Habitat preferences of Phoebetria albatrosses in sympatry and allopatry
Abstract
Aim
Competition is often proposed to drive niche segregation along multiple axes in speciose communities. Understanding spatial partitioning of foraging areas is particularly important in species that are constrained to a central place. We present a natural experiment examining variation in habitat preferences of congeneric Southern Ocean predators in sympatry and allopatry. Our aim was to ascertain consistency of habitat preferences within species, and to test whether preferences changed in the presence of the congener.
Location
Southern Hemisphere.
Taxon
Multiple colonies of both species within the genus Phoebetria (sooty albatrosses).
Methods
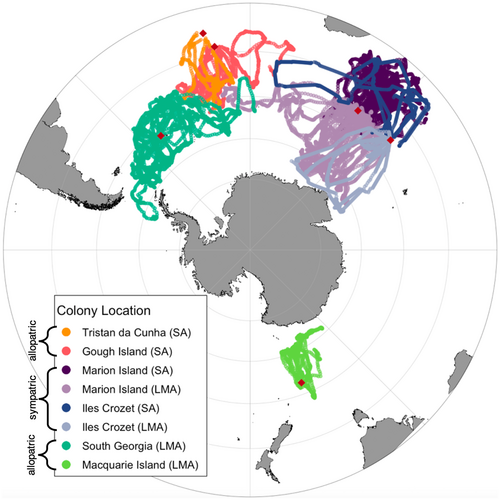
The two Phoebetria albatrosses breed on islands located from ~37–55°S – sooty albatrosses (P. fusca) in the north and light-mantled albatrosses (P. palpebrata) in the south – with sympatric overlap at locations ~46–49°S. We analysed GPS and PTT tracks from 87 individuals and multiple remotely sensed environmental variables using GAMs, to determine and compare the key factors influencing habitat preference for each species at each breeding colony.
Results
While foraging habitat preferences are consistent in light-mantled albatrosses, there is divergence of preferences in sooty albatrosses depending on whether they are in sympatry with their congener or in allopatry.
Main Conclusions
This study represents the most comprehensive work on this genus to date and highlights how habitat preferences and behavioural plasticity may influence species distributions under different competitive conditions.
1 INTRODUCTION
Interspecific competition occurs when species overlap in space and share the same habitats and resources, and can be particularly pronounced in closely related taxa (MacArthur & Levins, 1964; Svärdson, 1949; Tilman, 2007). Responses to interspecific competition vary, but include agonistic or territorial behaviour if species remain specialised on the same resources, or niche differentiation if either selection or plasticity results in one or both species shifting to alternative resources (Grant & Grant, 2006; Kokkoris et al., 1999; Stuart & Losos, 2013; Tarjuelo et al., 2017). The latter has been observed, for example, in hydrological niches in plants (Araya et al., 2011), feeding niches in predatory fish (Young et al., 2010), microhabitat use in reptiles (Pianka & Huey, 1978), and sensory abilities in bats (Siemers & Schnitzler, 2004). Segregation along multiple axes relating to habitat use, phenology, or trophic level has also been reported in speciose communities across diverse taxa (Croxall & Prince, 1980; Ito et al., 2021; Kiszka et al., 2011). Habitat segregation of mobile predators is well-studied in terrestrial and marine systems as an adaptive response to interspecific competition, which allows for competitors to coexist (Jankowski et al., 2010; Martin & Martin, 2001; Morris, 2003; Ziv et al., 1993).
For highly mobile species, foraging habitat selection is of critical importance, particularly when the environment is dynamic and prey are unpredictable at small spatial scales (Weimerskirch, 2007). Whether we consider the drivers of habitat selection at the proximate level (the environmental cues used to locate prey patches) or ultimate level (the evolutionary costs and benefits of using a particular habitat), it is assumed that individuals should forage such that their expected fitness is maximised (Hutto, 1985; Pyke, 1984). It has been suggested as a general rule that competitors with similar ecological niches are more confined to their specific, divergent niches when in sympatry, but expand into the niche of their absent competitor when in allopatry (Hildén, 1965). While often argued that niche divergence between competitors is evidence of competition driving coevolution (e.g. Cloyed, 2014; Jones & Barmuta, 2000; Salewski et al., 2003), demonstrating the supposed coevolutionary shaping of niches is difficult, particularly as there are other potential drivers (Connell, 1980). When studying an ecological community, it is often impossible to tell whether observed traits are truly coevolved – that is, that they represent reciprocal evolutionary change in traits between two co-occurring species (Janzen, 1980) – or instead are adaptations over shorter timescales (Connell, 1980, 1985). If habitat preferences have coevolved at a species level due to historical competition, they are likely to be less variable than those that emerged within populations due to behavioural plasticity or density-dependent mechanisms.
Seabirds are a good model to study effects of competition on habitat preference, because they breed in densely populated communities with highly constrained central-place foraging (Antolos et al., 2017; Phillips et al., 2017). As well as limitations in transit time, the high levels of intra- and interspecific competition associated with high densities of other animals foraging from the same central place leads to local prey depletion (Ashmole, 1963; Weber et al., 2021). This forced overlap during an energetically expensive life stage has led to segregation across multiple axes, both within and between species, as animals trade-off competing demands on their time and the costs and benefits of habitat specialisation (Bolton et al., 2019; Campioni et al., 2016; Cooper & Klages, 1995; Granroth-Wilding & Phillips, 2019; Navarro et al., 2013; Reisinger et al., 2020). However, almost all studies to date have focused on competition within a single breeding community, thus making it difficult to ascertain the drivers of observed niche differentiation at a broader level. A suitable study system to investigate the impact of competition on habitat preference exists in the Phoebetria albatrosses (sooty albatross P. fusca and light-mantled albatross P. palpebrata). This genus has a circumpolar distribution, and the two species breed both in sympatry and allopatry. Sooty albatrosses breed north of the Antarctic Polar Front (37–49°S), whereas light-mantled albatrosses breed in the subantarctic region (46–55°S); the species co-occur at their respective southern/northern limit, at the Prince Edward Islands and Iles Crozet (~ 46°S) (Berruti, 1979; Phillips et al., 2016; Schoombie et al., 2017). There is extensive overlap of the ~7 months of breeding seasons in both species, although on average sooty albatrosses lay ~2 weeks earlier than light-mantled albatrosses (Tickell, 2000). This allochrony means that there is little overlap of the ~3-week brood-guard stage, but significant overlap of the longer incubation and chick-rearing periods.
In this study, we compare the foraging habitat preferences of the Phoebetria albatrosses at multiple colonies across their breeding ranges, including in sympatry and allopatry (Figure 1). Our aim was to ascertain consistency in these habitat preferences within species and to test whether preferences changed in the presence of the congener. We hypothesised that if foraging habitat preference coevolved in sympatry or was otherwise innate, we would observe consistent preferences within both species across breeding sites. The alternative is that habitat preferences are shaped on shorter timescales by interspecific competition, in which case we hypothesised that colony-specific preferences would be apparent in one or both species. Understanding the habitat selection of these highly mobile predators is important not only to answer these ecological questions, but also to identify key areas for conservation and management interventions.
2 MATERIALS AND METHODS
2.1 Data collection
Light-mantled and sooty albatrosses were tracked during the breeding season at multiple islands from the years 2002 to 2017 (for full details, see Table 1).
| Site name | Latitude, longitude | Species | Est. population (breeding pairs) | % of global population | Sympatric/allopatric | Year(s) | Device | Further information |
|---|---|---|---|---|---|---|---|---|
| Tristan da Cunha | −37.11°, −12.28° | SA | 2000–3000 | ~18.7% | Allopatric | 2015 | GPS: CatTraQ (Mobile Action Technology, Inc. 2013) loggers, 42 × 25 × 10 mm, 25 g (incl. tape) | Schoombie et al. (2017) |
| Gough Island | −40.31°, −9.91° | SA | <5000 | <37.5% | Allopatric | 2013 | GPS: CatTraQ (Mobile Action Technology, Inc. 2013) loggers, 42 × 25 × 10 mm, 25 g (incl. tape) | Schoombie et al. (2017) |
| South Georgia | −54.0°, −38.03° | LMA | 5000 | ~24.5% | Allopatric | 2009, 2014 | GPS: i-gotU GT-120 (MobileAction Technology, Taiwan; 25 g) | BAS (this study) |
| Marion Island | −46.89°, 37.75° | LMA | 507 | ~2.4% | Sympatric | 2015–17 | GPS: CatLog-S loggers, Perthold Engineering LLC USA, 50 × 22 × 8 mm, 34 g | Carpenter-Kling et al. (2020) |
| Marion Island | −46.89°, 37.75° | SA | 1838 | ~9.6% | Sympatric | 2015–17 | GPS: CatLog-S loggers, Perthold Engineering LLC USA, 50 × 22 × 8 mm, 34 g | Carpenter-Kling et al. (2020) |
| Iles Crozet | −46.42°, 51.98° | LMA | 2300 | ~11.2% | Sympatric | 2008 | PTT: Microwave Telemetry, Solar, duty cycle 10/24, 62 × 18 × 14 mm, 18 g | CNRS (this study), see Delord et al. (2014) |
| Iles Crozet | −46.42°, 51.98° | SA | 2040 | ~15.8% | Sympatric | 2008 | PTT: Microwave Telemetry, Solar, duty cycle 10/24, 62 × 18 × 14 mm, 18 g | CNRS (this study), see Delord et al. (2014) |
| Macquarie Island | −54.61°, 158.85° | LMA | 1281 | ~6.3% | Allopatric | 2002 | PTT: Microwave Telemetry. 50 × 15 × 15 mm, 30 g | Cleeland et al. (2019) |
2.2 Track processing
Tracks were visually inspected and removed from analysis if they were incomplete due to device malfunction or early battery failure. Trips were counted as incomplete if fixes were missing for extended periods during the trip or were missing such that it was impossible to estimate the times of departure and return to the colony. Trips were taken during incubation (or chick-rearing at Iles Crozet only). Where multiple trips for the same individual were successfully recorded, only the first trip was selected from each bird to avoid pseudo-replication. The first trip, rather than a random trip, was selected for birds tracked for multiple trips to be consistent with the first, and only trip recorded for the remainder of the sample. All tracks were filtered (trip R package [Sumner et al., 2009]) to remove points indicating biologically implausible flight speeds >90 km/h (Phillips et al., 2007), and interpolated to 60-min intervals for consistency, as this was the coarsest temporal resolution of data collected. Interpolating locations at regular intervals ensures that bias is not introduced by comparing data with different temporal resolutions. Given that foraging trips of Phoebetria albatrosses last for over a week, hourly points were considered sufficient to capture movement patterns at an appropriate spatiotemporal scale. Characteristics (duration, distance travelled, and maximum displacement) were calculated from the interpolated tracks. In order to determine habitat preferences from presence-only data from tracked seabirds, it is necessary to generate pseudoabsences that represent the available environment within the foraging range. For each track, 20 pseudoabsence tracks were generated by randomising the departure direction from the colony, while retaining step length and turning angle to ensure flight patterns were biologically appropriate (Bentley et al., 2023). Various remote sensing and other environmental variables known for their importance in habitat selection for Southern Ocean predators (Reisinger et al., 2018) were extracted for each presence and pseudoabsence location (Table 2).
| Variable (units) | Data source | Temporal scale | Spatial scale | Relevance |
|---|---|---|---|---|
| Sea-surface temperature (SST,°C) | Global ocean ensemble physics reanalysis, CMEMS (Global Monitoring and Forecasting Centre, 2021) | Monthly composite | 0.25 × 0.25 degrees | Indicative of fronts and water mass |
| log(Chlorophyll a gradient) | Calculated from Global ocean biogeochemistry hindcast, CMEMS (Global Monitoring and Forecasting Centre, 2021) using R package ‘grec’ (Lau-Medrano, 2020) | Monthly composite | 0.25 × 0.25 degrees | Chlorophyll a is a proxy for marine productivity |
| Bathymetry (m) | Global Bathymetric Chart of the Oceans (GEBCO Compilation Group, 2020) | Static | 0.00833 × 0.00833 degrees, resampled to 0.25 × 0.25 degrees using the ‘terra’ package (Hijmans et al., 2022) | Identifies shelf and pelagic zones, and potential upwellings |
| log(eddy kinetic energy) | Calculated from north and east current velocities, Global ocean ensemble physics reanalysis, CMEMS (Global Monitoring and Forecasting Centre, 2021) | Monthly composite | 0.25 × 0.25 degrees | Index of mesoscale oceanic activity, often indicative of prey aggregations |
2.3 Modelling
Binomial generalised additive models (GAMs) using the environmental variables extracted at the presence and pseudoabsence locations were constructed for each species. Additive models are appropriate when relationships with predictors are likely to be non-linear and were constructed using the R package ‘mgcv’ (Wood, 2011). GAMs generate smooth terms that are simple to interpret, even when dealing with multiple variables, and are robust to overfitting. Full models were constructed for each species, using all environmental variables (see Table 2) by colony type (sympatric or allopatric), and model selection was undertaken using AICc values. Models for each colony were subsequently constructed using the environmental variables selected for the full model. Spatial autocorrelation was accounted for in all cases using a Gaussian correlation structure on the latitude and longitude terms. All model formulae and outputs are available in in Supplementary Tables 1–6. All data manipulation and analyses were undertaken in R version 4.0.3 (R Core Team, 2020). Means are provided ± SD unless indicated otherwise.
3 RESULTS
3.1 Trip characteristics
Trips of light-mantled albatrosses were on average 12.66 ± 4.62 days and covered on average 5723 ± 2151 km, whereas those of sooty albatrosses were on average 11.25 ± 4.58 days and covered an average 5154 ± 2132 km. The average maximum range flown from the colony was 1562 ± 675 km for light-mantled albatrosses and 1318 ± 495 km for sooty albatrosses (see Table 3). When pooling trips from all years, light-mantled albatrosses from South Georgia took the shortest trips (10.17 ± 4.57 days), and those from Marion Island the longest (12.72 ± 4.57 days). There was much higher variation in average trip lengths among sooty albatross colonies, with birds from Gough Island foraging for 8.45 ± 4.54 days, and birds from Tristan da Cunha (16.17 ± 2.16 days) and Iles Crozet foraging for almost twice as long (16.5 ± 10.4 days). However, the longest-lasting foraging trips were not always the ones where birds reached the greatest distances from their breeding colonies. In both light-mantled and sooty albatrosses, birds from Crozet attended the most distant foraging areas, 2456 ± 161 and 2210 ± 1091 km from their respective colonies. Light-mantled albatrosses from Macquarie Island (1203 ± 267 km) and sooty albatrosses from Marion Island (1145 ± 323 km) had the shortest maximum displacements. Finally, the average distance travelled in a foraging trip was greatest for Marion Island light-mantled albatrosses (6255 ± 2205 km) and Crozet Island sooty albatrosses (6913 ± 5019 km). The shortest average distance travelled was 4810 ± 399 km for Macquarie Island light-mantled albatrosses and 4826 ± 2328 km for Gough Island sooty albatrosses.
| Season | Breeding site | Species | n | Mean trip length (days) | Mean distance travelled (km) | Mean max displacement (km) |
|---|---|---|---|---|---|---|
| 2015 | Tristan da Cunha | SA | 3 | 16.17 ± 2.16 | 6339 ± 986 | 1682 ± 227 |
| 2013 | Gough Island | SA | 11 | 8.45 ± 4.54 | 4826 ± 2328 | 1307 ± 327 |
| 2009 | South Georgia | LMA | 6 | 7.61 ± 3.83 | 4828 ± 2128 | 1546 ± 633 |
| 2014 | South Georgia | LMA | 12 | 11.45 ± 4.50 | 5715 ± 2600 | 1259 ± 474 |
| 2015 | Marion Island | LMA | 4 | 14.48 ± 5.58 | 5782 ± 2308 | 1312 ± 704 |
| 2015 | Marion Island | SA | 3 | 11.72 ± 2.09 | 4402 ± 1101 | 1225 ± 360 |
| 2016 | Marion Island | LMA | 6 | 17.00 ± 3.99 | 7696 ± 2057 | 2135 ± 690 |
| 2016 | Marion Island | SA | 10 | 12.05 ± 2.11 | 5601 ± 1568 | 1273 ± 237 |
| 2017 | Marion Island | LMA | 11 | 13.56 ± 4.45 | 5640 ± 2064 | 1548 ± 762 |
| 2017 | Marion Island | SA | 8 | 10.10 ± 2.29 | 4226 ± 1296 | 953 ± 347 |
| 2008 | Iles Crozet | LMA | 4 | 13.84 ± 2.54 | 5666 ± 1938 | 2455 ± 161 |
| 2008 | Iles Crozet | SA | 3 | 16.50 ± 10.40 | 6913 ± 5018 | 2210 ± 1090 |
| 2002 | Macquarie Island | LMA | 6 | 12.17 ± 2.29 | 4809 ± 399 | 1202 ± 267 |
3.2 Habitat preferences
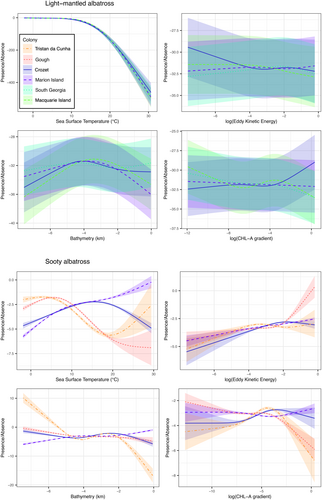
Light-mantled albatrosses in both sympatry and allopatry with sooty albatrosses preferentially foraged in cold-water areas of 0–5°C, and avoided water temperatures >15°C. At Crozet, Marion, and Macquarie Islands, light-mantled albatrosses mainly travelled south to forage in areas close to the ice edge, whereas at South Georgia, they foraged at the ice edge and to as far north as the Antarctic Polar Front. There was a slight tendency to use areas with high chlorophyll a gradients (indicating frontal zones), particularly at Iles Crozet. Preferred water depths were 2000–4000 m (Figure 2c). All environmental variables modelled had a significant influence on habitat preference, though the most influential was sea-surface temperature (Supplementary Tables 1 and 2).
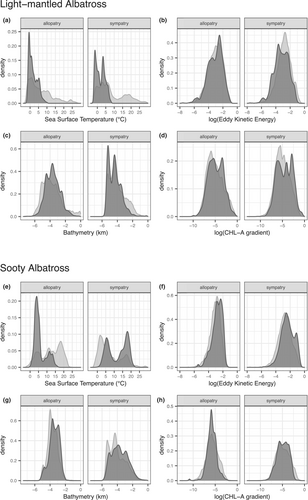
In contrast to light-mantled albatrosses, sooty albatrosses did not show consistent preferences across sites. When in allopatry, sooty albatrosses foraged preferentially in cold (0–5°C) and cool waters (10–15°C), even though most available habitat was 15–25°C (Figure 2e). However, sooty albatrosses breeding in sympatry with light-mantled albatrosses foraged preferentially in waters of 15–20°C and targeted areas with high chlorophyll a gradients (Figure 2h). Sooty albatrosses avoided the cold-water areas (<5°C) frequented by their congener, even though sites where the two species breed in sympatry are further south than those where only sooty albatrosses are present. Zones of high eddy kinetic energy were targeted by sooty albatrosses across their range (Figure 2f). Again, all environmental variables modelled significantly influenced habitat preference, and there were different relationships displayed at sympatric and allopatric colonies (Supplementary Tables 4 and 5).
Sea-surface temperature was the most important predictor in models for both species, with similar relationships for all environmental variables seen across colonies of light-mantled albatrosses, and clear differences in these relationships seen between colonies of sooty albatrosses, depending on whether they were breeding in sympatry or allopatry (Figure 3, Supplementary Tables 3 and 6).
4 DISCUSSION
Despite their morphological and behavioural similarities, the foraging habitat preferences of the two species of Phoebetria albatross differ significantly from one another when breeding in sympatry. The preference of light-mantled albatrosses for cold-water areas (either cold upwelling zones or the ice edge) remains consistent throughout their range. In contrast, habitat preferences of sooty albatrosses differ whether they breed in sympatry with their congener or in allopatry. Even though the islands where they breed in sympatry are further south, the sooty albatrosses at those sites foraged preferentially in warm water areas, which was not a preferred habitat when breeding in allopatry in the northern portion of their range. This suggests that there is a competitive mechanism at work driving habitat segregation.
Given the absence of consistent habitat preferences in both species across their global distributions, the observed niche segregation between sympatric Phoebetria albatrosses is unlikely to be driven by coevolution under competition. Competition is often considered to drive coevolved niche specialisation in speciose communities, but demonstrating coevolutionary shaping of the niches of competitors is challenging, especially as there are alternative, more plausible drivers (Connell, 1980). In general, ecological niche theory predicts that differentiation of some kind (e.g. prey specialisation, spatiotemporal segregation) should occur when multiple species with similar niches compete for resources (Tilman, 2007). Specialist foraging strategies that coevolved at a species level due to historical competition (i.e. following reciprocal divergent selection for different phenotypes between species) are likely to be less flexible than particular strategies that emerge within populations due to behavioural plasticity or density-dependent mechanisms. In an ecological community, it is often impossible to tell whether the observed specialisations are coevolved or are adaptations that developed over shorter timescales. In general, sympatric speciation – particularly on isolated islands where the population is under divergent ecological selection – is accepted as a plausible route to the formation of novel species (Jiggins, 2006). Indeed, there is recent empirical evidence for this in some seabird species (Friesen et al., 2007). Had Phoebetria speciation occurred in sympatry – with competition driving selection for resource segregation and leading to subsequent reproductive isolation – one would expect more consistent habitat preferences within each species that carried over to subsequently established allopatric populations. However, this ‘ghost of competition past’ (Connell, 1980) was not observed in our study: although the foraging habitat preferences of light-mantled albatrosses were consistent across sites, those of sooty albatrosses were not. If sympatric speciation did occur in these species, it is therefore unlikely to have been driven by competitive segregation of foraging niches. Based on simulation models, the alternative mechanism – speciation in allopatry with subsequent reestablishment of sympatric populations – is considered to be the more common mode of avian speciation (Phillimore et al., 2008). If this is the case, the observed niche segregation in sympatry cannot be the product of coevolution, as co-occurrence is a critical requirement for reciprocal selection to occur (Connell, 1980, 1985; Janzen, 1980).
Even if the habitat preferences of the Phoebetria albatrosses are not the result of coevolved niche differentiation, this does not preclude the possibility that competition influences their realised niches in sympatry. As such, we see three alternative mechanisms for the patterns observed. Firstly, ecological character displacement – the concept that sympatric species competing for limited resources should experience selection for divergent resource use (Brown Jr. & Wilson, 1956) – may be occurring at a population level. There are few cases with truly unequivocal support for ecological character displacement, with one review finding only nine of 144 case studies convincingly ruled out alternative explanations (Stuart & Losos, 2013). In our study, character displacement is difficult to confirm as the trait in question is behavioural rather than morphological, and multiple measures from the same individuals through time are required to confirm whether it is fixed or plastic.
Secondly, habitat segregation of the sympatric populations may be demonstrating a socially mediated variation of the ideal free distribution (IFD), the theory assuming that equally competitive organisms will act to maximise their foraging efficiency by moving to areas with decreased densities of competitors (Fretwell, 1969). It is theoretically possible that the observed segregation developed because individuals avoid areas of highest competition and is now maintained through social learning or preferred associations. However, multiple studies have shown that the space-use of seabirds and their prey rarely appears as expected under the IFD (Logerwell & Hargreaves, 1996; Swartzman & Hunt, 2000). This is most likely because seabirds rarely conform with the associated IFD assumptions that individuals have perfect environmental information and can engage in cost-free movement (Fauchald, 2009). In many colonial seabirds, density-dependent segregation by colony has been observed (e.g. Wakefield et al., 2013), but we did not find this. The speciose nature of seabird breeding aggregations means both intra- and interspecific competition are high. There is evidence in geese that the density of multispecies assemblages can influence fitness more than the density of conspecifics alone (Schmutz & Laing, 2002), and it is plausible that similar effects occur at seabird breeding islands; however, this has not been formally tested. In our study, we observed segregation between presumably unequal competitors (i.e. between the two species) and use of the same foraging areas by equal competitors (i.e. highly consistent habitat preferences within species, even among colonies). There was geographic overlap of foraging areas (within species) in birds from Marion and Crozet (Figure 1), although they were tracked in different years. Recent work on wandering albatrosses Diomedea exulans at Marion and Crozet has also shown no foraging area segregation between these two colonies (Orgeret et al., 2021). Future tracking of birds from neighbouring colonies in the same year is needed to rule out among-colony habitat partitioning in the Phoebetria albatrosses.
Thirdly, the observed differences in habitat preference between populations of sooty albatrosses may reflect behavioural plasticity. This we consider to be the most likely explanation. Strict resource preferences have been shown to relax in conditions of scarcity (Bergström et al., 2004; Snell-Rood & Papaj, 2009), which is adaptive in novel or dynamic environments where preferred resources are unavailable. Indeed, there should be selection for reversible phenotypic plasticity in environments that vary within the lifetime of an individual, due to the high costs of mismatch between preference and availability of resources (Snell-Rood, 2013). This is particularly relevant for long-lived species such as albatrosses, which can live for >40 years (Froy et al., 2017), as they presumably encounter greater environmental variability than shorter-lived species. There is evidence that habitat preferences in other Procellariiformes are not consistent between breeding populations (Clay et al., 2016; Péron et al., 2018; Torres et al., 2015). This suggests that flexibility of habitat preferences is adaptive at a species level. Furthermore, evidence for plasticity in habitat preference comes from Marion Island, where sooty albatrosses tracked during incubation showed high interannual variability in foraging behaviour, and light-mantled albatrosses targeted specific eddy fields only in years when eddy kinetic energy was particularly high (Carpenter-Kling et al., 2020). Given the dynamic environments in which albatrosses forage, we would expect foraging success (and subsequently, reproductive success) to be higher when preferences are plastic. We observed divergent habitat preference among colonies of sooty albatross, which supports the conjecture that this trait is behaviourally plastic. The consistency of preferences among light-mantled albatross colonies does not, however, confirm that this species is inflexible. To truly understand the flexibility of albatross habitat preferences, we would require repeated measures on individuals (to determine whether there is individual-level specialisation), across years (to determine the response within populations to changes in local conditions) and a combination of both (to understand within-population variation of individual responses to changing conditions).
Flexibility in habitat preferences can buffer the effects of anthropogenic environmental change, which is occurring at an unprecedented rate (Gruber et al., 2019). If colony-level habitat preferences are fixed, further research is required to ascertain whether this inflexibility is genetic (due to past selection) or cultural (due to learning). Furthermore, studies of ontogenetic changes in habitat preferences are important for understanding the potential flexibility within individuals (Frankish et al., 2020). Unfortunately, albatrosses are unsuitable for cross-fostering studies, which could explore the varying influences of genotype and the local environment on habitat preferences. High philopatry in seabirds minimises gene flow across breeding ranges, and there has been some suggestion that differentiation is occurring in sooty albatrosses, although citing unpublished data (Robertson, 1998). If this is the case, we are more likely to see differential responses among colonies of this species to environmental change. Importantly, even if birds are flexible in their habitat preferences, this may not be sufficient to compensate for poor environmental conditions: evidence from South Georgia shows that in years when grey-headed albatrosses foraged mostly on krill Euphausia superba in Antarctic waters – rather than cephalopods in the Antarctic Polar Frontal Zone, which is more common in years with favourable environmental conditions – they experienced poorer breeding success (Xavier et al., 2013). Flexible preferences do not always manifest as a shift to a generalist niche, as there is also evidence that when foraging conditions deteriorate, the diets of sympatric species show greater divergence (Barger & Kitaysky, 2012). Increased niche segregation in response to stress, such as greater anthropogenic pressures, can exacerbate impacts affecting particular aspects of the niche space. Threats may have synergistic effects, for example, if climate change causes shifts in habitat preference leading birds to choose foraging areas with an increased risk of incidental mortality in fisheries.
Foraging habitat location influences exposure to threats and, ultimately, the population trend. Sooty albatross are listed as Endangered, and light-mantled albatross as Near-threatened by the International Union for Conservation of Nature (IUCN, 2022), and both species are listed by the Agreement on the Conservation of Albatrosses and Petrels (Phillips et al., 2016). Decreasing population trends have been observed at almost all sooty albatross colonies, with the exception of Marion Island (Agreement on the Conservation of Albatrosses and Petrels, 2010a; Delord et al., 2008; Schoombie et al., 2016; Weimerskirch et al., 2018). This is proposed, in part, to be the result of increased overlap with subtropical tuna fisheries (Delord et al., 2008). It is also possible the extreme southerly latitudes of cold-water habitats near Marion and Crozet (~60° S) are generally inhospitable to a species such as the sooty albatross, which presumably evolved in the subtropics. Conversely, the light-mantled albatross population has likely increased, or at least remained stable, at Iles Crozet in recent years (Weimerskirch et al., 2018). However, the cold-water specialism of light-mantled albatrosses may increase their vulnerability to warming seas due to climate change (Inchausti et al., 2003; Schoombie et al., 2016). The distribution of Antarctic krill, an important prey item for this species (Green et al., 1998; Jaeger et al., 2010), is rapidly contracting southwards (Atkinson et al., 2019), and the increased costs associated with foraging even further to the south may reduce fitness in light-mantled albatrosses and contribute to future population declines.
Evidence from stable isotopes indicates that the cold-water preferences observed in incubating light-mantled albatrosses likely persist through chick-rearing and the non-breeding season. By measuring the isotopic ratios in chick and adult feathers, one can approximate the latitude of foraging during chick-rearing and moult, respectively (Jaeger et al., 2013), assuming that isotopic differences are caused by dietary origin and not by other factors known to affect isotopic composition (Shipley & Matich, 2020). Studies from both Crozet and Marion Islands show that light-mantled albatrosses forage further to the south than sooty albatrosses during chick rearing (Connan et al., 2014, 2018; Jaeger et al., 2010), as well as in incubation (this study). Interestingly, there is some indication that sooty albatrosses from Crozet foraged in subantarctic waters during chick-rearing (Jaeger et al., 2010), rather than the subtropical waters observed in this study. It may be that they consumed squid and penguin carrion taken from near Crozet Island, whereas light-mantled albatrosses showed relatively greater reliance on Antarctic krill, which is not available outside of the Antarctic zone (Jaeger et al., 2010). However, dietary studies on Crozet sooty albatrosses also showed squid beaks from subtropical species during this time, so their dietary niche remains equivocal (Connan et al., 2014). The reasonably consistent isotope ratios found in adult light-mantled albatross feathers indicate an annual fidelity to the Southern Ocean (Connan et al., 2014), and recent dietary studies emphasise the importance of Southern Ocean squid in the diet of this species across its range (Cherel et al., 2023). Sooty albatrosses, however, mostly overwinter in the subtropics (Schoombie et al., 2022), with the exception of those from Gough Island, which join their congener at higher latitudes (Connan et al., 2018). Given that breeding sooty albatrosses from Gough Island also target cold-water areas, this may indicate some consistency between breeding and non-breeding habitat preferences across the genus, which merits further study. Indeed, further work on foraging habitat choice in the non-breeding season is important to understand habitat preferences when these species are not constrained to a central place.
Finally, we acknowledge that comparisons of species pairs in sympatry and allopatry do not account for the reality that resource competition occurs within a wide community of ecologically similar species (Bodey et al., 2014). Ecological conditions are complex, and unquantified factors are likely to influence the foraging distributions of pelagic predators. The speciose nature of seabird breeding assemblages results in multiple layers of morphological segregation between, for example, small petrels, penguins, and albatrosses (Abrams & Griffiths, 1981), but more detailed comparative studies are required to identify how niche space is partitioned among sympatric (and morphologically more similar) Thalassarche and Phoebetria albatross species.
ACKNOWLEDGEMENTS
We are grateful to all fieldworkers, particularly Claudia Mischler, Derren Fox, and Stacey Adlard (Bird Island); Delia Davies, Mara Nydegger (Gough Island); Janine Schoombie, Jessie Berndt, Albert Snyman, Makhudu Masotla, and David Green (Marion Island); and Y Charbonnier and JB Thiebot (Crozet). We thank Rosemary Gales and Rachael Alderman for sharing data from Macquarie Island. LKB was supported by the Gates Cambridge Trust. This study has been conducted using E.U. Copernicus Marine Service Information. Bird Island tracking was approved by the British Antarctic Survey Animal Welfare and Ethics Committee and carried out with the permission of the Government of South Georgia and the South Sandwich Islands (permits nos. BAS 09-10, SCI-2014-014, and WPA-2014-016). Research was approved by the University of Cape Town's Animal Ethics Committee (2017/V10REV/PRyan). Work on Marion Island received ethics clearance from the Nelson Mandela University Animal Ethics Committee (A14-SCI-ZOO-012), and clearance from the Department of Forestry, Fisheries and the Environment (permit EC-2016-11-25). The Ethics Committee of IPEV and the Comité Environnement Polaire approved the field procedures for the French Southern Territories.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Birdlife Seabird Tracking Database at https://www.seabirdtracking.org, dataset ids: 1312, 1208, 1313, 1290, 420, 443, 650, 651, 1292, 1384, 1529, 1530).
REFERENCES
BIOSKETCH
Lily K. Bentley is a movement ecologist. She is interested in how and where highly mobile predators travel, what their journeys can teach us about their evolutionary histories, and how to translate research findings into effective conservation policies.
Author contributions: Lily K. Bentley, Richard A. Phillips, and Andrea Manica conceived the ideas and designed methodology; Richard A. Phillips, Tegan Carpenter-Kling, Robert J. M. Crawford, Richard J. Cuthbert, Karine Delord, Ben J. Dilley, Azwianewi B. Makhado, Peter I. Miller, Steffen Oppel, Pierre A. Pistorius, Peter G. Ryan, Stefan Schoombie, and Henri Weimerskirch collected and managed the data; Lily K. Bentley with supervision from Andrea Manica analysed the data and produced the figures; Lily K. Bentley led the writing of the manuscript with supervision from Andrea Manica and Richard A. Phillips. All authors contributed critically to the drafts and gave final approval for publication.




