Shifting seas: the impacts of Pleistocene sea-level fluctuations on the evolution of tropical marine taxa
Abstract
Aim
Pleistocene glacial cycles reduced global sea level by up to 130 m below present levels. These changes had profound impacts on coastal marine life, including a reduction of habitable area, changes in ocean currents, and shifts in water column thermal dynamics. We provide a comprehensive review of the impact of glacial sea-level changes during the Pleistocene on tropical coastal marine life and a set of maps showing how coastlines worldwide changed during periods of low sea levels.
Location
We focused on coastal marine taxa within tropical latitudes, with deeper coverage of the world's major coral reef biogeographical provinces.
Methods
We examined recent and historical literature that alluded to the effects of Pleistocene sea-level fluctuations in a variety of common marine clades. Data for shelf habitat area and map construction were obtained from the NOAA ETOPO1 database, with final manipulations carried out in Adobe Illustrator CS6.
Results
Drops in sea level led to a decrease in available coastal habitat and fragmented populations in many taxa, potentially resulting in high population genetic structuring. Habitable shelf area during sea-level lows was reduced as much as 92% from present-day values in some regions. Genetic evidence of population bottlenecks can be seen in many coastal marine taxa worldwide.
Main conclusions
Pleistocene sea-level fluctuations seem to be linked to population bottlenecks worldwide, and influenced connections among populations separated by barriers that are affected by sea levels. Despite decreased habitat availability, very few species became extinct, and several species may have been formed due to restrictions in water (and consequently larval) flow between regions that are now connected. A variety of interdisciplinary studies have significantly increased our understanding of how Pleistocene sea-level changes have shaped the marine landscape that we see today.
Introduction
Throughout the Pleistocene (2.6 Ma–11.7 ka), global sea level fluctuated following climatic oscillations (Pillans et al., 1998). Approximately 50 glacial cycles during this period constantly altered coastal habitat, influencing the biota and landscape throughout the planet (Woodruff, 2010). Low sea levels culminated in coastlines 115–130 m below current level, reaching a minimum during the Last Glacial Maximum (LGM) c. 17–19 ka (Fairbanks, 1989; Hanebuth et al., 2000; Voris, 2000; Bintanja et al., 2005; Bintanja & van de Wal, 2008; Clark et al., 2009). Generally, sea-level estimates are greatly impacted by subsidence, tectonic uplifting and isostatic loading/unloading, yet results from studies conducted across the Indian Ocean (West Indian Ocean: Camoin et al., 2004; Maldives: Fürstenau et al., 2010) and Pacific Ocean (Tahiti: Bard et al., 1996; Indonesia: Hanebuth et al., 2000; Papua New Guinea: Chappell & Polach, 1991), as well as the Red Sea (Siddall et al., 2003) and the Caribbean Sea (Fairbanks, 1989), all indicate relatively uniform heights across all tropical basins. While sea-level lows lasted for relatively short periods (approximately 6% of the time during the last 250 kyr), sea level remained at intermediate depths (< 40 m below present day) for the majority of the Pleistocene (Voris, 2000).
Here we provide a review of how glacial sea-level fluctuations affected the population dynamics in a wide range of tropical marine organisms during the Pleistocene. This review will focus primarily on extant taxa that have records dating to the Pleistocene. While both fossil and genetic evidence are useful in studying the impacts of eustatic sea-level shifts, many coastal taxa lack substantial fossil records for this time period, making comparisons difficult. Therefore, we will highlight important fossil evidence in several groups where substantial work has been done (e.g. cnidarians and molluscs), and then shift the focus of the review to phylogenetic, phylogeographical and population genetic studies, which are comparable across all groups. We will then transition to studies that examine the importance of certain life-history traits (e.g. spawning type, larval dispersal or larval feeding mode) and how habitat preference may drive genetic patterns associated with sea-level change. The relationship between sea-level change, speciation and demographic changes for various taxa is emphasized, exposing common patterns observed in specific groups and revealing knowledge gaps that point towards future research directions.
Materials and methods
We examined recent and historical literature for a variety of common marine clades that alluded to Pleistocene sea-level fluctuations impacting their main findings. Maps (shown in the cylindrical-equidistant projection) comparing present and historical sea levels were drawn using the ETOPO1 database from NOAA (http://www.ngdc.noaa.gov/mgg/global/: Amante & Eakins, 2009; accessed 1 November 2013), viewed with GEODAS Hydro-Plot 5.0.24.1 (available as part of the NOAA GEODAS-NG software package: http://www.ngdc.noaa.gov/mgg/geodas/geodas.html), with final manipulations made in Adobe Illustrator CS6. Our maps focus on both present-day sea levels, as well as glacial maximum sea level of 120 m below present based on the estimates presented in Bintanja et al. (2005) and Bintanja & van de Wal (2008). Shelf habitat area was estimated using the histogram function of GEODAS Hydro-Plot 5.0.24.1. Briefly, this function provides an estimate of the total seafloor area at specific bathymetric depths. We compared the amount of available habitat in the upper 60 m of the water column at current sea levels (0–60 m), or at levels reached during glacial maxima (120–180 m). It is within this upper 60 m of the photic zone where hermatypic corals thrive, supporting a diverse tropical biota (Fricke & Meischner, 1985). A variety of factors in addition to sea-level fluctuations can influence the amount of exposed land during sea-level lows, including subsidence, lithospheric flexing, surficial processes, and the loading/unloading of the seabed (for an example see Ali & Aitchison, 2014). However, implementing the effect that these factors have on exposed land could be the topic of several independent papers given the global scope of this review. The estimates presented are based on current bathymetric maps, and do not take into account these factors; therefore these values should be regarded as rough estimates.
Results
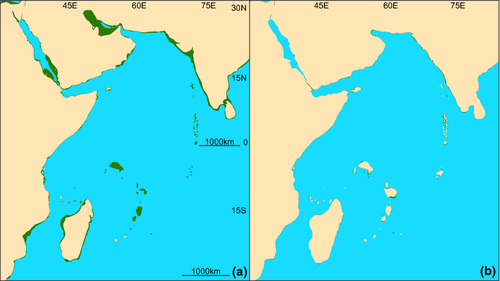
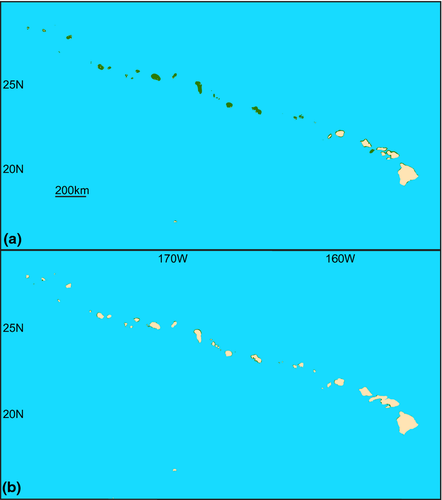
As the average depth of the continental shelf break is 135 m (Cook & Carleton, 2000), eustatic lows during the LGM reduced the area of coastal habitat by approximately 90% within tropical latitudes in the Pacific (Fig. 1) and Indian (Fig. 2) Oceans and upwards of 92% in the Gulf of Mexico and Caribbean Sea (Fig. 3; Bellwood & Wainwright, 2002). These habitat reductions were also observed on oceanic islands, with a 75% reduction of coastal habitat in Hawaii (Fig. 4), and an approximate 92% loss of shallow coastal habitat among south Pacific islands (Fig. 5). Furthermore, even intermediate depths of sea-level fall strengthened some biogeographical barriers, fragmenting populations of many coastal species. For example, in the Indo-Pacific, the shallow Sunda and Sahul shelves were exposed, connecting several islands in the region between Indonesia, Papua New Guinea and the Philippines, known as the Coral Triangle, with the nearby Asian continent (Fig. 1b). Lower sea levels almost doubled the exposed land in Southeast Asia (Woodruff, 2010) and resulted in restricted connections between the Indian and Pacific Oceans. Similarly, the Red Sea was at least partially isolated during sea-level lows, both geographically, at the narrow and shallow Strait of Bab al Mandab (Siddall et al., 2003), and environmentally, through regional temperature and salinity shifts generated by altered oceanic currents (Bailey, 2009). As with the Sunda and Sahul shelves, this barrier restricted connectivity between species inside and outside of the Red Sea (for examples see DiBattista et al., 2013).
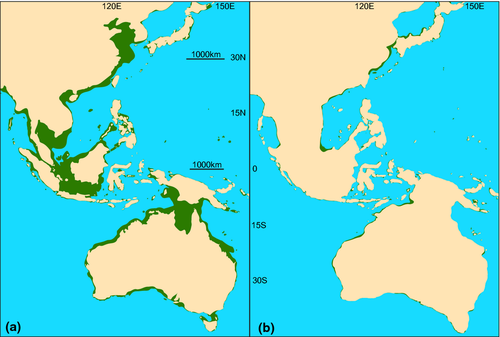
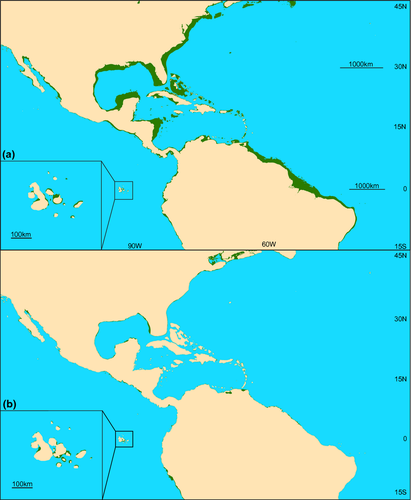
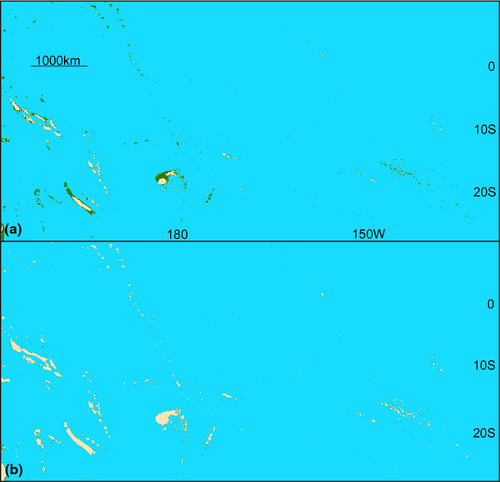
Barriers in the Atlantic also increased in strength during low sea levels, and the main barrier separating Brazil and the Caribbean, the Amazon outflow, probably became less permeable because a deep reef corridor where sponges provide structure, which presently serves as a stepping stone between those regions, was closed (Rocha, 2003). In addition, shallow marine taxa were restricted to narrowing coastal margins as c. 92% of modern habitable shelf became exposed (Fig. 3). Therefore, receding waters are likely to have reduced the overall quantity of available shallow habitat for near-shore species and severely impacted connections between marine regions. The timing of sea-level fluctuations also shifted throughout the Pleistocene, occurring approximately every 41 kyr prior to the mid-Pleistocene transition 900 ka, and every 100 kyr since (Elderfield et al., 2012). It is thought that this transition period had a large influence on deep ocean temperatures (Elderfield et al., 2012). Sea-surface temperature in the tropics, on the other hand, fluctuated on average between 1 °C and 3 °C between glacial and interglacial periods (Herbert et al., 2010). This had an unknown effect on tropical marine taxa; however, it may be important for the interhemisphere movement of temperate fishes (Hubbs, 1952; Burridge, 2002), invertebrates (Lindberg, 1991), and marine mammals (Fordyce & Barnes, 1994; Hare et al., 2002). Despite these temperature and habitat changes during the LGM, few marine species went extinct (Wise & Schopf, 1981; Valentine & Jablonski, 1991; Ávila et al., 2008).
Discussion
Fossil evidence
Corals dominate many coastal ecosystems in the tropics, adding structural complexity to the surrounding substrate and creating reef habitat that is critical for high biodiversity marine ecosystems (e.g. Bell & Galzin, 1984; Öhman & Rajasuriya, 1998; Graham et al., 2006). Melt water pulse (MWP) events 1a and 1b, approximately 14,000 and 11,300 years before present (Fairbanks, 1989; Bard et al., 2010), raised sea levels at a rate of approximately 40–50 mm year−1 (Hanebuth et al., 2000). Despite these rapid changes in sea level, coral community composition from the Pleistocene was relatively stable. Towards the end of the Pliocene and into the beginning of the Pleistocene there was a clear signal of coral faunal turnover in the Caribbean Sea from reefs dominated by agariciids and poritids in the Pliocene to Acropora- and Diploria-dominated reefs in the Pleistocene (Budd et al., 1996). However, as the Pleistocene progressed, global coral community composition appears to have stabilized both in the Caribbean and in the Indo-Pacific (Jackson, 1992; Pandolfi, 1996, 1999). For example, while investigating fossil corals in the Ryukyu Islands of Japan, Humblet et al. (2009) showed that both species-level and generic richness in the past 410,000 years did not differ from present-day patterns.
Molluscs have also left substantial fossil records during the Pleistocene, and corroborate coral studies showing compositional stability through glacial cycles. For example, 54 mollusc species were examined from two deposits at the Bahamas from the last major interglacial period, showing stability in taxonomic composition between reef-building events (Gardiner, 2001). While local factors such as wave regimes (Pandolfi & Jackson, 2001) and freshwater inputs (Pandolfi, 1996) have been identified as important driving forces in the community assembly of corals on small scales, the overall evidence suggests that structurally complex habitats for reef-dependent species were maintained throughout the Pleistocene, irrespective of the rate and style of sea-level change, supplying habitat for many marine species.
Phylogeographical and population genetic evidence
Whereas fossils provide locality and community composition data through geological time, genetic approaches can estimate connectivity and demographic history (Rogers, 1995; Hellberg, 2007), and using these we can estimate the location of potential refugia during sea-level lows. One of the earliest population genetic studies within the Coral Triangle focused on the crown-of-thorns starfish, Acanthaster planci, using allozymes to distinguish major breaks between Indian and Pacific Ocean populations resulting from the exposure of much of the Sunda and Sahul shelves (Fig. 1; Benzie, 1999). This pattern has been observed repeatedly in a variety of other organisms across most tropical marine phyla, and is common in both invertebrate (Table 1) and vertebrate species (Table 2).
| Species | Impact of sea-level change | Location | Reference |
|---|---|---|---|
| Cnidaria: Anthozoa | |||
| Multiple | Community structure change from the Pliocene to Pleistocene | Caribbean Sea | Budd et al. (1996) |
| Multiple | Stable species composition | Caribbean Sea; Papua New Guinea; Ryukyu Islands, Japan | Jackson (1992), Pandolfi (1996, 1999), Humblet et al. (2009) |
| Multiple | Environmental factors (e.g. freshwater inputs, wave regimes) on small scales are important for local species composition | Caribbean Sea; Papua New Guinea | Pandolfi & Jackson (2001), Pandolfi (1996) |
| Mollusca | |||
| Multiple | Stable species composition | Caribbean Sea | Gardiner (2001) |
| Pinctada maxima | IP-S, FST = 0.027a RST = 0.023a | Indo-Pacific junction | Lind et al. (2007) |
| Tridacna crocea | IP-S, ΦST = 0.28a. Population expansion | Indo-Pacific junction | Kochzius & Nuryanto (2008) |
| Multiple Echinolittorina snails | IP-S in continental species (ΦST = 0.86a, 0.91a), but no genetic structure in oceanic species (ΦST = 0.005) | Indo-Pacific junction | Reid et al. (2006) |
| Haliotis asinina | IP-S, FST = 0.2901a | Indo-Pacific junction | Imron et al. (2007) |
| Nerita albicilla and N. plicata | Habitat effects found | Indo-Pacific junction | Crandall et al. (2008a) |
| Coralliophila abbreviata | No genetic structure, but evidence of population bottlenecks, FST = 0.0002 ΦST = −0.0062 | Caribbean Sea | Johnston et al. (2012) |
| Multiple | Habitat effects found | Pacific | Paulay (1990) |
| Arthropoda: Crustacea | |||
| Birgus latro | IP-S, GST = 0.37a | Indo-Pacific junction | Lavery et al. (1996) |
| Scylla serrata | Multiple isolated populations. Population expansion. | Indo-Pacific junction | Fratini et al. (2010) |
| Tetraclita squamosa | Up to 19% sequence divergence between populations | Indo-Pacific junction | Chan et al. (2007a,b) |
| Chthamalus malayensis | Up to 14% sequence divergence between populations. Population expansion | Indo-Pacific junction | Tsang et al. (2008) |
| Haptosquilla glyptocercus, H. pulchella and Gonodactylellus viridis | Genetic structure across small geographical distances, ΦST = 0.527a, 0.8a and 0.674a, respectively | Indo-Pacific junction | Barber et al. (2006) |
| Phycomenes zostericola | Genetic structure across small distances, ΦST = 0.3a. Population expansion | Northeast Australia | Haig et al. (2010) |
| Eriocheir sensu stricto | Genetic structuring between the E. China Sea, S. China Sea, and Sea of Japan, FST = 0.29a | Northwest Pacific | Xu & Chu (2012) |
| Multiple decapod shrimps | Population bottlenecks in all species | Caribbean Sea | Cook et al. (2010) |
| Echinodermata | |||
| Acanthaster planci | IP-S seen in allozyme data | Indian Ocean and West Pacific | Benzie (1999) |
| Acanthaster planci | Divergence up to 10% between populations | Red Sea, Indo-Pacific | Vogler et al. (2008) |
| Diadema paucispinum and D. setosum | IP-S, divergence of 1.76% and 8.18%, respectively | Indian and Pacific Ocean | Lessios et al. (2001) |
| Tripneustes ventricosus | Reciprocally monophyletic clades in Western and Eastern Atlantic | Atlantic Ocean | Lessios et al. (2003) |
| Protoreaster nodosus and Linckia laevigata | Habitat effects found | Indo-Pacific junction | Crandall et al. (2008b) |
| Multiple brittle stars | Structure associated with larval feeding type found | Indian and Pacific Ocean | Hoareau et al. (2013) |
| Porifera | |||
| Leucetta chagosensis |
IP-S, ΦST = 0.419a Detection of local glacial refugia along the Great Barrier Reef |
Indo-Pacific junction Eastern Australia |
Wörheide et al. (2008) Wörheide et al. (2002) |
| Hymeniacidon flavia and H. sinapium | Genetic structure across small distances, FST = 0.214a and 0.6a, respectively | Northwest Pacific | Hoshino et al. (2008) |
| Pericharax heteroraphis | Low genetic structure across the Great Barrier Reef | Southwest Pacific | Bentlage & Wörheide (2007) |
- a P < 0.05.
- FST, ΦST and GST are fixation indexes that approximate genetic distances (Hendrick, 2011).
| Species | Impact of sea-level change | Location | Reference |
|---|---|---|---|
| Reptilia | |||
| Aipysurus laevis | IP-S, ΦST = 0.78a. Population expansion | Indo-Pacific junction | Lukoschek et al. (2007, 2008) |
| Cerberus rynchops | Multiple isolated populations, sequence divergence up to 11.8% | Indo-Pacific junction | Alfaro et al. (2004) |
| Mammalia | |||
| Trichechus manatus | Population expansion, isolated populations, ΦST = 0.18a | Caribbean Sea | Vianna et al. (2006) |
| Dugong dugong | IP-S, FST = 0.251a. Population expansion | Indo-Pacific junction | McDonald (2005), Blair et al. (2013) |
| Dugong dugong | Isolated population in Andaman Sea | Thailand | Palmer (2004) |
| Condrichthyes and Actinopterygii | |||
| Carcharhinus amboinensis | Isolated populations, ΦST = 0.029a | Northern Australia | Tillett et al. (2012) |
| Naso brevirostris and N. unicornis |
IP-S in N. brevirostris, ΦST = 0.0184a Non-significant genetic structure in N. unicornis, ΦST = 0.0299 |
Indian and Pacific Oceans | Horne et al. (2008) |
| Naso vlamingii | Temporal genetic structure detected, ΦST = 0.0766a | Indian and Pacific Oceans | Klanten et al. (2007) |
| Scarus ghobban | Low genetic structure, ΦST = 0.0105a | Western Indian Ocean | Visram et al. (2010) |
| Chlorurus sordidus | IP-S, ΦST = 0.5910a | Indian and Pacific Oceans | Bay et al. (2004) |
| Scarus psittacus | Evidence of population expansion, ΦCT = −0.099 | Indian and Pacific Oceans | Winters et al. (2010) |
| Scarus rubroviolaceus | Genetic isolation of peripheral populations, FST > 0.1a | Indian and Pacific Oceans | Fitzpatrick et al. (2011) |
| Halichoeres hortulanus and Lutjanus kasmira | No structure, ΦST = 0.27 and 0, respectively | Red Sea and Indian Ocean | DiBattista et al. (2013) |
| Acanthurus nigrofuscus, Cephalopholis argus and Chaetodon auriga | Genetic structure between the Red Sea and Indian Ocean, ΦST =0.23a, 0.28a and 0.2a, respectively | Red Sea and Indian Ocean | DiBattista et al. (2013) |
| Neoniphon sammara and Pygoplites diacanthus | Genetic structure between the Red Sea and Indian Ocean, ΦST = 0.34a and 0.69a, respectively | Red Sea and Indian Ocean | DiBattista et al. (2013) |
| Amphiprion ocellaris | IP-S, ΦST = 0.241a | Indo-Pacific junction | Timm & Kochzius (2008) |
| Amphiprion ocellaris | IP-S, ΦCT = 0.56a | Indo-Pacific junction | Nelson et al. (2000) |
| Multiple species | Habitat effects found | French Polynesia | Fauvelot et al. (2003) |
| Gnatholepis anjerensis, and G. scapulostigma | ΦST = 0.261a and 0.066a, respectively | South Pacific | Thacker (2004) |
| Halichoeres margaritaceus, H. trimaculatus, H. claudia, and H. ornatissimus | Population expansion for all species. Habitat effects found | Indo-Pacific junction | Ludt et al. (2012) |
| Acanthemblemaria aspera and A. spinosa | Population bottleneck in deeper living A. aspera | Caribbean | Eytan & Hellberg (2010) |
| Sicyopterus lagocephalus | Population expansion | Indian and Pacific Oceans | Hoareau et al. (2012) |
- a P < 0.05.
- FST and ΦST are fixation indexes that approximate genetic distances (Hendrick, 2011).
Population structuring at smaller geographical scales has also been found in this region. The damselfish Amphiprion ocellaris separates into four contemporary populations, with mixed historical genetic signals (Timm & Kochzius, 2008) possibly reflecting isolation during the Pleistocene with later re-colonization of shelf habitats as the Sunda Shelf was flooded by sea-level rise (Nelson et al., 2000). Genetic variation in this region among stomatopod and caridean shrimps suggests limited connectivity across relatively small spatial scales dating back to the Pleistocene (Barber et al., 2006; Haig et al., 2010); this is also the case for the sea snake Cerberus rynchops, with semi-isolated populations inferred from the Philippines, Thailand, the Greater Sunda Islands and the rest of the Indian Ocean, including Myanmar (Alfaro et al., 2004). North of the Coral Triangle, divergence on small geographical scales can be seen in the mitten crab, Eriocheir sensu stricto, corresponding to possible glacial refugia in the East China Sea, the Sea of Japan, and the South China Sea (Xu & Chu, 2012), and several sponges of the genus Hymeniacidon show genetic structuring over small geographical scales off Japan, reflecting isolation of parts of the Seto Inland Sea from the Pacific during sea-level lows (Hoshino et al., 2008). Population breaks are also found at other locations bordering the Indo-Pacific, such as the Red Sea where the crown-of-thorns starfish (Vogler et al., 2008) and several fishes (DiBattista et al., 2013) exhibit population breaks.
In addition to population-level divergences, the exposure of the Sunda and Sahul shelves may have been critical for speciation in the Indo-Pacific (Rocha & Bowen, 2008; Gaither & Rocha, 2013), driving allopatric partitioning in marine snails (Frey & Vermeij, 2008) and barnacles (Chan et al., 2007a,b; Tsang et al., 2008). Furthermore, each population of the barnacle Tetraclita squamosa has unique genetic signatures of expansion, suggesting distinct evolutionary histories (Tsang et al., 2008). Unique genetic signatures of population expansion are also found in nesting colonies of loggerhead turtles (Caretta caretta) in Japan (Hatase et al., 2002). In the Pacific, unique genetic signatures of population expansion led Hoareau et al. (2012) to conclude that the amphidromous goby Sicyopterus lagocephalus survived glacial fluctuations in refugia located on Melanesia and Polynesia. In the Caribbean, population bottlenecks associated with glacial cycles are also found in marine gastropods and shrimps, but do not date to the most recent glacial maximum (Cook et al., 2010; Johnston et al., 2012).
Several studies have found evidence of Pleistocene refugia around Australia. Phylogeographical studies of the olive sea snake, Aipysurus laevis, found structure across northern Australia: the western Australian population was a source to the eastern population, and high genetic diversity in comparison to the eastern clades suggests that this population experienced several size fluctuations, whereas populations in the Gulf of Carpentaria and the Great Barrier Reef were composed of recent migrants from the western population and have only been in place since the LGM (Lukoschek et al., 2007, 2008). This suggests that the olive sea snake may have been restricted to glacial refugia in western Australia during sea-level lows. The common pig-eye shark, Carcharhinus amboinensis, in northern Australia also shows genetic breaks between eastern and western populations (Tillett et al., 2012). In eastern Australia, the sponge Leucetta chagosensis separates into two divergent clades, north and south, which also may reflect local refugia (Wörheide et al., 2002).
For marine mammals, one study in Thailand suggested that dugongs (Dugong dugong) founded populations in the Gulf of Thailand after being constrained to the Andaman Sea during the LGM (Palmer, 2004). Refugia have also been hypothesized for related manatees in the Atlantic. A phylogenetic analysis of the West Indian manatee Trichechus manatus found three distinct clades, one of which (in Guyana and Brazil) shared no haplotypes with the other populations. As habitable area decreased with lower sea levels, populations were isolated in several refugia in the southern Caribbean, from which they expanded as sea levels rose (Vianna et al., 2006). Migration was probably restricted between refugia in the eastern populations (Brazil/Guyana) and the western Caribbean when low sea level caused several islands in the Lesser Antilles to merge and form land barriers (Fig. 3b).
Habitat preference and life-history correlations
Phylogeographical and population genetic studies have exposed a variety of patterns common to shallow water marine taxa but do not address why similarly related taxa might have vastly different histories over the course of the Pleistocene. An assortment of hypotheses regarding the mechanisms that influence these observed responses has driven comparative studies that look for commonalities between certain aspects of an organism and their environment.
In French Polynesia, correlations between genetic diversity and habitat preferences (lagoon versus outer reef) were found among eight reef fishes (Fauvelot & Planes, 2002; Fauvelot et al., 2003). The authors hypothesized that during sea-level lows lagoon habitats were exposed and dried up (Fig. 5), causing extirpation of strictly lagoon species. This extirpation resulted in lower genetic diversity in lagoon species (Chaetodon citrinellus, Chrysiptera glauca, Dascyllus aruanus and Pomacentrus pavo) and genetic signatures of population expansion when sea levels rose and the lagoons were re-colonized (Fauvelot & Planes, 2002; Fauvelot et al., 2003).
The impact that habitat preference can have has been corroborated by several studies on fishes (Thacker, 2004; Eytan & Hellberg, 2010; Ludt et al., 2012) and invertebrates (Paulay, 1990; Crandall et al., 2008a,b). Fossil evidence suggests that, while lagoon-inhabiting species were extirpated from areas during sea-level lows, these species did not go extinct. However, it is hypothesized that some populations found refuge on continental margins in the West Pacific and then recolonized oceanic lagoons when sea level rose (Paulay, 1990). At local scales this has caused reduced genetic diversity for lagoon species (Fauvelot et al., 2003; Thacker, 2004). However, at the scale of ocean basins, Ludt et al. (2012) found evidence that species living in shallow lagoons had persisted in refugia during glacial sea-level lows and that isolation of these refugia led to higher overall genetic diversity than the outer reef species, promoted by genetic mixing of expanding refugial populations as sea level rose. Results based on habitat preferences have also been detected in Caribbean blennies living at different depths, where a recent population bottleneck associated with sea-level fluctuations was detected in a deeper-water species, Acanthemblemaria aspera, but not in the shallower A. spinosa (Eytan & Hellberg, 2010). This is counter to the above examples, where shallow-water species are more affected than deeper species, highlighting that depth range is not the only factor driving species demographic history.
Genetic differences in continental and oceanic species of Echinolittorina snails in the Indo-Pacific also strengthen the notion that habitat preference has a large effect on how species respond to sea-level fluctuations (Reid et al., 2006). Oceanic species show little to no population structuring, while continental species are highly structured, possibly because of ecological differences between continental and oceanic-adapted species (Reid et al., 2006).
Fauvelot et al. (2003) found no correlation with reproductive strategy (demersal versus pelagic eggs) and genetic demographic history in fishes; however, life-history strategies of larvae seem to be important in southern Indian Ocean brittle stars. By comparing 10 currently recognized brittle stars, Hoareau et al. (2013) found no structure between Pacific and south-western Indian Ocean populations for species that have planktotrophic larvae, but significant structure (divergence at the species level) for those that have lecithotrophic larvae. Furthermore, population expansion was seen in each species, dating to the early Pleistocene, which suggests regional persistence through several sea-level changes (Hoareau et al., 2013).
Other life-history traits, such as pelagic larval duration (PLD), have been hypothesized as mechanisms regulating species dispersal and range size. For example, both Naso surgeonfishes and parrotfishes have a long PLD (c. 90 and 60 days, respectively; Leis & Rennis, 1983; Klanten et al., 2007; Horne et al., 2008) that has explained a general lack of structure across the central Indo-Pacific in Naso unicornis (Horne et al., 2008) and Scarus ghobban (Visram et al., 2010). However, other closely related surgeonfishes, such as Naso brevirostris and Naso vlamingii (Klanten et al., 2007; Horne et al., 2008), and other parrotfishes, including Scarus psittacus, Scarus rubroviolaceus and Chlorurus sorididus (Bay et al., 2004; Winters et al., 2010; Fitzpatrick et al., 2011), show more complicated patterns ranging from population breaks between the Indian and Pacific Oceans, to isolated populations at the periphery of ranges, to non-geographical population structuring which may be due to isolation during the Pleistocene followed by mixing as sea levels rose, thus erasing the geographical signature. This complicated pattern of population structure overlapping in the Indo-Pacific has been summarized by Gaither & Rocha (2013), and has ultimately led to the acceptance that PLD is not a strong predictor of population structure in the Indo-Pacific (Weersing & Toonen, 2009).
A comparative study of seven species of fish in the Red Sea and adjacent Indian Ocean found multiple patterns in response to Pleistocene isolation (DiBattista et al., 2013). Some species showed no genetic structure (Halichoeres hortulanus and Lutjanus kasmira), some exhibited shallow genetic structure (Acanthurus nigrofuscus, Cephalopholis argus and Chaetodon auriga), and two species showed deep genetic divergence possibly at the species level (Neoniphon sammara and Pygoplites diacanthus: DiBattista et al., 2013). However, the underlying causes of these species-specific differences are not yet known.
Conclusions
Over the past few decades, investigations of intraspecific and higher-level genetic differences between species in different biogeographical areas have laid the foundation for the study of organismal responses to sea-level fluctuations. Fossil evidence has been useful in some groups (e.g. anthozoans, bivalves, gastropods), but is scarce or absent in many others (e.g. fishes, sponges). Furthermore, because of the technical and logistical challenges of sampling currently deeper water sediments and populations, it is likely that the picture presented here is incomplete and many gaps need to be filled.
At present, several phylogeographical patterns have been observed across a broad range of marine invertebrate (Table 1) and vertebrate (Table 2) taxa. Genetic structure between populations has been detected at both large and small geographical scales. This includes isolated populations that are geographically close to one another (such as in shrimps, barnacles, sponges and some fishes), populations that are structured between the Indian and Pacific Oceans (a pattern observed at some level in most clades), and cases where no, or very little, genetic structure is found across the entire range of a species (e.g. Lessios et al., 2003 for sea urchins; Bowen et al., 2001; Craig et al., 2007; Reece et al., 2010 for fishes). Demographically, most marine clades exhibit patterns indicative of population expansion following bottlenecks associated with low sea-level stands.
Studies designed to examine species-specific life-history traits have shown that habitat preference correlates strongly with how a species is impacted, with species living in lagoons or other shallow areas being more susceptible to large population bottlenecks (Crandall et al., 2008a for marine snails; Crandall et al., 2008b for star fish; Fauvelot et al., 2003; Thacker, 2004; Ludt et al., 2012 for fishes). These studies have also found that, at least in fishes, PLD and spawning mode (pelagic versus demersal egg spawning) do not correlate well with genetic signatures caused by sea-level change (Fauvelot et al., 2003; Weersing & Toonen, 2009).
Although much progress has been made, there are some areas that will benefit from additional studies. Research on the effects of Pleistocene sea-level fluctuations is somewhat concentrated in the Indo-Pacific. This may be due to the well-known exposure of the Sunda and Sahul shelves during sea-level lows, which left a clear genetic signature in many species. Alternatively, the abundance of studies in the Indo-Pacific may reflect its status as a modern-day biodiversity hotspot, which causes it to receive more attention from researchers worldwide. Regardless of cause, focus on the Indo-Pacific has left many tropical areas, such as the Red Sea, Persian Gulf (which was completely dry during the LGM), regions abutting Africa, the tropical eastern Pacific, and even the Caribbean, wide open for new investigations.
Population genetic data are absent or scarce for some groups owing to a lack of sampling (e.g. marine snakes) or a lack of appropriate molecular markers (e.g. cnidarians and poriferans; Hellberg, 2006). However, the advent of new sequencing techniques is already beginning to improve analytical success of studies of some of these unresolved groups (Baums et al., 2012; Andras et al., 2013). Next-generation sequencing techniques, in particular, are likely to prove very useful to bridge these gaps by identifying appropriate genomic regions for comparative approaches and by increasing the amount of data that are available (Rocha et al., 2013).
Furthermore, continued investigation of the role of life-history characteristics (e.g. mutualistic relationships, habitat preference, etc.), especially in shaping species demographic history, will help to improve our understanding of species–habitat (Fauvelot & Planes, 2002; Fauvelot et al., 2003; Thacker, 2004; Ludt et al., 2012) and symbiotic relationships (Crandall et al., 2008b). Notably, the investigation of species-specific traits influencing demographic history is still relatively unexplored. Future studies using a multi-species comparative approach may differentiate the impact of traits, in contrast to analyses of single species, by allowing the comparison of closely related species that differ in their life history. Additionally, comparisons between more distantly related taxa, and across several phyla, may further expose common patterns across all marine life.
By filling in these gaps we will gain a more holistic view on how eustatic sea-level change altered the demographic history of marine organisms. The inclusion of additional taxa will help to determine whether marine species responded to Pleistocene sea-level fluctuations individually, or whether there are general ecological or biogeographical rules that apply to the tropics. This might give us general expectations of how rapid sea-level fluctuations impact on coastal marine systems. In the process of adding taxa we may provide new testable hypotheses regarding the impact of future sea-level change on marine biota, and inform management plans for targeted conservation efforts.
Acknowledgements
We thank Benjamin Walther and Deana Erdner for reviewing early versions of this manuscript and Corinne E. Myers for substantial help in later manuscript revisions. Comments from Jason Ali and three anonymous referees greatly improved this review. Financial support was provided by Louisiana State University, the California Academy of Sciences and the National Science Foundation (NSF DEB-1257630 to L.A.R.).
References
Biosketches
William B. Ludt is a graduate student at Louisiana State University working in the LSU Museum of Natural Science. His research interests centre on the molecular systematics, evolutionary patterns and biogeography of coastal marine fishes.
Luiz A. Rocha is the Curator of Ichthyology at the California Academy of Sciences. His research focuses on the evolution, biogeography, genomics and systematics of coral reef fishes.




