SNP partitions (ARI values generally below 0.25), suggesting some influence of environmental gradients on genetic structure. Morphometric traits were less consistently aligned with genetic or environmental clusters, although a few moderate agreements emerged, such as measurement of bract size in the 3rd row with taxonomy (ARI: ~0.24).
Under UPGMA, the overall pattern persisted, with SNP and taxonomy maintaining good agreement (ARI: ~0.60) and cpDNA and taxonomy also displaying moderate congruence (ARI: ~0.28). Environmental clustering exhibited somewhat closer alignment with genetic variables and taxonomy compared to PAM, reflecting a potentially enhanced capacity of hierarchical clustering to capture subtle environmental–genetic associations. Nonetheless, environmental and morphometric clusters generally remained less synchronised than the genetic–taxonomy link.
In the single-species dataset, clustering patterns were strongly shaped by geographic structure and genetics (Figure 2d). Using PAM, SilicoDArT- and geographic-derived clusters aligned exceptionally well (ARI = 1.00), indicating virtually identical partitioning of individuals. Similarly, SNP and geographic clusters displayed substantial overlap (ARI: approximately 0.60), and environmental clustering showed moderate agreement with both SNP and SilicoDArT partitions (ARI: approximately 0.38 and 0.42, respectively), suggesting that local environmental variation influences genetic structure within the species. cpDNA-based clusters were less strongly aligned with geography or genetics (ARI values generally below 0.24), and morphometric traits again displayed lower concordance, as seen in the moderate overlap between measurement of bract size in the 3rd row and SNP (ARI: ~0.13).
Applying UPGMA to the single-species dataset generally reinforced these patterns. SilicoDArT and geographic clusters remained perfectly matched (ARI = 1.00), SNP and environmental partitions continued to show moderate agreement (ARI: ~0.29), and environmental and SilicoDArT clusters aligned strongly (ARI: ~0.59). While these UPGMA results suggest that certain clustering methods can enhance the apparent concordance between environmental, genetic and geographic partitions, the relatively low ARI values for morphometric traits persisted, underscoring their more complex or independent patterns of variation.
A strong spatial signal emerged from several datasets, particularly SNP, Silico-DArT, cpDNA and environmental variables (Figure 3a–d). In contrast, soil, topography, HDI and morphometric data showed less spatial consistency, often clustering individuals from geographically distant regions and yielding overlapping group structures (Figure 3e–l). All methods consistently singled out the Vranica Mountains' population as a distinct cluster. The most nuanced subdivision appeared in the SNP dataset, which identified 10 clusters in total (Figure 3a). Specifically, one cluster encompassed populations from the Western Carpathians and the North-Eastern Carpathians, while the Apuseni Mountains formed a separate cluster, followed by three more clusters in the Eastern Carpathians and four additional clusters in the Southern Carpathians. Silico-DArT similarly grouped the Western and North-Eastern Carpathians together but merged the Southern and Eastern Carpathians into one large cluster (Figure 3b). Environmental variables produced broadly comparable results to Silico-DArT, although one high-elevation population in the Tatra Mountains was separated, and the North-Eastern Carpathians diverged from the Western Carpathians (Figure 3d). The Southern Carpathians and Apuseni Mountains formed another large group. By contrast, cpDNA data highlighted a single large cluster comprising the Western Carpathians, Eastern Carpathians, Apuseni Mountains and Banat Mountains, a second cluster uniting the Southern Carpathians with part of the South-Eastern Carpathians, and a small, localised cluster in the Rakhiv Mountains (Figure 3c). When considering the congruence of these patterns across datasets, six primary groups emerged: Western Carpathians, three subdivisions in the Eastern Carpathians, the Southern Carpathians (after merging individual SNP clusters) and Vranica Mountains.
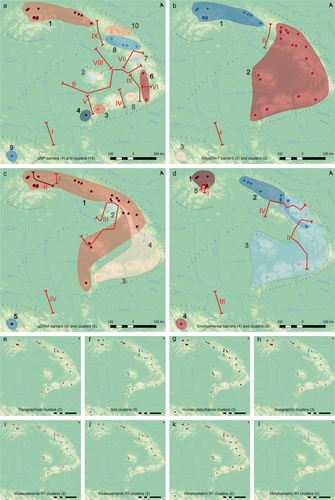
Inferred barriers closely mirrored the clustering patterns in the SNP and Silico-DArT data (Figure 3a,b). Although cpDNA and environmental barriers did not align perfectly, they largely separated similar regional blocks (Figure 3c,d). For cpDNA, a barrier was detected within the Western Carpathians and around the northern Apuseni Mountains and Rakhiv Mountains; for environmental data, the high-elevation Tatra Mountains' population and portions of the Eastern and South-Eastern Carpathians were distinctly partitioned. Notably, all datasets consistently indicated a clear barrier between the Vranica Mountains and the main Carpathian arc.
3.3 PCA Patterns
For single-species dataset comprising different Leucanthemum rotundifolium individuals, the strength of separation among regional populations varied depending on the type of variable analysed through Principal Component Analysis (PCA) (Data S5). PCA revealed clear patterns of population structure, particularly when based on SNP, SilicoDArT, cpDNA, environmental and geographic data. In contrast, morphometric traits, morphometric shape descriptors, topographic and soil variables produced more diffuse patterns, often showing considerable overlap among individuals. The Vranica population was consistently well-separated across most PCA plots, indicating its distinctiveness. Individuals from the Eastern and Western Carpathians frequently clustered together, while those from the Southern Carpathians and Apuseni Mountains tended to group more closely with one another.
4 Discussion
4.1 Comparison of Spatial Scales
Our analyses revealed that taxonomy exerts a strong influence when examining multiple Leucanthemum taxa. Taxonomic identity strongly correlates with environmental variables and human disturbance levels, suggesting that each species occupies distinct ecological niches and experiences varied degrees of anthropogenic impact. Likewise, genetic differentiation (especially in the SNP and cpDNA datasets) aligns closely with taxonomic boundaries—even after controlling for geography—underscoring that historically divergent lineages maintain unique genetic signatures.
Morphological traits, though also shaped by taxonomy, exhibit somewhat weaker associations. For example, Mantel correlations for bract shape (rows 1 and 3) with taxonomic distance are significant but comparatively low (e.g., r values often below 0.2), while Partial Mantel tests show these relationships weaken further once geographic distance is taken into account. This finding implies that spatial structure can obscure or interact with taxonomic signals (Rodriguez-Peña and Wolfe 2023). Soil, topography and environment also display significant intercorrelations (some with r values above 0.3 in Partial Mantel tests) but offer less direct discrimination among species.
Within a single species, genetic variation correlates strongly with geography (e.g., r > 0.70 for SNP vs. geographic distance in Mantel tests), suggesting classic isolation-by-distance. Environmental variables also drive intraspecific genetic variation independently of geography. In contrast, morphometric traits, although sometimes statistically significant, generally exhibit lower correlations with genetic or environmental distances. Nevertheless, upper-row bracts (particularly row 3) occasionally show moderate links to SNP or environmental distances (Partial Mantel r ~ 0.13–0.20), hinting at local adaptation or drift in certain morphological features.
Overall, spatial scale emerges as a key factor in how genetics, environment and morphology contribute to adaptation (Becker et al. 2006; Volis et al. 2015). In the multispecies context, taxonomic divergence and phylogeographic history predominate, whereas in a single species, geographic distance and environmental variation play more substantial roles. Despite the relatively low morphological variance within a single species, it remains possible that SNP, SilicoDArT, or cpDNA haplotypes underlie subtler morphological traits not captured by our current measurements (Konowalik 2022; Konowalik et al. 2025; Zelený 1970).
4.2 Insights From ARI and Clustering
Cluster analyses based on the Adjusted Rand Index (ARI) confirm that SNP and SilicoDArT consistently produce groupings mirroring taxonomic distinctions across species (e.g., ARI values often > 0.50 with taxonomy). These same markers also reflect strong geographic structuring within single-species datasets. Meanwhile, environmental variables show moderate yet consistent overlap with genetic partitions, particularly in Partial Mantel tests (e.g., r ~ 0.50–0.60 for SNP or cpDNA vs. environment). In contrast, morphometric traits generally return lower ARI values and weaker Mantel correlations, highlighting a less direct relationship to genetic or environmental clustering. Moreover, differences between the PAM and UPGMA clustering algorithms further underscore that analytical choices can influence the extent of observed congruence among datasets.
4.3 Implications for Morphological Traits and Local Adaptation
These findings suggest that certain morphological characters—especially bract measurements—largely capture historical lineage divergence rather than ongoing local adaptation. The evolutionary canalisation of these traits may render them less sensitive to contemporary environmental gradients or finer-scale genetic variation, and in cases where populations remain isolated, morphological differentiation may require more time to emerge (Song et al. 2024). This underscores important timescale differences in how traits respond to selection or drift and highlights that genetic markers evolve more rapidly than morphological characters. While genetics and some environmental parameters clearly shape population structure and differentiation, morphological change can lag behind, particularly when traits are functionally conserved or historically constrained (Buffalo and Coop 2020; Sork et al. 2016; Ye et al. 2014).
Taken together, our results show that molecular markers (SNP and SilicoDArT) and environmental datasets consistently outperform broad morphological measures in detecting population-level or taxonomic divergence. Nonetheless, subtle morphological traits do exhibit some degree of association with genetic or ecological variation and may eventually differentiate more distinctly if present isolation or environmental pressures persist. Additionally, when analysing individuals consistently assigned to the same genetic clusters, noticeable trends are observed, especially between the Carpathian and Vranica populations (Data S6), even if these differences are not statistically significant.
4.4 Biogeographical Structure and Barriers
Overall, the barriers inferred in this study align well with patterns reported by previous research (Mráz and Ronikier 2016), although the position and number of these divisions vary depending on the marker system (Figure 4). The SNP dataset captures the highest number of subdivisions, especially in the Southern Carpathians, where multiple clusters and barriers suggest an ancient colonisation history coupled with a rugged topography of deep valleys and high mountain ranges (Konowalik 2022; Urdea et al. 2022). This fine-scale resolution in the South echoes findings from other studies emphasizing the region's long-term role as a refugium and a hotspot of genetic differentiation (Hurdu et al. 2016; Șuteu et al. 2023).
Interestingly, the often-discussed biogeographical break between the Western and Eastern Carpathians (Mráz and Ronikier 2016) is not as pronounced here, since Western and North-Eastern Carpathian populations frequently group together. cpDNA results corroborate this configuration and reinforce the notion of northward migrations originating from southern refugia via the Dacian route (Hendrych 1996; Hendrych and Hendrychová 1979; Konowalik 2022; Sramkó 2004). Within the Eastern Carpathians, SNP markers further reveal substantial subdivision in the South, differentiating three clusters and one additional barrier that aligns with geomorphological features. Although environmental data produce slightly different clustering patterns, a major barrier nonetheless separates cluster 2 from 3, roughly coinciding with the inner–outer Carpathian boundary.
The Apuseni Mountains emerge as a unique cluster only in SNP-based analyses, whereas cpDNA, SilicoDArT and environmental data associate Apuseni populations with the Eastern and Southern Carpathians, suggesting their role as a corridor rather than a refugium (Krascsenitsová et al. 2013; Pop et al. 2010; Sutkowska et al. 2017). Notably, SilicoDArT and geographic data produce a robust match, delineating three principal groups—(1) Western and Northern-Eastern Carpathians, (2) Central and Southern-Eastern Carpathians plus the Southern Carpathians and Apuseni, and (3) Vranica Mountains, which consistently stand apart. These contrasts among datasets highlight how different markers can capture distinct temporal or demographic processes, underscoring the value of multiple data sources for reconstructing the phylogeographic history and population structure (Kumar and Kumar 2018; Ronikier and Zalewska-Gałosz 2014; Těšitel et al. 2018). They also illustrate the complex interplay of topography, historical refugia and climatic factors in shaping the current distribution of diversity across the Carpathian arc (Mráz and Ronikier 2016; Ronikier 2011).
Based on the patterns identified in this study, we propose several evolutionarily significant units (ESUs, Casacci et al. 2014) within L. rotundifolium, which may also guide conservation strategies for other Carpathian taxa (Figure 4). First, populations in the Western Carpathians (‘Western’ in Figure 4) form a relatively cohesive unit, characterised by consistently low genetic and environmental differentiation. While certain subsets, such as high-mountain populations, display minor distinctions, these groups remain broadly similar and collectively distinct from populations in other regions.
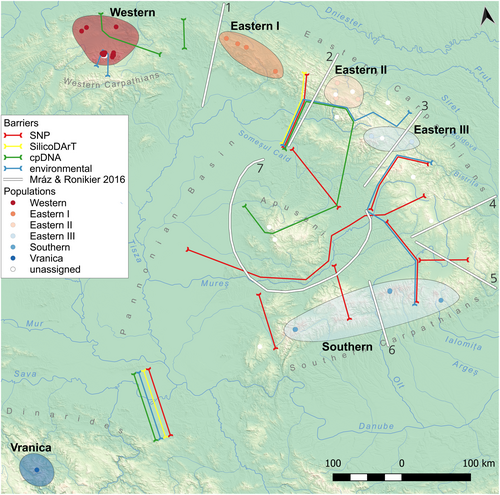
In the northern part of the Eastern Carpathians, another group emerges (‘Eastern I’ in Figure 4), showing significant connections with Western Carpathian populations. This relationship is reflected in partial overlaps observed in SNP and cpDNA clusters. Further south, the central part of the Eastern Carpathians contains a distinct ESU (‘Eastern II’ in Figure 4). Its separation from the Eastern I group is supported primarily by SNP and SilicoDArT datasets and corresponds to the barrier identified as ‘Barrier 2’ in (Mráz and Ronikier 2016).
Another ESU is located in the southeastern part of the Eastern Carpathians, near the sources and upper reaches of the Moldova and Bistrița rivers (‘Eastern III’ in Figure 4). This group is separated from Eastern II by an environmental barrier, potentially located along the Tisza and Moldova rivers. To the south, this ESU is further distinguished from several unassigned populations by SNP and environmental barriers, partially coinciding with ‘Barrier 3’ from (Mráz and Ronikier 2016) and the Călimani Mountains.
The Southern Carpathians stand out due to their high genetic variability and differentiation. However, when SNP clusters are consolidated, the populations can be grouped into a single distinct ESU (‘Southern’ in Figure 4). Finally, geographically disjunct or isolated populations that exhibit pronounced divergence across genetic, environmental and morphological metrics should be treated as independent ESUs (Casacci et al. 2014). In our study, this is exemplified by the population in the Vranica Mountains (‘Vranica’ in Figure 4, Data S5 and S6), which consistently forms a unique and isolated gene pool, highlighting its critical need for targeted conservation efforts. It would be valuable for future studies to investigate whether similar patterns of divergence and isolation are observed in other Dinaric and Carpathian taxa, recognizing that microevolutionary processes may vary among species depending on traits such as dispersal mechanisms and ecological specialisation (Müller et al. 2020).
Author Contributions
Kamil Konowalik: conceptualization, data curation (lead), formal analysis, funding acquisition, investigation (lead), methodology (lead), project administration, resources, software, supervision, validation, visualization, writing – original draft (lead), writing – review and editing (lead). Olga Łuczak: data curation (supporting), investigation (supporting), methodology (supporting), writing – original draft (supporting), writing – review and editing (supporting).
Acknowledgements
We express our gratitude to the curators of the KRA and KRAM herbaria for granting us access to their specimens. Plants were collected under permission no. DOP-WPN.286.142.2017.MŚ and in compliance with applicable regulations for non-protected species. Our thanks also go to Sandra Wydra and Marcin Graczyk from the Institute of Environmental Biology and Dominika Reszewicz from the Department of Botany and Plant Ecology, Wrocław University of Environmental and Life Sciences (WUELS), for technical assistance in molecular laboratory work. We extend our sincere gratitude to Denise Crampton, whose exceptional morphometric guides and engaging video tutorials have significantly benefited this research, and deeply admire her passion, expertise and engaging teaching style. We gratefully acknowledge the financial support provided by the National Science Centre (Poland) for the project ‘Phylogeography and Hybridization Within Carpathian Oxeye Daisies (Leucanthemum, Compositae)’, grant no. 2016/23/D/NZ8/00935. The APC is financed by Wroclaw University of Environmental and Life Sciences.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All additional data supporting this study are available in the Supporting Information accompanying this manuscript. The raw data have been deposited in the Zenodo repository and are accessible under the DOI: 10.5281/zenodo.14658454.




