Association between mitochondrial DNA copy number and production traits in pigs
Abstract
Mitochondria are essential organelles in the regulation of cellular energetic metabolism. Mitochondrial DNA copy number (mtDNA_CN) can be used as a proxy for mitochondria number, size, and activity. The aims of our study are to evaluate the effect of mtDNA_CN and mitochondrial haploblocks on production traits in pigs, and to identify the genetic background of this cellular phenotype. We collected performance data of 234 pigs and extracted DNA from skeletal muscle. Whole-genome sequencing data was used to determine mtDNA_CN. We found positive correlations of muscle mtDNA_CN with backfat thickness at 207 d (+0.14; p-value = 0.07) and negative correlations with carcase loin thickness (−0.14; p-value = 0.03). Pigs with mtDNA_CN values below the lower quartile had greater loin thickness (+4.1 mm; p-value = 0.01) and lower backfat thickness (−1.1 mm; p-value = 0.08), which resulted in greater carcase lean percentage (+2.4%; p-value = 0.04), than pigs with mtDNA_CN values above the upper quartile. These results support the hypothesis that a reduction of mitochondrial activity is associated with greater feed efficiency. Higher mtDNA_CN was also positively correlated with higher meat ultimate pH (+0.19; p-value <0.01) but we did not observe significant difference for meat ultimate pH between the two groups with extreme mtDNA_CN. We found no association of the most frequent mitochondrial haploblocks with mtDNA_CN or the production traits, but several genomic regions that harbour potential candidate genes with functions related to mitochondrial biogenesis and homeostasis were associated with mtDNA_CN. These regions provide new insights into the genetic background of this cellular phenotype but it is still uncertain if such associations translate into noticeable effects on the production traits.
1 INTRODUCTION
Mitochondria are subcellular organelles that provide most of the energy needed for animal cell metabolism, as well as being involved in many other metabolic pathways, which include signalling, ion homeostasis, lipid metabolism, redox control, cell growth, and cell death (McBride et al., 2006). Mitochondria generate adenosine triphosphate (ATP), the main source of chemical energy of animal cells, by oxidative phosphorylation of adenosine diphosphate (ADP) in the mitochondrial membrane during the TCA cycle and the electron transport chain. It has been hypothesized that defective proteins in the electron transport chain could lead to a reduction of energy efficiency of animals (Bottje, 2019). Suboptimal mitochondrial function could lead to an increase of reactive oxygen species (Tait & Green, 2012), which might start a cascade of oxidative damage to proteins, and specifically to the electron transporter chain, further reducing ATP production (Iqbal et al., 2005).
Several studies reported a relationship between mitochondrial activity and feed efficiency in the context of livestock production. Bottje et al. (2002) reported that less feed-efficient broilers had increased electron leakage in breast mitochondria and lower activities of protein complexes I and II. This study was followed by several more in poultry (Bottje et al., 2017; Iqbal et al., 2005; B.-W. Kong et al., 2016; Lassiter et al., 2006), cattle (Dorji et al., 2021; Kelly et al., 2011; R. S. G. Kong et al., 2016), and pigs (Carmelo & Kadarmideen, 2020; Fu et al., 2017; Gondret et al., 2017; Grubbs et al., 2013; Jing et al., 2015). Most studies have concluded that individuals with high feed efficiency show a reduction in the expression of mitochondrial genes (Dorji et al., 2021; Iqbal et al., 2005) and a reduction in the expression of genes involved in mitochondrial biogenesis (Jing et al., 2015) and in oxidative phosphorylation (Fu et al., 2017). Nevertheless, there is some controversy about these results because other researchers have reported contradictory results, such as that there are no clear differences in mitochondrial abundance and activity between cattle with different feed efficiency (McKenna et al., 2020), or that animals with high feed efficiency have increased expression of mitochondrial genes (Gondret et al., 2017; R. S. G. Kong et al., 2016) and reduced production of reactive oxygen species, which indicates less electron leakage and, thus, more efficient electron transport chain proteins (Bottje et al., 2002; Grubbs et al., 2013). Thus, the direction of the association between mitochondrial activity and feed efficiency remains inconclusive and seems dependent on the species and sampled tissue.
Mitochondrial activity can be measured using laboratory methodologies such as the determination of oxygen consumption rate or the production of ATP or reactive oxygen species. However, mitochondrial DNA (mtDNA) copy number (mtDNA_CN), which can be easily derived from raw whole-genome sequence data, can be used as a proxy for mitochondrial activity. This cellular phenotype is tissue-dependent and it should preferably be determined in a tissue of metabolic relevance such as the muscle or liver. Variation in mtDNA_CN in humans has been associated with diverse pathologies, such as cardiomyopathy, diabetes and cancer (Abd Radzak et al., 2022; Dillon et al., 2012; Holmström et al., 2012; Kaaman et al., 2007; Pirinen et al., 2020), and positively correlated with mitochondrial mass and the respiratory capacity (D'Erchia et al., 2015). Three studies in livestock species have associated mtDNA_CN with carcase and breast meat yield and abdominal fat, in chickens (Reverter et al., 2016), and feed efficiency and birth and weaning weight, in cattle (R. S. G. Kong et al., 2016; Sanglard et al., 2023). This cellular trait is moderately heritable, with heritability estimates in humans of 0.33 to 0.65 (López et al., 2012; Xing et al., 2008). Several genes located in the nuclear DNA act as master regulators of muscle mitochondrial content. Genes such as TFAM (Ekstrand et al., 2004; Ikeda et al., 2015), TWNK (Ikeda et al., 2015; Tyynismaa et al., 2004), and PPARGC1A (Chaudhary et al., 2021; Gouspillou et al., 2014) have been observed to regulate mtDNA_CN in humans and mice. To our knowledge, there is no information about the relationship between mtDNA_CN and production traits in pigs and only scarce information on its genetic determinism in this species (Yang et al., 2024).
The aims of this study were (i) to evaluate the effect of mtDNA_CN on growth, carcase composition, and meat quality traits in pigs, (ii) to evaluate the effect of mitochondrial haploblocks on the same traits, and (iii) to identify the genetic background of this cellular phenotype.
2 MATERIALS AND METHODS
2.1 Animals and phenotypes
A total of 234 Duroc pigs from the same genetic line were raised in 19 batches between 2002 and 2021 following a common protocol for data recording and tissue sampling (Ros-Freixedes et al., 2016). Pigs from each batch were raised from around 75 days until slaughter age (at 213 days, SD 7.8 d) under identical conditions. During this time, animals had ad libitum access to commercial feed (Esporc, Riudarenes, Spain). At 207 days of age (SD 8 d), pigs were weighed and the backfat and loin thickness were measured at 5 cm off the midline at the position of the last rib using a portable ultrasonic scanner (Piglog 105; Frontmatec, Kolding, Denmark). All pigs were slaughtered in the same abattoir, where carcase weight was recorded. Backfat and loin thickness at 6 cm off the midline between the third and fourth last ribs were recorded using an on-line ultrasound automatic scanner (AutoFOM; Frontmatec, Kolding, Denmark). Ultimate pH of meat was measured in muscle semimembranosus after chilling for approximately 24 h at 2°C with a pH-meter equipped with a spear-tipped probe (Testo 205; Testo AG, Lenzkirch, Germany). Then, for a subset of 205 pigs (15 batches), samples of muscle gluteus medius were taken, vacuum packaged and stored at −20°C until required for chemical analyses. Intramuscular fat content and fatty acid composition was determined in duplicate by quantitative gas chromatography (Bosch et al., 2009). Fatty acids were expressed as percentages relative to total fatty acid content. The proportion of saturated fatty acids (C14:0, C16:0, C18:0 and C20:0), monounsaturated fatty acids (C16:1n-7, C18:1n-7, C18:1n-9 and C20:1n-9), and polyunsaturated fatty acids (C18:2n-6, C18:3n-3, C20:2n-6 and C20:4n-6) were calculated.
2.2 Whole-genome sequencing
Extraction of DNA from all pigs was carried out following a conventional phenol: chloroform protocol (Green & Sambrook, 2017) from skeletal muscle (n = 234). An additional set of 48 individuals, from the last 5 batches (between 2019 and 2021) and that followed the same rearing and sampling protocol as the other 234 pigs, had DNA extracted from blood at 85.6 days of age (SD 2.4 d) for the purpose of assessing the value of blood as a non-target tissue for mtDNA_CN determination.
The DNA samples were submitted to the Centre Nacional d'Anàlisi Genòmica (CNAG-CRG, Barcelona, Spain) for whole-genome sequencing on a NovaSeq 6000 instrument (Illumina, San Diego, CA, USA) in paired-end mode, as described by Molinero et al. (2022).
Trimmomatic (Bolger et al., 2014) was used to remove adapter sequences from the FASTQ sequence files. The trimmed reads were aligned to the reference genome Sscrofa11.1 (GCA_000003025.6; Warr et al., 2020) using the BWA-MEM algorithm (Li, 2013). The average realized sequencing coverage depth was 7.3× (SD 2.0×). After using Picard (http://broadinstitute.github.io/picard) to tag duplicates, the variant calling was performed separately for each individual with GATK HaplotypeCaller (GATK 3.8.0) (DePristo et al., 2011) using default settings. Then, all individuals were jointly genotyped for all variant positions. We retained all biallelic variants, including single nucleotide polymorphisms and short insertions/deletions, for further analyses with VCFtools (Danecek et al., 2011).
The mtDNA_CN was calculated as the natural logarithm of the ratio between mitochondrial and nuclear DNA coverage depths. Mitochondrial haploblocks were obtained using Haploview 4.2 (Barrett et al., 2005) with the standard method described by Gabriel et al. (2002).
2.3 Relationship with production traits and meat quality
We used a linear model to adjust mtDNA_CN for the batch effect. The effect of mtDNA_CN on each production trait was then tested with an F-test after fitting a linear model that included mtDNA_CN as a covariate as well as the effects of batch and other covariates. For carcase weight, we included the age of slaughter as a covariate, whereas for the other production traits we included carcase weight as a covariate. For fatty acid composition traits, carcase weight and intramuscular fat content were both included as covariates. We also generated two divergent groups for mtDNA_CN by selecting the 50 animals with the highest and 50 with the lowest values among the 205 pigs with complete fat content and fatty acid composition data (i.e., approximately all pigs below the lower quartile or above the upper quartile). These two groups were subsequently used to study potential associations between mtDNA_CN and production traits. The difference between the two mtDNA_CN groups for each trait was tested with an F-test using a linear model that also included the effects of batch and covariates as described above. To assess the value of non-target tissues for mtDNA_CN determination, we compared the correlations between the preadjusted values of mtDNA_CN, quantified either from muscle or from blood samples, and the phenotypes for the different production traits, which were also preadjusted in a similar way using a model with the effects of the batch and the same covariates as detailed. The effect of mitochondrial haploblocks and the rs709596309 leptin receptor (LEPR) genotypes (Ros-Freixedes et al., 2016) were similarly tested with models that included the same effects. The heritability of mtDNA_CN was estimated using a sire model with the batch as a fixed effect and the sire as a random effect. The heritability estimate was calculated as: 4·/( + ), where is the between-sire variance and is the residual variance. All analyses were performed using the statistical package JMP PRO 16 (SAS Institute Inc., Cary, NC).
2.4 Genome-wide association study
3 RESULTS
3.1 Association between mtDNA_CN and production traits
The regression coefficients of the production traits on mtDNA_CN are shown in Table 1. The mtDNA_CN showed a positive effect on both live and carcase backfat thickness (+1.5 ± 0.9 mm/unit mtDNA_CN, p-value = 0.09, and +1.2 ± 0.6 mm/unit, p-value = 0.06, respectively), and negative on loin thickness (−3.7 ± 1.7 mm/unit; p-value = 0.03), and carcase lean percentage (−2.3 ± 1.2 mm/unit; p-value = 0.06), as well as a positive effect on pH (+0.11 ± 0.04 unit−1; p-value = 0.01). The regression coefficients of fatty acid composition in muscle gluteus medius on mtDNA_CN are shown in Table 2, but we did not observe any relevant effect of mtDNA_CN on fatty acid composition.
| Trait | Regression coefficient | p-Value | Low | High | p-Value |
|---|---|---|---|---|---|
| mtDNA_CN | – | – | 5.24 ± 0.02 | 6.18 ± 0.03 | <0.001 |
| Live measurements at 207 d | |||||
| Body weight, kg | −2.7 ± 2.5 | 0.28 | 127.3 ± 2.0 | 125.2 ± 2.1 | 0.49 |
| Backfat thickness, mm | +1.5 ± 0.9 | 0.09 | 21.4 ± 0.6 | 22.7 ± 0.7 | 0.19 |
| Loin thickness, mm | −0.8 ± 1.2 | 0.53 | 48.9 ± 0.8 | 48.3 ± 1.0 | 0.71 |
| Carcase measurements | |||||
| Carcase weight, kg | −1.5 ± 1.8 | 0.41 | 98.1 ± 1.3 | 97.3 ± 1.5 | 0.69 |
| Backfat thickness, mm | +1.2 ± 0.6 | 0.06 | 22.6 ± 0.4 | 23.7 ± 0.5 | 0.08 |
| Loin thickness, mm | −3.7 ± 1.7 | 0.03 | 47.6 ± 1.1 | 43.5 ± 1.2 | 0.01 |
| Carcase lean percentage, % | −2.3 ± 1.2 | 0.06 | 43.1 ± 0.8 | 40.7 ± 0.9 | 0.04 |
| Intramuscular fat content, % | −0.2 ± 1.0 | 0.82 | 17.8 ± 0.8 | 18.6 ± 0.9 | 0.42 |
| pH | +0.11 ± 0.04 | 0.01 | 5.77 ± 0.04 | 5.81 ± 0.04 | 0.49 |
- Note: In bold, p-values <0.10.
| Trait | Regression coefficient | p-Value | Low | High | p-Value |
|---|---|---|---|---|---|
| Saturated fatty acids | −0.09 ± 0.40 | 0.81 | 37.9 ± 0.3 | 38.1 ± 0.3 | 0.63 |
| C14:0 | −0.03 ± 0.05 | 0.57 | 1.74 ± 0.05 | 1.68 ± 0.05 | 0.37 |
| C16:0 | +0.09 ± 0.23 | 0.70 | 24.3 ± 0.2 | 24.5 ± 0.2 | 0.58 |
| C18:0 | −0.15 ± 0.22 | 0.50 | 11.7 ± 0.2 | 11.8 ± 0.2 | 0.54 |
| Monounsaturated fatty acids | +0.39 ± 0.40 | 0.33 | 49.3 ± 0.3 | 49.6 ± 0.3 | 0.52 |
| C16:1n-7 | +0.08 ± 0.10 | 0.43 | 3.56 ± 0.09 | 3.57 ± 0.09 | 0.97 |
| C18:1n-7 | +0.15 ± 0.10 | 0.10 | 4.45 ± 0.06 | 4.52 ± 0.07 | 0.47 |
| C18:1n-9 | +0.24 ± 0.40 | 0.55 | 41.4 ± 0.3 | 41.5 ± 0.3 | 0.67 |
| Polyunsaturated fatty acids | −0.29 ± 0.26 | 0.25 | 12.8 ± 0.2 | 12.3 ± 0.2 | 0.06 |
| C18:2n-6 | −0.19 ± 0.20 | 0.35 | 10.2 ± 0.2 | 9.9 ± 0.2 | 0.15 |
| C18:3n-3 | −0.02 ± 0.02 | 0.29 | 0.59 ± 0.01 | 0.55 ± 0.02 | 0.07 |
| C20:4n.6 | −0.08 ± 0.07 | 0.22 | 1.40 ± 0.05 | 1.28 ± 0.05 | 0.12 |
- Note: In bold, p-values <0.10.
When we compared two groups of animals with the highest and lowest mtDNA_CN scores. The high group had an average mtDNA_CN score of 6.21 (SD 0.15) and the low group had an average of 5.25 (SD 0.17). In line with the correlation data, we observed differences between the two groups for carcase composition traits (Table 1). The pigs with low mtDNA_CN had greater loin thickness (+4.1 mm; p-value = 0.01) and lower backfat thickness (−1.1 mm; p-value = 0.08), which resulted in greater carcase lean percentage (+2.4%; p-value = 0.04) than pigs with high mtDNA_CN. Similar trends were observed in the live body composition traits, although not significant. However, both point towards pigs with more mtDNA_CN being leaner. No association between mtDNA_CN groups was detected for carcase weight, pH, or intramuscular fat content and fatty acid composition traits, except for a suggestive trend for the pigs with low mtDNA_CN to contain more polyunsaturated fat (Table 2).
The mtDNA_CN variation has a genetic additive component. We estimated a heritability for mtDNA_CN of 0.60 ± 0.46. We previously described that the recessive missense variant rs709596309, which is a missense variant of the LEPR gene, is associated with feed intake, mobilization of energy reserves, and fat deposition in this population (Ros-Freixedes et al., 2016; Solé et al., 2021). We confirmed that the LEPR genotype affected carcase backfat thickness, loin thickness, and lean percentage in the sequenced pigs, but we did not find any evidence that these effects are underlain by any differences in mtDNA_CN (Table 3).
| Trait | C– | TT | p-Value |
|---|---|---|---|
| mtDNA_CN | 5.78 ± 0.03 | 5.77 ± 0.04 | 0.92 |
| Carcase measurements | |||
| Carcase weight, kg | 99.1 ± 0.8 | 99.4 ± 1.1 | 0.79 |
| Backfat thickness, mm | 24.0 ± 0.3 | 25.9 ± 0.4 | <0.001 |
| Loin thickness, mm | 44.6 ± 0.7 | 40.7 ± 1.0 | <0.001 |
| Carcase lean percentage, % | 42.3 ± 0.5 | 37.9 ± 0.7 | <0.001 |
| Intramuscular fat content, % | 18.1 ± 0.4 | 19.5 ± 0.6 | 0.05 |
| pH | 5.77 ± 0.02 | 5.80 ± 0.03 | 0.39 |
- Note: In bold, p-values <0.10.
The tissue that was used for extracting DNA had a clear effect on the estimated mtDNA_CN. Using raw sequencing data from DNA extracted from skeletal muscle resulted in greater mtDNA_CN (5.79; SD 0.37) than from blood samples (4.09; SD 0.34; p-value <0.01). However, we observed similar correlation structures for mtDNA_CN estimated from skeletal muscle or blood samples, at least for pH and carcase composition traits. Due to the small sample size, the correlations for blood samples were not significant, yet the sign of the correlation for these traits was largely maintained with respect to skeletal muscle (Table 4).
| Trait | Muscle (n = 234) | p-Value | Blood (n = 48) | p-Value |
|---|---|---|---|---|
| Live measurements | ||||
| Body weighta | −0.09 | 0.23 | +0.09 | 0.56 |
| Backfat thickness at 207 d | +0.14 | 0.07 | – | – |
| Loin thickness at 207 d | −0.06 | 0.48 | – | – |
| Carcase measurements | ||||
| Carcase weight | −0.06 | 0.37 | +0.16 | 0.31 |
| Backfat thickness | +0.08 | 0.26 | +0.10 | 0.53 |
| Loin thickness | −0.14 | 0.03 | −0.06 | 0.69 |
| Carcase lean percentage | −0.09 | 0.19 | −0.05 | 0.74 |
| Intramuscular fat content | −0.02 | 0.76 | – | – |
| pH | +0.19 | <0.01 | +0.13 | 0.43 |
- a Body weight was registered at 207 and 85 days for muscle and blood, respectively.
- Note: In bold, p-values <0.10.
3.2 Effect of mtDNA haploblocks on traits
Twenty-six mitochondrial variants, all SNPs, segregated across the sequenced pigs. These variants formed 7 mitochondrial haploblocks, of which 4 had a frequency equal or greater than 0.10 and represented 98% of our population (Tables 5 and S1). No association was detected between the common haploblocks and mtDNA_CN or the production traits (Table 5).
| Trait | Hap1 | Hap2 | Hap3 | Hap4 | p-value |
|---|---|---|---|---|---|
| Frequency | 0.41 | 0.30 | 0.17 | 0.10 | – |
| mtDNA_CN | 5.71 ± 0.04 | 5.69 ± 0.05 | 5.75 ± 0.06 | 5.67 ± 0.08 | 0.81 |
| Carcase measurements | |||||
| Carcase weight, kg | 98.6 ± 1.0 | 100.3 ± 1.2 | 99.1 ± 1.5 | 98.7 ± 1.9 | 0.72 |
| Backfat thickness, mm | 23.3 ± 0.3 | 23.6 ± 0.4 | 24.3 ± 0.5 | 23.4 ± 0.6 | 0.37 |
| Loin thickness, mm | 43.9 ± 0.9 | 44.7 ± 1.1 | 44.1 ± 1.4 | 43.8 ± 1.8 | 0.94 |
| Carcase lean percentage, % | 42.0 ± 0.7 | 41.5 ± 0.8 | 39.7 ± 1.0 | 41.6 ± 1.3 | 0.28 |
| Intramuscular fat content, % | 19.0 ± 0.5 | 18.5 ± 0.6 | 18.1 ± 0.8 | 18.3 ± 1.0 | 0.77 |
| pH | 5.79 ± 0.02 | 5.79 ± 0.03 | 5.80 ± 0.04 | 5.74 ± 0.05 | 0.73 |
3.3 Genome-wide association study for mtDNA_CN
Over 20 million variants were called in the Duroc pigs. We discarded variants that presented a genotyping rate below 0.9 and a minor allele frequency below 0.2 so that all genotypes were sufficiently represented in the 234 pigs. After the filtering, 7.05 million variants remained. The GWAS for mtDNA_CN did not detect any significant variants after applying multiple testing corrections, but we identified 27 variants at Sus scrofa chromosomes (SSC) 3, 4, 5, 7, 10, 11, and 13 that had p-values <10−5 as those with the highest signal-to-noise ratio (Figure 1). Eleven of them were located at genomic regions where no annotated genes have been described. The remaining 16 were grouped into 9 genomic regions (Table 6) at SSC 3 (3 regions), 4, 5, and 10 (4 regions). A literature search pinpointed several candidate genes that have been previously associated with mitochondria homeostasis and activity, such as ALODA, MAPK3, MTHFD2, HFM1, several genes of the keratin (KRT) gene family, HLX, and NEBL.
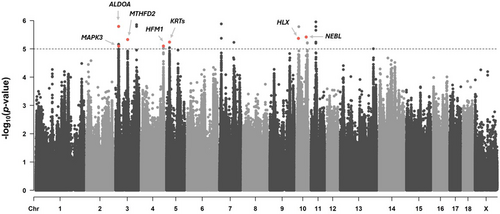
| SSC | Region (Mb) | No. of SNPs | Position of top SNP (bp) | p-Value of top SNP | Genes |
|---|---|---|---|---|---|
| 3 | 17.82–17.94 | 4 | 17,876,257 | 1.61 × 10−6 | ITGAL, DCTPP1, ZNF771, ZNF48, SEPTIN1, MYL11 |
| 3 | 18.25–18.35 | 1 | 18,296,353 | 8.01 × 10−6 | ALDOA, PPP4C, TBX6, YPEL3, GDPD3, MAPK3, CORO1A, BOLA2 |
| 3 | 68.75–68.85 | 1 | 68,803,893 | 4.70 × 10−6 | SLC4A5, MTHFD2, MOB1A |
| 4 | 125.48–125.58 | 3 | 125,528,343 | 7.93 × 10−6 | HFM1 |
| 5 | 17.56–17.74 | 2 | 17,613,902 | 5.76 × 10−6 | KRT7, KRT85, KRT84, KRT82, KRT81, KRT87, KRT83, KRT86, KRT88, KRT90 |
| 10 | 10.17–10.27 | 1 | 10,224,678 | 4.26 × 10−6 | MTARC2, MTARC1, HLX |
| 10 | 11.76–11.86 | 1 | 11,806,923 | 1.65 × 10−6 | WDR64, CHML |
| 10 | 53.92–54.02 | 1 | 53,973,228 | 3.82 × 10−6 | NEBL |
| 10 | 60.36–60.46 | 2 | 60,410,715 | 6.06 × 10−6 | USP6NL |
- Note: In bold, functional candidate genes for mtDNA_CN.
4 DISCUSSION
We used whole-genome sequence data to determine mtDNA_CN as a proxy of mitochondrial activity in skeletal muscle of Duroc pigs. Our findings suggested that this cellular phenotype exhibited an association with carcase lean percentage. Pigs with lower mtDNA_CN displayed reduced fat content and increased loin mass. Additionally, we analysed the genetic variation within the mtDNA and conducted a GWAS to pinpoint specific genomic regions harbouring promising functional genes that could regulate mitochondrial activity.
4.1 Association between mtDNA_CN and production traits
Our study represents the first attempt to explore the relationship between mtDNA_CN and production traits in pigs. Two previous studies delved into this association in chickens and cattle. In chickens, Reverter et al. (2016) used quantitative polymerase chain reaction (qPCR) to quantify various mtDNA segments, normalizing their abundance against a nuclear DNA segment. Their research spanned different tissues and showed a positive correlation of mtDNA_CN among various muscles, suggesting a coordinated regulation of mitochondrial content across skeletal muscles. Moreover, they found that higher muscular mitochondrial content was linked to lower levels of abdominal fat, breast meat yield, and carcase yield. Another study, conducted by Sanglard et al. (2023) in cattle, utilized low-pass sequencing data from blood samples. Their investigation revealed a negative correlation between mtDNA_CN and birth and weaning weights, but not perceptible in mature animals. Both of these studies appear to indicate that reduced mitochondrial activity contributes to an increase in body weight and fat deposition, although, for cattle, this effect was only observable before weaning. Moreover, the study of Kaaman et al. (2007) concluded that humans with less mtDNA_CN tend to have a higher body mass index (i.e., greater body fat content). These findings align with existing fat metabolism models, which have noted a higher susceptibility to obesity in individuals with lower rates of oxidation (Tanner et al., 2002) or low mitochondrial activity (Holmström et al., 2012). In our study, we found that a reduction of mtDNA_CN produced an increase in muscle yield, which is consistent with observations on breast meat yield in poultry. Moreover, it has been described that myostatin-deficient mice, which display skeletal muscle mass hypertrophy, show mitochondrial depletion (Amthor et al., 2007; Ploquin et al., 2012), and that genes with electron transport function are downregulated in callipyge lambs (Fleming-Waddell et al., 2009). Pig skeletal muscle is predominantly composed by type IIb muscle fibres (Park et al., 2024). Among the different fibre types, type IIb displays the lowest mitochondrial content (Gouspillou et al., 2014). Thus, an increase of muscle mass would ultimately increase the proportion of type IIb fibres, which could explain the reduction of mitochondrial content in a similar way as observed in mice (Amthor et al., 2007), double-muscled cattle (Fiems, 2012), or horses (Rooney et al., 2017). However, contrary to what has been described, we observed a trend towards increased backfat thickness in the pigs with high mtDNA_CN.
In pigs, high feed efficiency lines tend to show less backfat thickness and more loin thickness (Faure et al., 2013; Hoque et al., 2008). Consequently, our research outcomes support the hypothesis that a reduction of mtDNA_CN or activity is associated with an increase of feed efficiency (R. S. G. Kong et al., 2016), which would produce leaner animals. Although we do not have data on feed intake or feed efficiency, we have demonstrated that a missense variant in the LEPR gene affects feed efficiency in our population through interfering with the satiety of the pig (Solé et al., 2021). However, we found no relationship between the genotypes for this variant and mtDNA_CN and adding the LEPR genotype into the model did not alter the estimated differences between mtDNA_CN groups (Table S2), indicating independent modes of action. We used data from pigs born during almost two decades from a line that was selected mainly for backfat thickness and intramuscular fat content (Estany et al., 2017). The batch effect captured differences between time points, including genetic differences due to selection for these traits, mainly backfat thickness. Thus, the batch effect may capture part of the variance of backfat thickness and result in an underestimation of its correlation with mtDNA_CN. The mtDNA_CN least-square mean of the batches fitted well against the batch leastsquare means of backfat thickness (R2 = 0.24) and intramuscular fat content (R2 = 0.49), which would be compatible with a correlated response of mtDNA_CN to selection for fat-related traits.
We found a positive association between mtDNA_CN and meat ultimate pH. It is well-established that mitochondria play a role in muscle-to-meat conversion (Dang et al., 2020, 2022; Hudson, 2012; Popp et al., 2015). Mitochondrial content has been previously associated with dark-cutting beef. Ramanathan et al. (2020) observed a greater mitochondrial content and a downregulation of metabolites involved in glycolytic pathways in dark-cutting beef, which can explain the lower lactic acid formation during anaerobic metabolism; thus, resulting in a higher pH. An in vitro study using mitochondria isolated from porcine longissius lumborum, detected that mitochondria have a positive impact on ATP maintenance, whereas mitochondrial inhibitors promote ATP loss, glycogen degradation and lactate accumulation, ultimately reducing the pH (Scheffler et al., 2015). The positive correlation that we observed aligns with these studies, suggesting that a higher mtDNA_CN is associated with an elevated pH. However, we did not observe any substantial difference for meat ultimate pH between the extreme mtDNA_CN groups.
The cellular phenotype mtDNA_CN is sensible to the sampled tissue from which DNA was extracted. As expected, we detected great differences for mtDNA_CN between skeletal muscle and blood samples (Picard, 2021; St John, 2019; Wachsmuth et al., 2016). However, a similar correlation structure of mtDNA_CN in both tissues with the main production traits suggests that non-target tissues might be similarly used, for example blood (Sanglard et al., 2023) or non-target skeletal muscles (Reverter et al., 2016). In our study, blood samples were collected at a much earlier age than skeletal muscle samples, which can impact those correlations, which in our case was most noticeable in carcase weight. Thus, the use of non-target tissues for mtDNA_CN estimation should be properly validated before it can be used as a predictor for other performance traits.
4.2 mtDNA_CN genetic background
We estimated a heritability for mtDNA_CN of 0.60 ± 0.46 using a sire model. Despite the large standard error, this estimate is larger than that of 0.36 reported in F6 crossbred pigs (Yang et al., 2024) but it falls within the range reported in the literature for humans (López et al., 2012; Xing et al., 2008) and confirms a genetic component for the variance of mtDNA_CN.
Mitochondrial genome polymorphisms had been previously related to production traits (Fernández et al., 2008; Liu et al., 2023; St John & Tsai, 2018). However, we did not observe any discernible impact of mitochondrial haploblocks on mtDNA_CN or on production traits. It is possible that lower levels of variation in the mtDNA genome within our purebred population, compared with commercial F1 or F2 hybrids, might account for the absence of a notable association with production traits.
Nuclear DNA also contributes to variation in mitochondrial activity and mtDNA_CN. The GWAS identified 27 nuclear DNA variants associated with mtDNA_CN. Of those, 16 were located at genomic regions with annotated genes. A literature search of the variants located at those regions revealed several functional candidate genes. We detected two genomic regions in chromosome SSC3. The first one, located at around 18 Mb, contains two genes that had been previously reported to be associated with mitochondria regulation. The first one is the gene coding for aldolase A (ALDOA), described as a regulator of mitochondrial respiration in cancerous pancreatic cells (Ji et al., 2016). Moreover, it has been recently demonstrated that ALDOA restricts PRKN-dependent mitophagy (Bai et al., 2022). Mitogen-activated protein kinase 3 (MAPK3), also known as extracellular signal-regulated kinase 1 (ERK1), is also associated with mitochondrial function; specifically with mitochondrial fission leading to a decrease in mitochondrial mass during cell reprograming (Prieto et al., 2016). Other studies have reported that activation of the ERK1/2 signalling pathway produces an inhibition of glycolysis and oxidative phosphorylation, which leads to mitochondrial dysfunction (Nowak, 2002; Zhou et al., 2023; Zhu et al., 2012). Another region located at 69 Mb of SSC3 has been associated with mtDNA_CN. Among the genes located at that region, methenyltetrahydrofolate cyclohydrolase (MTHFD2) stands out. In mouse, a knock-down of MTHFD2 produces a glucose metabolism shift, from oxidative phosphorylation to glycolysis. This change in the metabolism has been linked to the interaction between this gene and complex III member of the mitochondrial electron transport chain. A depletion of MTHFD2 reduces considerably the stability of the complex, leading to a mitochondrial dysfunction (Yue et al., 2020; Zhao et al., 2022).
On chromosome SSC4, at around 125 Mb, a signal has been detected. The helicase for meiosis 1 (HFM1) gene maps to this region. Although this gene might seem unrelated with mitochondrial function, it has been observed in Saccharomyces cerevisiae yeast that the overexpression of HFM1 is capable to restore respiratory capacity, cytochrome spectrum and oxygen consumption (Tigano et al., 2015).
On chromosome SSC5, at 17 Mb, we located a cluster of keratin genes. Keratin, which forms intermediate filaments found in all epithelial cells, plays a critical role for stress protection and cellular integrity. Cells from individuals affected by pachyonychia congenita, a disease caused by mutations in type II keratins 6a, 6b and 6c, and type I keratins 16 and 17, showed a reduction in mitochondrial–endoplasmic reticulum contact sites. The loss of these contact sites has been linked to a reduction in mitophagy, and, consequently, an accumulation of old and dysfunctional mitochondria (Lehmann et al., 2020). Additionally, dysmorphic mitochondria were detected in Krt5 and Krt16 knock-out mice (Alvarado & Coulombe, 2014; Takahashi et al., 1994), reinforcing its involvement in mitochondrial function.
On chromosome SSC10, at 10 Mb, there is located the gene HLX, which encodes a transcription factor that in mice is co-activated by Prdm16 to control brown adipose tissue gene expression and mitochondrial biogenesis (Huang et al., 2017), and which also regulates mitochondrial metabolic genes (Piragyte et al., 2018). Furthermore, in this chromosome we identified another region at 54 Mb where the nebulin gene (NEBL) is located. This gene is an important contributor on the maintenance of myofibrillar integrity during muscle contraction. Also, knock-out mice for this gene displayed abnormal accumulation of mitochondria within myofibres (Bang et al., 2006).
None of the regions that we identified overlapped with genomic regions associated with mtDNA_CN in other studies with F6 crossbred pigs (Yang et al., 2024).
5 CONCLUSIONS
Pigs with less mtDNA_CN produce leaner carcases than pigs with high mtDNA_CN. This result supports the hypothesis that a reduction of mitochondrial activity is associated with greater feed efficiency. Pork from pigs with less mtDNA_CN may also tend to have lower ultimate pH as mitochondria play a role in muscle-to-meat conversion. We found no association of the most frequent mitochondrial haploblocks with mtDNA_CN or the production traits, suggesting that mtDNA_CN may be primarily controlled by nuclear DNA. Several genomic regions that harbour potential candidate genes with functions related to mitochondrial biogenesis and homeostasis were associated to mtDNA_CN. These regions provide new insights into the genetic background of this cellular phenotype but it is still uncertain if such associations translate into noticeable effects on the production traits.
AUTHOR CONTRIBUTIONS
EM: Conceptualization, Methodology, Formal analysis, Investigation, Writing—Original Draft. RNP: Resources, Writing—Review and Editing. JE: Resources, Writing—Review and Editing, Funding Acquisition. RRF: Conceptualization, Methodology, Resources, Writing—Original Draft, Supervision, Funding Acquisition.
ACKNOWLEDGEMENTS
We acknowledge the personnel at Selección Batallé for their cooperation for the recording of on-farm data and sample collection. We gratefully acknowledge Pilar Sopeña from the Animal Breeding group, University of Lleida, for laboratory assistance.
FUNDING INFORMATION
This research was supported by the Spanish Ministry of Science, Innovation & Universities and the EU Regional Development Funds (grant PID2021-125689OB-I00). EM is recipient of a UdL-Santander Predoc scholarship.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




