Sequence variants associated with resilient responses in growing pigs
Abstract
The current work aimed to identify genomic regions and candidate genes associated with resilience in pigs. In previous work, we proposed the body weight deviation from the expected growth curve (ΔBW) and the increase of the positive acute-phase protein haptoglobin (ΔHP) after a vaccine challenge as resilience indicators which may be improved through selective breeding in pigs. Individuals with steady growth rate and minor activation of haptoglobin (high ΔBW and low ΔHP values) were considered resilient. In contrast, pigs with perturbed growth rate and high activation of haptoglobin (low ΔBW and high ΔHP values) were considered susceptible. Both ∆BW and ∆HP were simultaneously considered to select the most resilient (N = 40) and susceptible (N = 40) pigs. A genome-wide association study was carried out for the pigs' response classification to the challenge test using whole-genome sequence data (7,760,720 variants). Eleven associated genomic regions were identified, harbouring relevant candidate genes related to the immune response (such as pro- and anti-inflammatory responses) and growth pathways. These associated genomic regions harboured 41 potential functional mutations (frameshift, splice donor, splice acceptor, start loss and stop loss/gain) in candidate genes. Overall, this study advances our knowledge about the genetic determinism of resilience, highlighting its polygenic nature and strong relationship with immunity and growth.
1 INTRODUCTION
Selective breeding for improved animal resilience could enhance the animals' ability to cope with external perturbations such as environmental and social stresses or infectious challenges (Colditz & Hine, 2016). In the context of animal production systems, selective breeding for resilience would lead to positive consequences in improving animal welfare and well-being and production profitability (Mulder & Rashidi, 2017). However, selective breeding for improved resilience is currently limited by the absence of (i) a consensus on the definition of resilience and how to measure it and (ii) relevant information on the genetic determinism and background of resilience. Recently, the pig industry has expressed an interest in including resilience in selection indexes, being now a priority rather than a secondary trait (Knap, 2020). However, so far, only a few specific immune responses, such as antibody and cell-mediated immune responses, have been explored in experimental conditions of selection in pigs (Mallard et al., 1998; Wilkie & Mallard, 1999). Breeding for resilience is not straightforward, as resilience and productivity might be negatively correlated (Prunier et al., 2010) and selection for a specific immune response may have important drawbacks on general resilience (Nakov et al., 2019). Overall, resilient animals are expected to be those that are less affected by external stressors and produce more robust phenotypes, regardless of their ranking position in terms of performance on unchallenged conditions.
In the lack of consensus, multiple resilience indicators have been proposed in livestock species, mostly as deviations in production and/or immune-related traits in chickens (Berghof et al., 2019), rabbits (Argente et al., 2019), pigs (Chen et al., 2020; Putz et al., 2019) and cattle (Poppe et al., 2021, 2022). Genome-wide association studies (GWASs) were carried out for some of these resilience indicators to unravel the genetic background of resilience in rabbits (Casto-Rebollo et al., 2020) and pigs (Chen et al., 2020; Cheng et al., 2022). We recently suggested both the body weight deviation from the expected growth curve (ΔBW) and the increase of the positive acute-phase protein haptoglobin (ΔHP) after an attenuated vaccine challenge as resilience indicators in commercial growing pigs (Laghouaouta et al., 2021). Pigs that maintained their growth rate and showed a minor activation of haptoglobin (high ΔBW and low ΔHP values) were deemed resilient. In contrast, pigs showing restricted growth and a high activation of haptoglobin after the challenge (low ΔBW and high ΔHP values) were deemed susceptible. Both resilience indicators (ΔBW and ΔHP) showed substantial variability within the population and low-to-moderate heritability estimates (0.33 and 0.16, respectively), suggesting sufficient genetic variance to respond to selective breeding.
GWASs based on whole-genome sequencing (WGS) assess a high density of genetic variants, considering variants specific to each population and including deletions and insertions (indels). In recent years, reduction in WGS costs along with the availability of efficient bioinformatics tools has made such studies feasible in livestock species (Zhao & Grant, 2011). The aim of the current study was to investigate the genetic background of resilience by detecting variants and candidate genes for the pigs' response to the challenge, using the resilience indicators described in our previous experiment (Laghouaouta et al., 2021).
2 MATERIALS AND METHODS
2.1 Animals and phenotypes
A detailed description of the experimental design has been previously reported (Laghouaouta et al., 2021). Briefly, 540 Duroc pigs at 10 weeks of age (71.4 ± 2.4 days) were randomly placed into five fattening batches (108 ± 2.64 pigs per batch) and reared under the same conditions with ad libitum access to commercial diets. After 2 weeks (85.6 ± 2.4 days of age), 445 pigs were challenged with 2 mL (≥105.5 DICC50) of an attenuated Aujeszky vaccine (Auskipra, Laboratorios Hipra, Amer, Girona) administered intramuscularly and 95 control pigs were inoculated with phosphate-buffered saline. Each fattening batch contained approximately 89 challenged and 19 control pigs. Two resilience indicators, the body weight deviation from the expected growth curve (ΔBW) and the haptoglobin increment after the challenge (∆HP), were estimated for each challenged pig as described in Laghouaouta et al. (2021, 2022), using the data of the control pigs to establish growth and haptoglobin baselines in unchallenged conditions. Pigs that maintained their growth rate (high ΔBW) and had a minor activation of haptoglobin (low ΔHP) were considered resilient, whereas pigs with a reduced growth rate (low ∆BW) and a high activation of haptoglobin (high ∆HP) were considered susceptible.
2.1.1 Selection of pigs with extreme resilience indicators
Both ∆BW and ∆HP were simultaneously considered to select 80 pigs with extreme responses towards the challenge (40 resilient and 40 susceptible). To balance individuals across batches, the eight most resilient pigs and the eight most susceptible pigs from each batch were selected. Descriptive statistics for ∆BW and ∆HP of the selected extreme pigs are displayed in Table 1. On average, resilient pigs had positive ∆BW (+2.79 kg) and negative ∆HP (−0.60 mg/mL) values. In contrast, susceptible pigs had negative ∆BW (−5.15 kg) and positive ∆HP (+0.89 mg/mL) values. Resilience indicators had high standard deviations, which reflects their substantial variability. No significant correlations were observed between ∆BW and ∆HP within the resilient and susceptible groups.
| Resilience indicator | Group | Mean | Standard deviation |
|---|---|---|---|
| ∆BW (kg) | Resilient | +2.79 | 1.68 |
| Susceptible | −5.15 | 2.78 | |
| ∆HP (mg/mL) | Resilient | −0.60 | 0.22 |
| Susceptible | +0.89 | 0.44 |
- Abbreviations: ∆BW, body weight deviation from the expected growth curve at 28 days post-vaccination; ∆HP, increment of haptoglobin at 4 days post-vaccination.
2.2 Whole-genome sequencing
Blood samples (2–5 mL) were collected from the jugular vein into blood collection tubes coated with K2-EDTA (BD Vacutainer, Franklin Lakes, NJ, USA). Genomic DNA was isolated from blood samples collected from the most resilient and susceptible pigs following a standard phenol/chloroform procedure (Green & Sambrook, 2017). DNA samples were sequenced with paired-end reads using the NovaSeq 6000 platform (Illumina, San Diego, CA, USA) at an average depth of 8.5× (SD 1.9×) at Centre Nacional d'Anàlisi Genòmica (CNAG-CRG). Briefly, the short-insert paired-end libraries were prepared with PCR-free protocol using KAPA HyperPrep kit (Roche, Basel, Switzerland) with some modifications. In short, in function of the material availability, 0.4 to 1.0 μg of genomic DNA was sheared on a Covaris™ LE220-Plus (Covaris, Brighton, UK) to reach the fragment size of ~400 bp. The fragmented DNA was size-selected for the fragment size of 220–550 bp with AMPure XP beads (Agencourt, Beckman Coulter, Nyon, Switzerland), end-repaired and adenylated, and Illumina platform compatible adaptors with unique dual indexes and unique molecular identifiers (Integrated DNA Technologies, Leuven, Belgium) were ligated. The libraries were quality-controlled on an Agilent 2100 Bioanalyzer with the DNA 7500 assay (Agilent, Madrid, Spain) for size and quantified by Kapa Library Quantification Kit for Illumina platforms (Roche). The libraries were sequenced on NovaSeq6000 (Illumina) in paired-end mode with a read length of 2 × 151 + 17 + 8 bp following the manufacturer's protocol for dual indexing. Image analysis, base calling and quality scoring of the run were processed using the manufacturer's software Real Time Analysis (RTA 3.4.4) and followed by generation of FASTQ sequence files. Adapter sequences were removed using Trimmomatic (Bolger et al., 2014). Reads were aligned to the Sscrofa11.1 reference genome (GenBank accession: GCA_000003025.6) using the BWA-MEM algorithm (Li, 2013). Duplicates were tagged using Picard Tools (Broad Institute). Single nucleotide polymorphisms and short insertions and deletions (indels) were identified with the variant caller GATK HaplotypeCaller (GATK 3.8.0) (Depristo et al., 2011; Poplin et al., 2018) using default settings. Variant discovery with GATK HaplotypeCaller was performed separately for each individual and then jointly genotyped by extracting the variant positions from all the individuals. We retained all biallelic variants for further analyses with VCFtools v.0.1.17 (Danecek et al., 2011). Further, variants with a minor allele frequency below 0.2 and a genotyping rate below 0.9 were filtered out using PLINK v1.9 (Chang et al., 2015). Due to the limited sample size, we used a high minor allele frequency (0.2) to have a representation of all genotypes within the studied population. A total of 7,760,720 variants were analysed in this study.
2.3 Genome-wide association study
2.4 Permutation testing
As a high-throughput genotyping method was used in this study, permutation testing was performed to control for multiple testing (Rice et al., 2008). As suggested by Casto-Rebollo et al. (2020), in each of 100,000 permutations, phenotypic data were randomly shuffled across individuals to remove pedigree structure and any possible relationship between phenotypes and genotypes. In each permutation, the shuffled data were analysed correcting for the batch effect and the first four principal components of the genotypes. The empirical p-value (EMP) was estimated for each variant as K/N, where K is the number of times the variant was associated in the randomly generated datasets and N is the total number of permutations (100,000). As the minimum possible EMP is 10−5, variants with an EMP value above 10−4 were considered false positives. Permutation testing was performed using the PLINK v1.9 software.
2.5 Variant functional annotation, associated genomic regions and candidate genes
Annotation and functional prediction of the associated variants that passed permutation testing was performed using the variant effect predictor (VEP) (McLaren et al., 2016) from the Ensembl database (Howe et al., 2021). Associated genomic regions were determined by grouping significant variants within 500 kb and extending each genomic region by 500 kb on each side to consider nearby genes. BioMart (Smedley et al., 2015) was used to retrieve candidate genes within the associated genomic regions from the Ensembl database (Howe et al., 2021) using Sscrofa11.1 as the reference genome. Gene ontologies and functions were investigated using the Database for Annotation, Visualization, and Integrated Discovery (Jiao et al., 2012) and a literature search. Further, variants within the associated genomic regions and relevant candidate genes were retrieved and their functional consequences were predicted using VEP (McLaren et al., 2016).
3 RESULTS
3.1 Genome-wide association study
GWAS for the pigs' response to the challenge detected 69 variants at pig chromosomes (SSC) 1, SSC5, SSC8, SSC9, SSC10, SSC11, SSC13 and SSC15 (Figure 1). Fifty of them (13 indels and 37 SNPs) passed permutation testing and were combined into 11 genomic regions in five chromosomes (Table 2 and Table S1). Chromosome SSC8 accumulated five non-overlapping regions. Regions SSC1 (202.2–205.6 Mb) and SSC8 (128.7–130.0 Mb) held the largest number of associated variants (14 and 11, respectively). A total of 100 protein-coding and 94 non-coding genes were retrieved from the 11 associated genomic regions (Table S2). Most non-coding genes (85 out of 95) were long non-coding RNA genes, which are still poorly annotated regarding their functionality in the pig genome. About 31% of the protein-coding candidate genes were unannotated. Human or mouse orthologues were identified for 16 (52%) unannotated protein-coding genes. Promising candidate genes for resilience related to the immune response and growth pathways were identified within the associated genomic regions (Table 2), such as C-C motif chemokine ligand 20 (CCL20), nuclear factor kappa B subunit 1 (NFKB1), DEAH-box helicase 15 (DHX15), B-cell scaffold protein with ankyrin repeats 1 (BANK1) and coiled-coil serine rich protein 1 (CCSER1), which are explained in more detail in Section 4.
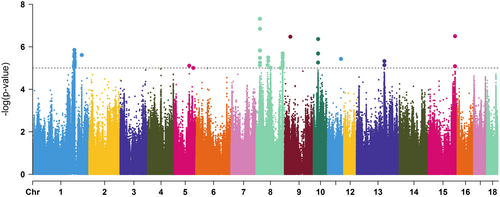
| SSCa | Region (Mb)b | #Variantc | Top variant | p-value | #Genesd | Candidate genese |
|---|---|---|---|---|---|---|
| 1 | 199.6–200.6 | 1 | 1:200118233 | 8.23E-06 | 2 | – |
| 202.2–205.6 | 14 | 1:203237353 | 1.40E-06 | 18 | ACER2, PLIN2, RRAGA, SH3GL, SLC24A2 | |
| 238.6–239.6 | 1 | 1:239066015 | 2.45E-06 | 12 | SHB | |
| 8 | 17.9–19.2 | 7 | 8:18681343 | 1.42E-07 | 8 | DHX15, SOD3, PPARGC1A, SEPSECS, PI4K2B |
| 55.1–56.1 | 1 | 8:55591974 | 7.21E-06 | 15 | SRP72, REST, HOPX | |
| 58.4–59.6 | 2 | 8:59089727 | 3.18E-06 | 3 | – | |
| 118.3–119.3 | 4 | 8:118805694 | 9.92E-06 | 4 | NFKB1, SLC39A8, BANK1 | |
| 128.7–130.0 | 11 | 8:129375919 | 2.05E-06 | 3 | SNCA, MMRN1, CCSER1 | |
| 9 | 27.1–28.1 | 1 | 9:27639801 | 3.37E-07 | 9 | ENDOD1, SESN3, MAML2 |
| 13 | 135.1–136.1 | 3 | 13:135634196 | 4.75E-06 | 9 | MUC13, ITGB5 |
| 15 | 128.3–129.5 | 5 | 15:129005040 | 3.19E-07 | 17 | IRS1, CCL20, RHBDD1 |
- a Pig chromosome.
- b Associated genomic region.
- c Number of associated variants within each associated genomic region.
- d Number of protein-coding genes within each associated genomic region.
- e Most relevant candidate genes related to the immune response or growth pathways.
3.2 Polymorphisms within the associated genomic regions
In order to further investigate the genetic basis of these traits, we screened for variants in the selected regions that could explain the detected effects. In total, 115,340 variants were called within the 11 genomic regions associated with the pigs' response to the challenge (Table 3 and Table S3). As expected, most of the detected variants are located at intergenic (42.4%) and intronic (42.3%) regions. Within the associated genomic regions, a total of 41 potentially damaging variants (frameshift, splice donor, splice acceptor, start lost, stop lost and stop gained) were detected within 20 positional candidate genes, which might deserve further study. Specifically, 11 variants with expected high impact and 67 with moderate impact over the encoded protein functionality were identified in candidate genes with relevant function within immune or fat accretion pathways (Table 4). The number of synonymous variants in these genes was moderate (70 in total), while there were close to 600 variants in 5′ and 3′ sequences with potential cis-regulatory activity over the expression of the flanking genes.
| Consequence | Impact | SSC1a | SSC8 | SCC9 | SCC13 | SCC15 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 200–201 | 202–206 | 239–240 | 18–19 | 55–56 | 58–60 | 118–119 | 129–130 | 27–28 | 135–136 | 128–129 | |||
| Frameshift | High | 0 | 1 | 0 | 0 | 2 | 0 | 6 | 0 | 1 | 4 | 13 | 27 |
| Splice donor | High | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 3 |
| Splice acceptor | High | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Start lost | High | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Stop lost | High | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Stop gained | High | 0 | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 6 |
| Inframe deletion | Moderate | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 6 | 10 |
| Inframe insertion | Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 5 |
| Missense | Moderate | 2 | 16 | 7 | 7 | 24 | 13 | 20 | 27 | 28 | 36 | 107 | 287 |
| 5′ UTR | Modifier | 2 | 63 | 9 | 22 | 15 | 17 | 3 | 9 | 1 | 2 | 23 | 166 |
| Upstream | Modifier | 15 | 535 | 48 | 385 | 351 | 75 | 206 | 264 | 155 | 182 | 2132 | 4348 |
| 3′ UTR | Modifier | 9 | 177 | 13 | 97 | 71 | 13 | 20 | 11 | 69 | 45 | 228 | 753 |
| Downstream | Modifier | 33 | 645 | 37 | 274 | 322 | 68 | 251 | 191 | 215 | 322 | 1307 | 3665 |
| Intronic | Modifier | 4 | 14,392 | 1835 | 2359 | 1492 | 1 | 5585 | 5037 | 1285 | 3969 | 12,794 | 48,753 |
| Intergenic | Modifier | 2685 | 4747 | 1090 | 7007 | 1041 | 4714 | 3665 | 15,025 | 2185 | 603 | 6202 | 48,964 |
| Splice region | Low | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 4 |
| Synonymous | Low | 0 | 28 | 14 | 8 | 15 | 12 | 11 | 29 | 12 | 38 | 154 | 321 |
| Non-coding | Low | 553 | 611 | 116 | 2663 | 609 | 141 | 674 | 1825 | 360 | 46 | 425 | 8023 |
- Note: Predicted consequences and impact as predicted by the variant effect predictor from the Ensembl database.
- a SSC: pig chromosome. The position of each associated genomic region is indicated in Mb below the corresponding chromosome.
| Consequence | Impact | PLIN2 | RRAGA | SHB | DHX15 | SOD3 | PPARGC1A | NFKB1 | SLC3918 | BANK1 | SNCA | MMRN1 | CCSER1 | ENDOD1 | SESN3 | MAML2 | MUC13 | ITGB5 | CCL20 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frameshift | High | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 7 |
| Splice donor | High | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Stop gained | High | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 3 |
| Inframe deletion | Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| Missense | Moderate | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 8 | 1 | 22 | 8 | 4 | 0 | 0 | 17 | 0 | 1 | 65 |
| Synonymous | Low | 7 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 4 | 0 | 27 | 5 | 9 | 0 | 2 | 7 | 3 | 0 | 70 |
| 5′ UTR | Modifier | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 6 | 2 | 1 | 2 | 0 | 1 | 0 | 1 | 1 | 21 |
| Upstream | Modifier | 0 | 0 | 0 | 0 | 0 | 27 | 0 | 0 | 72 | 0 | 0 | 0 | 0 | 22 | 0 | 0 | 0 | 0 | 121 |
| 3′ UTR | Modifier | 21 | 0 | 0 | 0 | 1 | 8 | 4 | 0 | 7 | 0 | 11 | 66 | 26 | 34 | 1 | 0 | 1 | 0 | 180 |
| Downstream | Modifier | 83 | 0 | 0 | 5 | 0 | 59 | 0 | 0 | 2 | 2 | 0 | 111 | 0 | 7 | 0 | 0 | 0 | 0 | 269 |
| Intronic | Modifier | 175 | 0 | 376 | 77 | 0 | 565 | 175 | 1100 | 3593 | 355 | 1211 | 20,701 | 244 | 374 | 871 | 651 | 846 | 51 | 31,365 |
| Total | – | 290 | 2 | 376 | 82 | 2 | 783 | 183 | 1103 | 3693 | 364 | 1274 | 20,894 | 285 | 438 | 876 | 677 | 851 | 53 | 32,226 |
4 DISCUSSION
Selection for production potential can compromise the so-called functional traits (survivability, longevity, immunocompetence). Therefore, selective breeding for resilience aims at selecting animals for steady production in changing environments or situations. In a previous work, we tested pigs by exposing them to a vaccine, which represents a mild immunological challenge. We suggested ΔBW and ΔHP as resilience indicators (Laghouaouta et al., 2021) as they represent both growth and immune response, which are known for their evolutionary and selection trade-offs. For instance, trade-offs between growth and immunity have been demonstrated in intensive poultry systems (Zerjal et al., 2021), sheep (Douhard et al., 2022) and pigs (Clapperton et al., 2006). During major infectious challenges, immune functions enter in competition with other energy-demanding processes, including growth. Not surprisingly, one of the first consequences of inflammation is to suppress appetite (and therefore productivity) and divert energy to support immune responses (Broom & Kogut, 2018). An optimal scenario to maintain an effective response to infections with minimal production losses relies on a strong inflammatory reaction (within seconds or minutes) transiting to an anti-inflammatory state within hours or few days (Broom & Kogut, 2018). The interaction between production (growth) and immunity is not only a matter of nutrient allocation: a surplus of fatty acids can mediate inflammatory reactions. Conversely, adipose-resident leucocytes have non-inflammatory roles that regulate energy utilization and glucose/fat balance (Lee & Dixit, 2020), while leptin from skin fat pads is needed to orchestrate monocyte healing after infections (Kratofil et al., 2022).
With this in mind, we turned our attention to the 11 genomic regions that were deemed associated with the pigs' response to the challenge in our experiment. The unbalance between pro- and anti-inflammatory responses has been linked to pathogen burden and disease persistency in parasitic, bacterial and viral infections (Cicchese et al., 2018). Several genes in the associated GWAS regions are related to the pro-inflammatory responses triggered by NF-κB signalling (Figure 2). In the SSC8 (118.3–119.3 Mb) region, NFKB1 encodes for the subunit 1 of the NF-κB factor. This pleiotropic transcription factor is a master regulator of multiple processes related to inflammation, cytokine production, and innate and adaptive immunity (Caamaño & Hunter, 2002; Gilmore et al., 2015). NF-κB modulates immune mediators and acute-phase proteins through binding enhancers or promoters of NF-κB target genes (Liang et al., 2004). At the same genomic region, solute carrier family 39 member 8 (SLC39A8) is a zinc transporter that modulates the inflammatory response through activating and translocating NF-κB to the nucleus (Liu et al., 2013). Following lipopolysaccharide exposure, SLC39A8 increases zinc uptake in human macrophages and regulates the host defence by promoting pro-inflammatory (TNFα, IL6 and IL8) and inhibiting anti-inflammatory (IL10) cytokine production (Pyle et al., 2017). Related to this, DHX15 in SSC8 (17.9–19.2 Mb) region initiates the innate immune response during RNA virus infections by activating NF-κB and MAPK signalling (Lu et al., 2014; Mosallanejad et al., 2014). Also related to innate immunity, Ras-related GTP binding A (RRAGA) in SSC1 (202.2–205.6 Mb) mediates the acute-phase inflammatory response through the regulation of TNFα (Lukashok et al., 2000). Lastly, the genomic region at SSC9 (27.1–28.1 Mb) included the endonuclease domain containing 1 (ENDOD1) gene, which is considered a modulator in the innate immune signalling pathways activated by foreign DNA. As such, ENDOD1 expression increased in fish exposed to bacterins (Lyu et al., 2016) and red deer susceptible to Mycobacterium avium subsp paratuberculosis (Mackintosh et al., 2016). Pro-inflammatory signals are key in reducing susceptibility to pathogens, but sustained signalling can have negative consequences on productivity (Broom & Kogut, 2018). As mentioned above, it is also essential to have coordinated anti-inflammatory signals to achieve pathogen control without inducing excessive inflammation and tissue damage. In this sense, in the SSC8 (17.9–19.2 Mb) region, superoxide dismutase 3 (SOD3) exerts anti-inflammatory actions by suppressing T-cell differentiation, reducing the levels of several cytokines (IL2, IL4, IL5, IL6, IL10, IL13 and TNFα) and inhibiting the NF-κB signalling pathway (Agrahari et al., 2021; Laurila et al., 2009). Lastly, the genomic regions at SSC15 (128.3–129.5 Mb) harbour ENSSSCG00000016254 which is an orthologous of the human CCL20 (also known as macrophage inflammatory protein 3 alpha MIP-3α). CCL20 binds to the CC chemokine receptor 6 (CCR6) (Schutyser et al., 2003). The CCR6-CCL20 complex regulates the homing of leucocytes to specific lymphoid tissues and stands out from most other chemokine axes for its broad leucocyte range and for promoting both pro- and anti-inflammatory actions (Comerford et al., 2010; Lee & Körner, 2019).
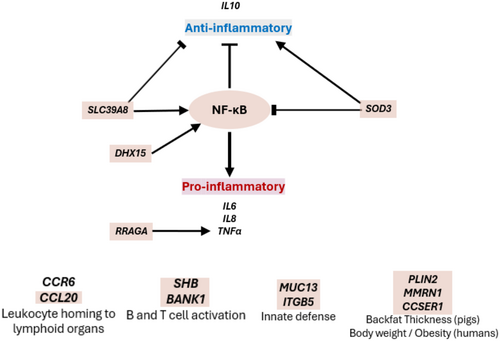
Another group of positional candidate genes had functions related to acquired immunity. One of the most interesting is the SH2 domain-containing adaptor protein B (SHB) in SSC1 (238.6–239.6 Mb). SHB is an adapter protein for the fibroblast growth factor (FGF)/T-cell receptor signalling (Lindholm et al., 2002). In mice, SHB knockout increased response to T-cell receptor stimulation and IL2 and IL4 release (Gustafsson et al., 2011). Also related to acquired immunity, SSC8 (118.3–119.3 Mb) region maps the B cell scaffold protein with ankyrin repeats 1 (BANK1) gene. This gene is expressed in mature B cells and acts downstream of the B cell and TLR7 receptors, triggering pro-inflammatory cytokines and increasing circulating levels of plasma and memory cells (Georg et al., 2019; Hernández et al., 2021). It is also considered a susceptibility gene for several human autoimmune diseases (Hernández et al., 2021). The genomic region at SSC8 (128.7–130.0 Mb) harbours synuclein-alpha (SNCA). SNCA is ubiquitously expressed in B cells, T cells and natural killer cells (Shin et al., 2000). It regulates T- and B-cell function and MHC expression (Harm et al., 2013; Pei & Maitta, 2019). Moreover, SNCA knockout mice showed impaired T-cell differentiation and development (Shameli et al., 2016). Also of interest in relation with immune response are mucin 13 (MUC13) and integrin beta-5 (ITGB5) in the SSC13 (135.1–136.1 Mb) region and mastermind-like protein 2 (MAML2) in the SSC9 (27.1–28.1 Mb). MUC13 encodes a cell surface glycoprotein expressed by ductal and glandular epithelial tissues, which has been shown to regulate the epithelial inflammatory response in humans (Sheng et al., 2012). Both MUC13 and ITGB5 were found essential for the immune response against enterotoxigenic Escherichia coli in pigs (Zhou et al., 2013). MAML2 is an essential activator of the Notch signalling pathway which has been initially associated with cellular differentiation and development (Kitagawa, 2016). Yet, recent studies have reported that Notch signalling also mediates the immune response through regulating B and T cells (Garis & Garrett-Sinha, 2021; Vanderbeck & Maillard, 2021).
Lastly, given the interaction between fatty acid accretion, growth and inflammation, we also selected a group of genes related to fattening and growth (Figure 2). For instance, PPARGC1A is a well-studied transcriptional mediator of mitochondria biogenesis and energy allocation. Expression in muscle is increased with regular exercise, and mutation in this gene can lead to excessive fattening in humans and in pigs. PLIN2 in SSC1 (202.2–205.6 Mb) has been previously associated with lipid droplet accumulation and growth (Gol et al., 2016). In addition to its role in lipid metabolism, PLIN2 has also been implicated in the inflammatory response (Najt et al., 2016; Norman et al., 2018). In SSC8 (128.7–130.0 Mb), two important candidate genes are multimerin 1 (MMRN1) and CCSER1. MMRN1 is associated with body fat mass and changes in body weight in humans (Rendo-Urteaga et al., 2015) and is differentially methylated and expressed in obese and non-obese individuals (Keller et al., 2017). CCSER1 was reported associated with growth-related traits such as backfat thickness in pigs (Xue et al., 2021) and feed efficiency (Abo-Ismail et al., 2018) and body weight (Smith et al., 2019) in cattle. Additionally, SESN3 at SCC9 (27.1–28.1 Mb) belongs to the sestrin family, a group of highly conserved stress-inducible proteins with protective roles in most physiological and pathological conditions. In response to oxidative stress, SESN3 regulates glucose metabolism and adipogenesis contributing to animal growth and fattening (Li et al., 2022).
An analysis of the sequence variability within these associated genomic regions has evidenced the presence of 41 potential functional variants with critical consequences on gene functions (frameshift, splice donor, splice acceptor, start lost, stop lost and stop gained) although they were not significant (Table 3 and Table S3). In addition, 11 mutations with potential effect over the protein function were detected within relevant candidate genes involved in growth and immune response pathways (NFKB1, BANK1, MUC13, MAML2 and SESN3) (Table 4 and Table S4). Further studies are needed to elucidate the effects of these potential mutations over a larger population of animals.
Improving animal's resilience is highly needed, since it would benefit (i) the animal through the improvement of its welfare and well-being, (ii) farmers through the reduction of economic losses and the increase of profitability, and in the long term, (iii) the environment by increasing sustainability and efficiency of production systems. Therefore, understanding the genetic background of resilience is essential to investigate its possible improvement through selective breeding. Our findings about the genetic background of resilience are in line with previous GWAS for resilience which identified candidate genes related to immune pathways (Casto-Rebollo et al., 2020; Chen et al., 2020; Cheng et al., 2022). Moreover, three associated genomic regions at 17.9–19.2, 55.1–56.1 and 58.4–59.6 Mb in SSC8 overlap with a previously reported haptoglobin concentration QTL in pigs (Wimmers et al., 2009). Notably, no significant associations were found with the MHC loci in SSC7. As in other species, the pig MHC region is rich in QTLs related to health, growth and reproduction (Cheng et al., 2022). Previous experiments have highlighted the relevance of MHC variation in treatment numbers, mortality rate and growth rate in nursery pigs exposed to a polymicrobial disease challenge (Cheng et al., 2022) and porcine reproductive and respiratory syndrome virus antibody production (Sanglard et al., 2020; Serão et al., 2014) and PCV2 viraemia (Walker et al., 2018) following experimental challenges. However, we did not detect an association of the MHC loci with the pigs' response to the challenge in our experiment. One possible explanation might be because we are not studying a specific immune response, but the animals' response to the attenuated Aujeszky vaccine plus all the possible perturbations that may have occurred during the experiment. Additionally, we are using a purebred Duroc line (instead of a commercial F1 or F2 hybrid). Therefore, the variation at the MHC site is expected to be lower in our population than for instance in hybrid commercial pigs.
One limitation of the current study is the relatively small sample size. WGS data provide a high genetic marker density, including indels and specific variants in the population (Zhao & Grant, 2011), with the potential to increase the resolution of the genomic background of the resilient response. For accurately estimating the effect of such a large number of variants, large sample sizes are required. The selective genotyping approach (i.e., using animals with extreme phenotypes) increases the detection power of GWAS by taking advantage of the differences in allele frequencies between the two groups (Darvasi, 1997). This approach has been previously applied to increase the detection power of GWAS in cattle (Kurz et al., 2019) and pigs (Fontanesi et al., 2016), and it may be effective for identifying those variants with the largest effects if they segregate at moderate or high allele frequency. However, this is performed at the expense of missing variants that might be present at a low allele frequency, but which might have relevant cumulative effects over the studied trait.
5 CONCLUSIONS
Our work identified 11 QTLs for the pigs' response to a mild immune challenge across the pig genome (SSC1, 8, 9, 13 and 15). These associated QTLs harbour relevant candidate genes involved mainly in the immune response and fatty acid metabolic pathways. To the best of our knowledge, this is the first WGS-based GWAS for the resilient response in pigs. Further analyses are needed to validate our results and investigate variants with potential damaging effects over protein functionality or patterns of gene expression activation.
AUTHOR CONTRIBUTIONS
LJF and RNP designed the experiment. LJF and RNP collected the phenotypes and performed the lab procedures. HL, ML and RRF performed the data analyses. All authors contributed to the discussion of the results and to manuscript drafting and writing.
ACKNOWLEDGEMENTS
The authors would like to acknowledge all the staff and veterinarians participating in the farm experiment and P. Sopeña for technical assistance in the lab.
FUNDING INFORMATION
The research was funded by the Spanish Ministry of Science, Innovation and Universities and the European Regional Development Funds (grant RTI2018-097700-B-I00 and PID2021-124149OB-I00). HL is a recipient of a PhD scholarship from the Department of Research and Universities of the Government of Catalonia.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
Data are available from the author(s) with the permission of Selección Batallé S.A.




