Improving production efficiency in the presence of genotype by environment interactions in pig genomic selection breeding programmes
Summary
We simulated a genomic selection pig breeding schemes containing nucleus and production herds to improve feed efficiency of production pigs that were cross-breed. Elite nucleus herds had access to high-quality feed, and production herds were fed low-quality feed. Feed efficiency in the nucleus herds had a heritability of 0.3 and 0.25 in the production herds. It was assumed the genetic relationships between feed efficiency in the nucleus and production were low (rg = 0.2), medium (rg = 0.5) and high (rg = 0.8). In our alternative breeding schemes, different proportion of production animals were recorded for feed efficiency and genotyped with high-density panel of genetic markers. Genomic breeding value of the selection candidates for feed efficiency was estimated based on three different approaches. In one approach, genomic breeding value was estimated including nucleus animals in the reference population. In the second approach, the reference population was containing a mixture of nucleus and production animals. In the third approach, the reference population was only consisting of production herds. Using a mixture reference population, we generated 40–115% more genetic gain in the production environment as compared to only using nucleus reference population that were fed high-quality feed sources when the production animals were offspring of the nucleus animals. When the production animals were grand offspring of the nucleus animals, 43–104% more genetic gain was generated. Similarly, a higher genetic gain generated in the production environment when mixed reference population was used as compared to only using production animals. This was up to 19 and 14% when the production animals were offspring and grand offspring of nucleus animals, respectively. Therefore, in genomic selection pig breeding programmes, feed efficiency traits could be improved by properly designing the reference population.
Introduction
Current intensive pork production systems are based on high-quality feed sources mostly imported from developing countries (SLF 2013). In the future, feed prices will increase due to effects of climate change, global human population growth and a higher wage demand in developing countries (Godfray et al. 2010). This calls for a change in feed source from high-quality grains to lower quality feeds that are locally accessible. However, using locally accessible feed sources as an alternative to high-quality feed sources would reduce production (Merks 2001). This is because animals raised in intensive production systems would have a high physiological demand (Prunier et al. 2010). Therefore, the performance of improved genotypes would not be the same under different feeding systems leading to a genotype by feed interaction (Bradshaw 1965; Hermesch 2004; Brandt et al. 2010). These interactions will have implications for breeding schemes if animals are selected in the nucleus feeding on a high-quality feed and their progeny are expected to perform under a low-quality feed sources. Hence, the progeny genetic potential will be impaired by genotype by environment interaction effects even though the progeny inherit superior genes. Breeding schemes so far have focused on improving performance of pig genotypes with no or little attention on the feeding regimes in the selection environment and production environment. To mitigate these challenges, breeders should target selection for robust animals that are genetically superior and performs best under lower quality feed resources. Selection for feed efficiency is beneficial for several reasons. Robust animals that perform best under lower quality feed sources can be developed. The costs of feed can reduce; therefore, any improvement in feed efficiency made will have a significant impact on the economic return. It is estimated for most breeding schemes feed cost amounts to 40–60% of all the production costs (Ho et al. 2005). In addition, there are also reported benefits where improvements in feed efficiency can reduce methane emissions in dairy cattle (de Haas et al. 2012).
Genomic selection (Meuwissen et al. 2001) increases genetic gain mainly because it generates higher accuracy of selection than traditional selection methods. A better estimation of genotype by environment estimate was observed with genomic selection as compared to conventional selection methods (Silva et al. 2014). In addition, genomic selection may help to improve feed efficiency traits in the future. This is because breeding programmes would not afford breeding under high-input production systems for reasons mentioned above. Instead, they will be forced to design a breeding programme that uses local feed resources. For this reason, future breeding programmes would consider the feeding system, that is producing feed-efficient animals. Proper designing of the reference population has been shown to increase the accuracy of selection (Nirea et al. 2012). Therefore, feed efficiency traits may be improved if the reference population is properly designed including records from the production environment. The objective of this study was to design a genomic selection breeding scheme for developing robust pigs that are less sensitive to a lower quality feed sources. In this case, the reference population will be designed based on nucleus and production environments which differs in feeding regimes. Finally, the total genetic gain generated in the production environment will be compared when different sources of information are used in the reference population.
Materials and methods
Historical population
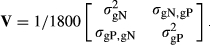
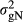 is the genetic variance of nucleus efficiency,
is the genetic variance of nucleus efficiency, 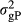 is the genetic variance for production efficiency and
is the genetic variance for production efficiency and 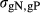 is the genetic covariance between nucleus and production efficiencies. The true breeding value for feed efficiency traits in the nucleus and production was the sum of QTL allele effects for each trait for each individual. The phenotypic records for both traits were simulated as the sum of the true breeding value and environmental normally distributed errors N(0, R), where
is the genetic covariance between nucleus and production efficiencies. The true breeding value for feed efficiency traits in the nucleus and production was the sum of QTL allele effects for each trait for each individual. The phenotypic records for both traits were simulated as the sum of the true breeding value and environmental normally distributed errors N(0, R), where
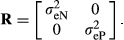
In which, 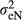 is the residual variance for nucleus efficiency trait and
is the residual variance for nucleus efficiency trait and 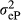 is the residual variance for production efficiency trait. Nucleus efficiency is a term used to describe for feed efficiency trait in the nucleus environment, and production efficiency is a term used to describe for feed efficiency trait in the production environment.
is the residual variance for production efficiency trait. Nucleus efficiency is a term used to describe for feed efficiency trait in the nucleus environment, and production efficiency is a term used to describe for feed efficiency trait in the production environment.
After simulating the historical population, we randomly sampled 200 females and 200 males to create a base generation. These animals were the foundation of all the selection schemes and served as parents for the first two rounds of selection before the breeding schemes had produced their own selection candidates. Each breeding schemes is replicated 100 times. For each replicate, a new historical population was simulated and SNP markers, QTL and QTL effects were resampled.
Breeding schemes: recording and reference population design
A pig breeding scheme was simulated with 25 breeding sires and 50 dams per generation for six distinct generations. The breeding schemes contained two distinct environments, a nucleus and a production. Breeding parents were selected based on genotype and phenotype information of either nucleus animals, production animals or a combination of nucleus and production animals. Animals in the production environment were cross-breeds, descendants of the nucleus. The breeding schemes were simulated under two different scenarios, where production animals are either offspring or grand offspring of nucleus animals (See Figure 1). In a three-way cross, the maternal line was cross-bred and the paternal line was purebred line.
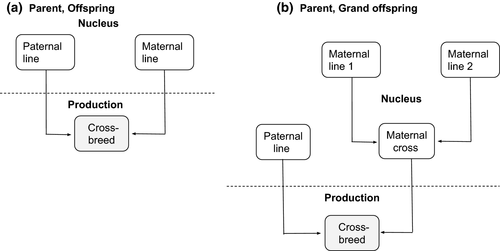
Nucleus efficiency (h2 = 0.3) and production efficiency (h2 = 0.25) were considered as two separate traits that are partly under the influence of different sets of genes related on a lower (rg = 0.2), medium (rg = 0.5) or higher (rg = 0.8) genetic correlation (Falconer 1952).
The breeding goal was to improve production efficiency by designing the reference population using different information sources for genetic evaluation of the selection candidates in the nucleus environment. Therefore, we simulated three different breeding schemes based on the reference population design: (i) nucleus (N), (ii) mixture of nucleus and production (N+P) and (iii) production (P) breeding schemes. In the N breeding scheme, the reference population contained only nucleus animals and all animals in the nucleus were genotyped and recorded for feed efficiency. In this case, the reference population was designed using the selection candidates own and relatives’ records from only the nucleus population. All animals in the production environment were genotyped and had no phenotype records. In the P breeding scheme, the reference population contained only production animals. In this case, the reference population was designed including only relatives information from the production environment. In the N+P breeding schemes, the reference population was a mixture of a fixed number of nucleus and various proportion of production animals. Both nucleus and production animals were genotyped and recorded for feed efficiency. The reference population was a mixture of own and relative's records from the nucleus and production environment. For a general overview of the breeding schemes, see Table 1.
| Schemes | Candidates | Selected | Feed efficiency records | ||
|---|---|---|---|---|---|
| Male | Female | Nucleus | Production | ||
| N | 400 | 25 | 50 | 2400 | 0 |
| 400 | 25 | 50 | 2400 | 600 | |
| 400 | 25 | 50 | 2400 | 1200 | |
| 400 | 25 | 50 | 2400 | 2400 | |
| N+P | 400 | 25 | 50 | 2400 | 3600 |
| 400 | 25 | 50 | 2400 | 4800 | |
| 400 | 25 | 50 | 2400 | 6000 | |
| 400 | 25 | 50 | 2400 | 7200 | |
| P | 400 | 25 | 50 | 0 | 2400 |
- The reference population included nucleus animals in the N breeding scheme; mixture of nucleus and production animals in the N+P breeding scheme; production animals in the P breeding scheme.
Breeding parents were always selected from the nucleus environment by truncation selection on a genomewide estimated breeding values (GEBV). In all breeding schemes, the breeding goal was to improve production efficiency and selection was always based on GEBV of production efficiency. A hierarchal mating structure was assumed in all breeding schemes, and generations were discrete.
Marker effects and breeding value estimation
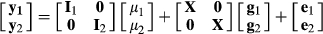
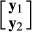 is the vector of response variables for nucleus and production efficiency traits, I1 and I2 are identity matrices relating to the vector of intercepts
is the vector of response variables for nucleus and production efficiency traits, I1 and I2 are identity matrices relating to the vector of intercepts 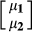 for nucleus and production efficiency.
for nucleus and production efficiency.  is the vector of random errors assumed to be drawn from a normal distribution with a mean 0 and variance R.
is the vector of random errors assumed to be drawn from a normal distribution with a mean 0 and variance R. 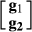 is vector of estimated SNP effects for nucleus and production efficiency.
is vector of estimated SNP effects for nucleus and production efficiency. 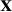 is matrix of standardized number of reference allele (allele ‘1’) for animal i and SNP j as assed by SNP i for nucleus and production efficiency, respectively, and was calculated as:
is matrix of standardized number of reference allele (allele ‘1’) for animal i and SNP j as assed by SNP i for nucleus and production efficiency, respectively, and was calculated as:
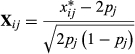
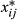 is the number of alleles ‘1’ genotyped for animal i at SNPj. The SNP effects were estimated as:
is the number of alleles ‘1’ genotyped for animal i at SNPj. The SNP effects were estimated as:
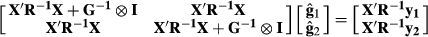
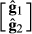 is the vector of estimated SNP effects at the marker loci for nucleus and production efficiency and
is the vector of estimated SNP effects at the marker loci for nucleus and production efficiency and  is inverse matrix of variance and covariance for nucleus and production traits. The variance of each marker effect was assumed to be
is inverse matrix of variance and covariance for nucleus and production traits. The variance of each marker effect was assumed to be 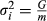 , where m is the number of SNP markers. Finally, the genomic EBV (
, where m is the number of SNP markers. Finally, the genomic EBV ( )of selection candidate i for both traits was estimated as:
)of selection candidate i for both traits was estimated as:
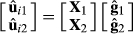
The accuracy of selection was obtained as the correlation between the predicted breeding values of the selection candidates with their true breeding values.
Results
Effect of environment-specific reference population on genetic gain
The genetic gain and accuracy of selection generated in the production environment for alternative reference population design breeding schemes when the production animals were offspring of the selection candidates are presented in Table 2.
| Breeding schemes | rg = 0.2 | rg = 0.5 | rg = 0.8 |
|---|---|---|---|
| Genetic gain | |||
| N | 0.724 | 0.889 | 1.080 |
| P | 1.307 | 1.2877 | 1.271 |
| Accuracy of selection | |||
| N | 0.128 | 0.250 | 0.403 |
| P | 0.385 | 0.399 | 0.381 |
- Animals in the production environment were offspring of nucleus animals, and standard errors were varying from 0.085 to 0.104.
A higher genetic gain was generated in the production environment when the reference population was designed using only production animals to predict the breeding value of selection candidates in the nucleus environment as compared to using only nucleus animals for both offspring (Table 2) and grand offspring (results not shown) scenarios. It should be noted that in the offspring scenario, the production animals were offspring of the selection candidates and in the grand offspring scenario, the production animals were grand offspring of the selection candidates. At generation 6, P breeding schemes, where production animals’ record were used for prediction of breeding value, generated 45% more production efficiency than the N breeding scheme when the genetic correlation (rg) between production and nucleus animals was 0.2. This figure decreased as the rg between the nucleus and production population increased, 31% when rg is 0.5 and 15% when rg is 0.8.
Effects of combined reference population on genetic gain
A higher genetic gain was generated in the production environment for breeding schemes with a combined environment reference population as compared to breeding schemes with a single-environment reference population. The increase in genetic gain was higher when large proportion of production animals were included into the reference population maintaining equal number of nucleus animal records. However, it should be noted that this was also accompanied by an increase of the genotyping and phenotyping costs (more reference animals). At generation 6, production efficiency increased by 46% (rg = 0.2), 34% (rg = 0.5) and 24% (rg = 0.8) in the N+P breeding schemes compared with the N breeding schemes and by 2% (rg = 0.2), 4% (rg = 0.5) and 10% (rg = 0.8) compared with P breeding schemes containing 2400 nucleus and 1200 production animals’ record in the reference population (Table 3). There was a substantial increase in production efficiency when more numbers of animals from the production environment were added to the reference population maintaining equal nucleus reference population size. Specifically, the increase in production efficiency was large when there were large numbers of production animals in the reference population. Similar patterns were observed with regard to selection accuracy (Table 4).
| Breeding schemes | rg = 0.2 | rg = 0.5 | rg = 0.8 |
|---|---|---|---|
| N + P600 | 1.017 | 1.087 | 1.245 |
| N + P1200 | 1.196 | 1.225 | 1.329 |
| N + P2400 | 1.334 | 1.344 | 1.416 |
| N + P3600 | 1.443 | 1.423 | 1.479 |
| N + P4800 | 1.478 | 1.464 | 1.520 |
| N + P6000 | 1.531 | 1.519 | 1.580 |
| N + P7200 | 1.560 | 1.535 | 1.587 |
- Notation of the breeding schemes (N+Px) show the combined reference population where 2000 animals were from the nucleus environment (N) and x animals from the production environment (P). Animals in the production were offspring of nucleus animals, and standard errors were varying from 0.085 to 0.104.
| Breeding schemes | rg = 0.2 | rg = 0.5 | rg = 0.8 |
|---|---|---|---|
| N + P600 | 0.280 | 0.343 | 0.433 |
| N + P1200 | 0.330 | 0.386 | 0.448 |
| N + P2400 | 0.380 | 0.415 | 0.472 |
| N + P3600 | 0.421 | 0.445 | 0.503 |
| N + P4800 | 0.441 | 0.467 | 0.512 |
| N + P6000 | 0.455 | 0.476 | 0.520 |
| N + P7200 | 0.471 | 0.499 | 0.541 |
- Notation of the breeding schemes (N+Px) show the combined reference population where 2000 animals were from the nucleus environment (N) and x animals from the production environment (P). Animals in the production environment were offspring of nucleus animals, and standard errors were 0.01 for all scenarios.
Effect of genetic distance between production and nucleus animals on genetic gain
Figures 2 and 3 show production efficiency and selection accuracy generated for breeding schemes when the production animals were offspring or grand offspring of the selection candidates. Results are only presented for rg = 0.2 because there was similar patterns when rg was medium (rg = 0.5) or higher (rg = 0.8).
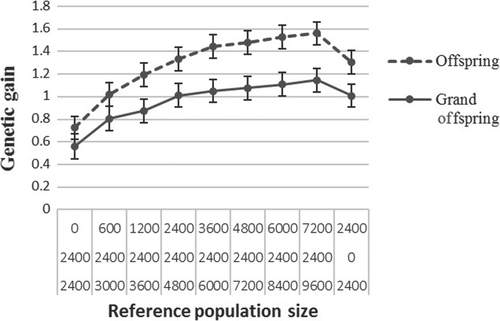
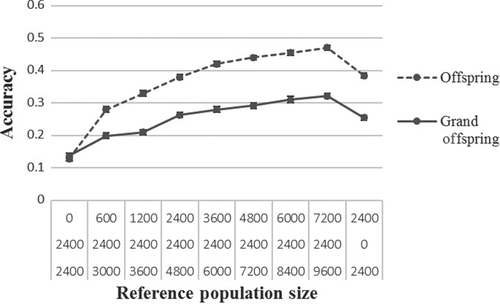
In the production environment, genetic gain and accuracy of selection increased when the production and nucleus animals were on average more related to each other than they were less related. At generation 6, genetic gain (Figure 2) and selection accuracy (Figure 3) were higher when the production animals were offspring of the selection candidates as compared to when they were grand offspring.
Discussion
Genomic selection (GS) (Meuwissen et al. 2001) may increase rates of genetic gain in pig nucleus breeding schemes (Lillehammer et al. 2011). However, in the presence of environmental differences between the nucleus and production environment, estimating genomic selection breeding value (GEBV) using nucleus records might be a poor predictor of production performance when the aim was to maximize feed efficiency in the production environment. This raises the possibility to address the environmental sensitivities due to feeding differences between the nucleus and production environments when designing a reference population. Hence, we evaluated the selection response achieved in the production environment for alternative reference population in GS pig breeding schemes. Our results showed in general that for subpopulations under the same breeding scheme and substantial feeding differences, design of the reference population determines the selection response achieved in a target subpopulation. This implies predicting GEBV based on a quality environment reference population might not lead to a substantial selection response in an inferior environment.
In this study, feeding differences between the nucleus and production environments was the main focus for designing the reference population. However, even if we use the same quality feed in both nucleus and production environments, it should be noted that the effect of genotype by environment interactions cannot be avoided. This is because that the genetic correlation between environments is a combined action of multiple environmental challenges. Therefore, a reference population design based on multiple environment records would account all the environmental challenges that exist.
Environment-specific reference population
Using only production animals in the reference population to estimate SNP marker effects provided a substantial genetic gain in the production environment than using nucleus animals when the breeding goal was to improve genetic gain in the production environment. Specifically, a larger selection response was observed when the genetic correlation (rg) between performances of production and nucleus environments was lower. When rg between nucleus and production environment was 0.2, genetic gain increased from 0.724 to 1.31 (81%) for offspring and from 0.561 to 1.01 (80%) for grand offspring scenarios. With rg = 0.8, production efficiency increased from 1.08 to 1.27 (18%) for offspring scenario and from 0.889 to 0.989 (11%) for grand offspring scenario. Similar findings were observed in a simulation study where a cross-breed reference population provided a higher selection response for cross-breed animals compared with using a pure breed reference population when the breeding goal was to improve selection response for cross-breed populations (Dekkers 2007; Esfandyari et al. 2015; Van Grevenhof & van der Werf 2015). Although a larger selection response was observed when the reference population was designed using production animals, technical challenges exist with regard to the large-scale genotyping and phenotyping of production animals. On the other hand, in a breeding scheme if the aim was often to achieve genetic change in the nucleus herds, afterwards disseminate to the production or commercial population, where the return of investment in a breeding scheme is achieved. Hence, we might not accurately estimate the breeding value of selection candidates in the nucleus with a reference population designed using only production animals. This is because the nucleus and production herds are at different genetic level. Therefore, the true genetic superiority might be impaired by environmental effects if we use production records to estimate breeding value of nucleus herds. The best option would be to find a balance between accounting environmental sensitivity and impairing genetic effects by environmental effects. The balance would be to design the reference population based on a mixture of nucleus and production records.
On the other hand, attempting to achieve similar selection response in the production population when nucleus animals were included in the reference population to estimate SNP marker effects provided a smaller genetic progress in the production population. This is a typical breeding scheme used in practice and such reference population designs would be ideal for populations where there is little interaction between genotypes and the environments. In a study using similar breeding structure, where there was more than 90% genetic correlation between nucleus and production environments, the accuracy of selection was higher for production traits when the reference population was designed using only nucleus animals (Hidalgo et al. 2016). In such cases, we expect less benefit by including animals from production environment into the reference population (Mulder et al. 2006). It would be even preferable to use more nucleus records for an accurate estimation of the genetic effects.
Mixed-environment reference population
Compared to the environment-specific reference population, selection response further increased in the production environment when the reference population was designed including a mixture of nucleus and production animals which fed on different quality diets. A highest selection response was observed in the production environment when various proportion of production animals were included into the reference population. At a lower genetic correlation between nucleus and production environments, a highest selection response occurred when large proportion of production animals were included in the reference population. When rg = 0.2, production efficiency increased in the production environment from 1.31 to 1.48 for offspring scenario and from 1.01 to 1.08 for grand offspring scenario when the number of production animals in the reference population increased from 2400 to 4800. In addition, there was a slight increase in production efficiency after the number of animals in the reference population was doubled. The increase in production efficiency was small in the production environment when rg was large. In a similar study, a higher selection response was observed for cross-breed population when the reference population to estimate marker effects contained a mixture of parental lines and cross-breed animals compared to using parental lines or cross-breeds (Dekkers 2007; Van Grevenhof & van der Werf 2015). Even though including production animals into the reference population increased genetic gain and selection accuracy, recording a large proportion of production animals may be a challenge for two reasons. First, feed efficiency is expensive to measure and it is not economical to record large number of production animals. Second, in most practical breeding schemes, performance test is based on a recording routine of nucleus animals and there is no individual feeding record in the production environment. Therefore, the mixture reference population would require the availability of individual feeding record in the nucleus and production environments.
The goal of any breeding scheme was to achieve a substantial selection response in the production environment. Breeding parents are normally selected from the nucleus environment, and genetic change is often realized in the production environment after disseminating from the nucleus to the production or commercial populations. Compared to the nucleus environment, a smaller genetic response is often observed in the production environment. This is because the realized genetic gain in the production environment depends on management practices, genetic distance, non-additive gene action and the interaction of genetic and management practices. To optimize the breeding goal in the production environment, various studies suggested different performance test designs for traditional breeding schemes (Hartmann 1992; Lo et al. 1993; Bijma & van Arendonk 1998) when the performances of nucleus and production animals are different.
For GS breeding schemes, Silva et al. (2014) reported a higher accuracy of selection when genotype by environment interaction was considered as compared to the traditional selection methods. In addition, recent studies have shown alternative reference population designs which optimizes the accuracy of selection and selection response for cross-breed animals when breeding parental lines (Dekkers & Chakraborty 2004; Dekkers 2007; Ibanz-Escriche et al. 2009; Esfandyari et al. 2015; Van Grevenhof & van der Werf 2015). On the other hand, designing the reference population using parental lines provided a higher selection response for cross-breed animals when the linkage disequilibrium (LD) between the SNP markers is higher (Esfandyari et al. 2015). One may argue that the consistency of the linkage phase in both populations is more important than the LD level, that is if the alleles of marker and QTL are in the same arrangement (coupling or repulsion). However, this was under the assumption of GS model, where performance differences between parental lines and cross-breed were due to direct genetic effect and non-additive gene action. The outcome might have been different if estimation was made in the presence of genotype by environment interaction effects. Although there are some reported results in the literature often based on simulation study, there is no unifying method to design the reference population which optimizes a breeding goal in an environment where selection is not taking place.
Our results are in agreement with Van Grevenhof & van der Werf (2015) who observed an increase in selection response for cross-breed population when the reference population included a combination of nucleus and various proportions of production animals. We found a higher selection response in the production environment for a mixture reference population as compared to only using nucleus or production animals. This is because the reference population design accounted the environmental sensitivity occurred due to feeding differences in the nucleus and production environments. The mixture reference population used an existing conventional performance testing structure and records obtained from both environments to improve the genetic gain in the production environment. In a reference population with only nucleus animals, which is the typical breeding scheme often implemented in practice, performance testing of selection candidates was basically using records obtained from animals which were fed high-quality feed sources in the nucleus. In this case, all the genetic improvements achieved in the nucleus environment were not realized in the production environment due to gene by environment interactions.
In our simulation, comparisons were not made at equal reference population size. The focus was to observe selection response in the production environment with increasing or decreasing number of production animals in the reference population at a fixed number of nucleus animals. In a mixture reference population, a larger number of animals were in the reference population compared to the environment-specific reference population breeding schemes. Therefore, selection response observed in breeding scheme with a mixture reference population might have been a contribution from a reference population design and the large number of records used in the reference population. This is because the number of records in the reference population is one factor which determines the accuracy of selection in GS breeding schemes (Goddard 2009).
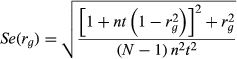
where t = ah2 and a is the additive genetic relationship between two individuals.
This was extended by Bijma & Bastiaansen 2014 to precisely estimate genetic correlation of cross-breed and pure breed lines performance and have shown to require more than 100 half-sib families. Genomic selection breeding programmes may require less number of families. This could relate to the increase in selection response observed in the production environment when large proportion of production animals were included into the reference population to estimate the breeding value of selection candidates. It should be noted that a larger selection response observed for a parent offspring relationship of nucleus and environment animals as compared to a grand offspring relationship. However, in practical pig breeding programmes, the nucleus and production animals are in different generations. Thus, we might not achieve similar genetic gain with the breeding schemes where the production animals are offspring of the nucleus animals.
With the mixed reference population design, a significant number of animals are required for accurate estimation of breeding value. Hence, this would increase the cost of genotyping as both the selection candidates and all animals included in reference population must be genotyped. To minimize the cost of genotyping, it is not recommended to genotype all animals in the nucleus environment. Genotyping could be performed in some animals from the nucleus environment and some animals from the production environment providing a mixture environment animal in the reference population.
Assumptions and implications
In both simulations and data analysis, we did not assume genetic lag between the nucleus and production populations. The main focus was to observe improvements in feed efficiency by designing a reference population including nucleus and production animals that had access to different quality diets. A further difference in genetic lag between the nucleus and production environment was assumed to complicate the simulated breeding schemes. Therefore, it was decided to focus on diet differences between the nucleus and production environments. It should be noted that the larger selection response observed in the simulated breeding schemes are only applicable in the absence of genetic lag. It should be noted that in practical pig breeding programmes, there exists a significant genetic lag between the nucleus and commercial animals. Thus, our results would not be replicated in breeding schemes where the nucleus and production animals are assumed to be at different genetic level. However, the outcome of this study might be considered as a maximum threshold that can be achieved for pig breeding programmes. The aim of this study was only intended to show a general overview how one can improve feed efficiency in pig genomic selection breeding programmes by designing the reference population. Therefore, implications of this study are only limited in the absence of genetic lag and a strong genetic relationship between a nucleus and production environments. A further research could be considered to verify the results including genetic lag between the nucleus and production environments.
Conclusion
In conclusion, when there is a significant environmental difference between the nucleus and production environment, the use of mixed reference population in genomic selection breeding programmes provides a higher genetic gain. Therefore, genomic selection has the potential to improve feed efficiency traits minimizing the cost of recording and accounting the environmental sensitivity that occurs due to differences in feeding system between environments. To utilize this potential, it is essential to include animals in the reference population from both the nucleus and production environments. One of the major challenges with the recommended approach would be that feed efficiency traits are expensive to measure and there is no individual feed recording scheme in the production environment. Thus, a scheme for individual feeding record should be devised and the total number of animals required to record should be distributed equally between the nucleus and production environments. If the above-mentioned challenges could be addressed, the recommended strategy accounts the genetic gain that would have been lost due to environmental sensitivity. Therefore, when implementing genomic selection for feed efficiency traits the reference population should be based on the feeding and management structure of the population and the reference population should involve animals from the environment where the breeding goal is targeted.
Acknowledgements
This study was funded by grant 233685/E50 from the Research Council of Norway.




