Everted gut sac model as a tool in pharmaceutical research: limitations and applications
Abstract
Objectives This review discusses the limitations and applications of the everted gut sac model in studying drug absorption, metabolism, and interaction.
Key findings The mechanism of drug absorption, interaction and the effect of factors such as age, sex, species, chronic therapy, and disease state on drug absorption have been summarized. The experimental conditions and their effects on the outcomes of trials have been discussed also.
Summary The everted sac model is an efficient tool for studying in-vitro drug absorption mechanisms, intestinal metabolism of drugs, role of transporter in drug absorption, and for investigating the role of intestinal enzymes during drug transport through the intestine.
Introduction
There are many factors affecting systemic availability of drug molecules through the oral route. Some of them are drug related and the others are systemic.[1,2] Drug related factors such as solubility, partition coefficient, ionic charge of molecule, penetrability, particle size and shape, salts, isomers, polymorphs, and stability, have been studied extensively and manipulated through material science and product technology.[3–5] However, the systemic factors such as membrane transporter, intestinal enzymes, membrane permeability, area or site of absorption, mechanism of absorption, etc. are difficult to manipulate. In last two to three decades drug interactions, metabolic pathways and absorption mechanisms have been extensively investigated through in-vivo as well as in-vitro models. Drug absorption, metabolism and drug interaction are relatively complex processes; detailed investigations are required to understand the involved mechanisms. In addition to passive absorption, intestinal absorption is facilitated through various carriers/pumps present in the intestinal epithelium. One type of pump transports drug substrates from intestinal mucosa to the serosal side (influx transporter), while another type of pump, known as an efflux pump, operates in the reverse direction, and transports drug substrates from the serosal side to the mucosal side (efflux transporter).[6–8] Several types of influx as well as efflux transporters have been identified in the intestine. The most commonly understood efflux transporters P-glycoprotein (P-gp), breast cancer resistance protein, and multidrug resistance-associated protein, impair intestinal absorption.[9] Generally, the interference of efflux transporters during drug absorption is undesirable, as it reduces bioavailability. The P-gp pump is involved in a number of interactions of different P-gp substrates.[10] Beside drug–drug interactions, P-gp also interacts with food components, e.g. apricot extract and herbal drugs.[11–13] The role of P-gp in the development of multidrug-resistance in cancer chemotherapy has been established and several modulators have been identified.[14] The influx transporters are members of the solute carrier transporter (SLC) family; a number of SLC transporters have been identified.[15]
This carrier-mediated transport is saturable and may be affected by external as well as internal factors. The external factors affecting carrier-mediated transport are food, drug molecules, ions, and other transport modulators.[11,16] The role of carrier-mediated transport in drug absorption and interaction can be established by using the everted sac model.[17–21]
Several in-vitro techniques have been utilized to investigate intestinal transport, including the everted gut sac technique, Ussing chamber, isolated epithelial cells, and brush–border and basolateral membranes isolated from enterocytes.[22]
In-vitro and in-vivo correlation has not been established between the everted sac and in-vivo model. In some cases, two different in-vitro models have shown the same or different absorption for a similar substance.[23] However, frequently results from the everted intestinal sac model have been in agreement with in-vivo findings.[24–28] The living system is more complex than in-vitro models, in an in-vitro condition there is probability of less or no enzymatic action. For example, after oral administration indometacin ethyl ester released from lipid-core nanocapsules hydrolysed into indometacin in the intestinal lumen and intestinal wall; but no indometacin was formed ex vivo.[29] For oral absorption studies Caco-2 cell line experiments were approved by the Food and Drug Administration, but these cell lines are not always the right substitute for the ex-vivo everted gut sac model.[30]
The in-vitro everted gut sac model was first introduced in 1954 by Wilson and Wiseman[31], since then modifications and improvements have been made to the model to increase the viability of tissue, and to maintain intact mucosal epithelium that mimic the in-vivo conditions. An improved everted gut sac model can be used as an in-vitro tool to study the mechanisms and kinetics of drug absorption.[32,33] The everted sac model has been extensively explored to carry out pharmacokinetic investigations such as drug absorption, drug metabolism or pro-drug conversion in gastrointestinal segments, efflux transport, multidrug resistance, drug interactions, and the impact of efflux transport modulators on the absorption of drugs. The advantages of this model are a relatively large surface area available for absorption and the presence of a mucus layer. However, the tissue viability is one of the limiting parameters. The recommended tissue viability and metabolic activity of intestine under physiological conditions is approximately two hours.[32] Another potential disadvantage of this approach is the presence of the muscularis mucosa, which is usually not removed from everted sac preparations. Muscularis mucosa might evoke an underestimation of the transport of compounds with a tendency to bind with muscle cells.
Different animals have been chosen for everted gut sac experiments, for example frog, catfish, rat, rabbit, sheep, chicken, goldfish, turtle, pigs, guinea pig and mice.[31,32,34–44] The everted rat intestinal sac is the most commonly used sac for in-vitro studies. Everted gut sac of rat was used to investigate P-gp-mediated efflux of [3H]vinblastine, [14C]doxorubicin and verapamil as reference compounds. The reproducibility of this in-vitro model suggests that the rat everted gut sac is a useful screening tool for studying transport of P-gp substrates and potential P-gp modifiers.[36]
Everted gut sac preparation
Under ether anaesthesia, rapidly remove the jejunum or duodenum or ileum of the intestine and divide into segments (5–6 cm each). Wash each segment with an ice-cold physiological solution (e.g. oxygenated Krebs solution (pH 6.5) containing 7 g/l sodium chloride, 0.34 g/l potassium chloride, 1.8 g/l glucose, 0.251 g/l disodium hydrogen phosphate, 0.207 g/l sodium dihydrogen phosphate and 46.8 mg/l magnesium chloride). Gently evert the washed intestine over a glass rod. Clamp one end of the everted intestine and tie with a silk braided suture and then fill with 600 µl Krebs solution at 37°C using a 1-ml micropipette. Seal the filled intestine segment with a second tie using a braided silk suture. Transfer the everted filled sac to the incubation flask containing drug in 15–25 ml oxygenated media at 37°C. The sampling can be done at different time intervals. The outline diagram of the everted gut sac model is given in Figure 1.
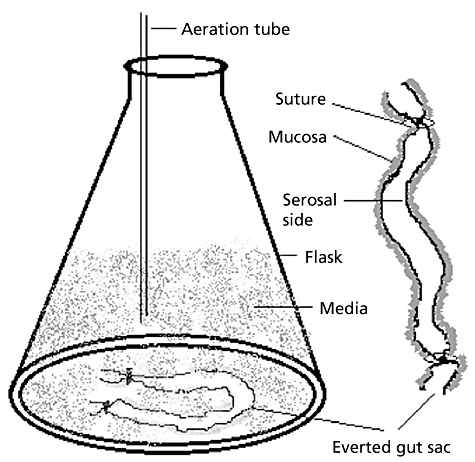
Outline diagram of the everted gut sac model.
The everted gut sac model is sensitive and specific; there are several factors which impact on the outcome and conclusion, e.g. animal factors (age, sex, species, diet, disease state, chronic treatment and toxicity), intestinal segment used (ileum, jejunum, duodenum and colon), and experimental factors (e.g. pH, aeration, temperature, concentration of substance); for other factors see Table 1)[45–69].
| Factors | Impact | |
|---|---|---|
| Animal factors | Age | Mechanism and preferred site of absorption varies with age[45,46] |
| Sex | Different physiology and hormonal stages[47] | |
| Species | Different species may have different enzymes and transporters. Different anatomy and physiology[48,49] | |
| Diet | Influences absorption. Starvation affects the enzymatic activity[50,51] | |
| Disease/toxicity | Affect the expression of transporters. Permeability also changes[52–55] | |
| Chronic treatment | Affect the absorption of subsequently used drug[56,57] | |
| Intestinal segment | Ileum | Drugs have different absorption window because of site specific presence of transporters, different intensity of transporters in segments, different mechanism of absorption, permeability, surface area, metabolic site, and anatomic and physiological variables[58–60] |
| Jejunum | ||
| Duodenum | ||
| Colon | ||
| Experimental factors | pH | Affects solubility, stability and ionization[63–65] |
| Aeration | Promote viability of tissue | |
| Temperature | Influence the viability and mechanism of transport[35,66,67] | |
| Substrate concentration | Affects mechanism of absorption[68] | |
| Other factors | Segment harvesting time | Quick harvesting provides maximum enzyme and transporter activity |
| Method of killing | Harvesting under anaesthesia avoid transporter deterioration[69] | |
Animal Factors
Animal age
Change in the pharmacokinetics of drug molecules with age is well known, its impact is more obvious when the drug belongs to a narrow therapeutic index category. Along with age, different developmental and maturation stages also affect intestinal absorption.[70,71]β-Lactoglobulin was more easily absorbed from the intestine of five-week-old rats than from rats of more than 20-weeks old.[72] In some cases the mechanism of absorption also changes with age; in the mature rat acyclovir is predominantly absorbed via passive diffusion.[45] For some drug molecules, the preferential sites of absorption also vary at different stages of age. In adult rats biotin is preferentially transported through the jejunum, while suckling rats transport biotin preferentially through the ileum. Those findings also suggested that the number of ileal biotin transporters decreases at maturation.[46] The rate of transepithelial transport of d-glucose, 3-O-methyl-d-glucose, and l-tyrosin was increased during the growth period, while old mouse showed a decrease in transepithelial transport of d-glucose, 3-O-methyl-d-glucose, and l-tyrosine. Along with drug absorption, age may impact on drug metabolism. The rate of d-glucose metabolism in very old mouse was only 45% of mature mouse.[73] The transport of riboflavin is similar in suckling, weanling, and adult rats and occurs by an energy, temperature, and Na+ dependent carrier-mediated process. However, the affinity and the activity of the transport carriers of riboflavin decrease with maturation.[74] Prolactin induced duodenal active transport in eight-week-old rats but not in three-week-old, five-week-old, or > 15-month-old rats, and enhances apical uptake of Ca2+ in oil-filled everted duodenal sac obtained from rats that received prolactin 5 and 10 min before the experiment.[75]
Sex and species
Due to physiological differences, different sex as well as species will influence drug pharmacokinetics. In female rat, transport rate of nimodipine in the jejunum was higher than ileum; and in contrast, nimodipine transport in male rat jejunum was lower than ileum.[47] In an everted gut sac study the transport, enzyme degradation and apparent permeation of leuprolide varied in different animal species (rabbits and rats). The enzyme degradation of leuprolide in rabbit gut was the highest and the apparent permeation coefficient in rat gut was the largest.[48] Some animal species may lack some transporter or some segment of intestine may lack some particular transporter.[49]
Pathological conditions and chronic therapy
Microbial infections or other pathological conditions may affect drug pharmacokinetics.[76] The possible impact of infections over intestinal transport/absorption can be investigated through the everted intestine sac model.[77] Recently, sepsis has been shown to impair intestinal amino acid absorption.[52] Sepsis depresses the expression of imino transporters by rabbit enterocytes, which may explain the reduced intestinal proline absorption.[53] Furthermore, the everted sac model can be used to investigate mucosal barrier function after several ailments and changed conditions after medical treatment.[78] The disease state may affect the transport by increasing or decreasing the number of transporters; for example, the chemically-induced acute diabetes mellitus is associated with an enhancement in folate absorption.[79] Intestinal toxicity can be assessed through the everted sac model by measuring uptake of d-glucose and l-histidine, and surface examination of apical membrane or measuring change in membrane composition after exposure to a substance will be helpful to assess the toxicity.[54,55] The measurement of drug transport through the everted sac might be useful as a qualitative index of gastrointestinal mucosal damage/integrity. The model was used to clarify the effects of pre-administration of mitomycin C on intestinal transport of drugs. Pretreatment with mitomycin C resulted in a loss of intestinal tissue weight and an increase in fluxes of passively-absorbed drugs.[80] Prolonged therapy in chronic diseases may influence the pharmacokinetics of subsequently used drugs. Absorption of ornidazole was decreased from different parts of the small intestine of rat pretreated with rifampicin and sodium butyrate.[56] Malnourishment significantly reduced the serosal fluid transfer, mucosal fluid transfer, gut fluid uptake as well as gut glucose uptake.[81] The effect of the disease state on absorption of ions and drugs has been investigated using the everted sac model.[82] The everted sac model can be exploited to study drug absorption in colon cancer and other intestinal diseasesl. Chronic treatment with chlorpromazine and phenobarbital decreased the transport of L-DOPA.[57]
Intestinal Segments
There are several factors responsible for the differences in drug absorption, interaction and metabolism from different intestinal segments, e.g. pH, enzyme activity, surface area, wall thickness, transporters, tight junctions. The impact of enzyme activity and the role of transporters are the most commonly investigated factors through the everted gut sac model.
It is a well established fact that different drugs have different absorption windows, even the same drug can be absorbed differently through different segments of intestine. This absorption variability is due to the site specific presence of transporters or different intensity of transporters in segments, different mechanism, permeability variations, surface area, metabolic site, and physiological variables (pH, fluid volume, viscosity, thickness of mucus layer, blood circulation network, thickness of muscularis, etc.). The best absorption site can be screened through the in-vitro everted gut sac model, and the effect of drugs or food extracts on drug absorption can be investigated.[83–85] Efflux-mediated saturable absorption of rifampicin was studied using the everted sac method.[86] In an in-vitro experiment, zidovudine showed saturable transport mechanisms in the jejunum and the proximal ileum, while no saturation was found in the distal ileum, the permeation of didanosine was higher through duodenum than across jejunum and ileum, while vitexin-2″-O-rhamnoside almost uniformly absorbed through four segments of the rat intestine (duodenum, jejunum, ileum, and colon).[58,61,87] The transport inhibitor may have the same or a different effect in the different segments of the intestine. For example, quercetin, diosmin, methyl hesperidin, gossypin and chrysin decrease the transport of nitrendipine (P-gp substrate) in the rat intestine. Diosmin and quercetin decreased the transport rate of nitrendipine to almost the same extent in the duodenum, jejunum and ileum, but chrysin and gossypin had greater impact in the ileum than in the duodenum and jejunum. Methyl hesperidin inhibited nitrendipine transport in the ileum and jejunum, but not in the duodenum.[88] Ketoconazole improved the absorption of ranitidine, while refampicin decreases the ranitidine absorption, through intestine.[28] The role of the luminally-exposed enzyme lactase phlorizin hydrolase (LPH; an enzyme expressed specifically at the small intestinal brush border) in drug metabolism was established through the everted sac model.[89] The efflux of rhodamine 123 (P-gp substrate) increased from the apex of the jejunum to the lower ileum as the expression of P-gp in the ileum was 2.31-fold higher than that in the jejunum.[59] Intestinal absorption inhibitors may inhibit the absorption in selected segments and may not have any effect on the absorption through other segments; it depends on the absorption mechanism as well as the mechanism of action of the inhibitor. Aluminum inhibits calcium absorption in the duodenum through an effect on active mucosa-to-serosa transport, but has no effect on ileal calcium absorption, which in the rat is not mediated by an active process.[90] The duodeno-jejunal absorption of morphine takes place through carrier-mediated transport stimulated by an H+ gradient, although morphine is passively absorbed from other sites.[60] Absorption of substrates may vary from segment to segment and from drug to drug. Some may absorb better in some parts whilst others may absorb better in other parts of the intestine.[91,92]
A very good example of segment selectivity in absorption of substrates is the absorption of K+ ions. The K+ ions absorb through the descending colon while the ascending colon does not absorb K+ ions. The quantitative differences were also observed in the absorption of Na+ ions through the ascending colon and descending colon.[62]
Experimental Factors
In in-vitro experiments, rate of tissue uptake may be affected by factors such as pH, incubation temperature, concentration of substrate, aerobic condition and the presence of metabolic inhibitors.[93]
pH and media
The intraluminal as well as media pH will affect stability, solubility, ionization and the absorption of substrates through the everted sac. pH plays an important role in the transport of drugs through the intestine.[63] Increased intraluminal pH promotes the absorption of HgCl2 accompanied by a change in the binding of HgCl2 to the everted tissue sac.[64] The media composition influences the substrate transport in an everted sac experiment. Uptake of manganese is higher from bovine milk or infant formula than human milk and its isolated manganese fractions.[65]
Temperature
Temperature is one experimental condition which may influence the outcome of in-vitro experiments.[94] The change in temperature will influence passive or active transport or both types of transport. Temperature higher than baseline temperature will promote passive diffusion, the permeability of rhodamine 123 through catfish everted sac was different at acclimation and acute temperature.[35,66] Hyperthermia affects the transport of glucose through intestinal sac; heat treatment causes mucosal damage, consisting of loss of epithelial cells and destruction of villi.[67]
Substrate related factors
Some drugs shows concentration-dependent absorption through the intestine. Absorption of curcumin at different concentration ranges (50–1000 µg in 10 ml incubation mixture) was investigated and the absorption of 100 µg curcumin was found to be the maximum.[68] In such cases, after a certain range absorption does not increase with substrate concentration.
Other Factors
Time lapse in the harvesting of the intestine and animal state (live/dead) affects active transport in duodenal segments of rat intestine. The transport of calcium and glucose was decreased significantly in intestinal segments of animals killed by cervical dislocation 10–20 min before tissue removal. Harvesting from animals under anaesthesia permits excision of intestinal segments before death and will avoid transporter deterioration.[69] The starved condition may have some effects on enzymatic activity in intestine, which may influence the absorption of enzyme substrates.[50] The results obtained through an everted sac experiment also showed that diet induced variations on the absorption of nutrients.[51] The volume filled in the sac affects the outcome, and non-uniformly or a tightly filled sac gives variable results.
Applications
Investigating drug interaction and mechanism of transport
The everted gut sac model helps researchers to understand the mechanism of drug absorption/transport/interaction. In an interaction study, lactic acid reduced accumulation of valproic acid on the serosal side of the intestinal sac against the concentration gradient by interfering with carrier-mediated transport of valproic acid. Imipenem inhibited the intestinal absorption of valproic acid after oral administration, but imipenem did not affect the active transport of valproic acid. Therefore, the inhibition of valproic acid absorption by imipenem was caused by a mechanism different from that of lactic acid.[95] In an everted sac experiment, the absorption of methochlorpromazine was slightly inhibited by choline, but more markedly inhibited by more hydrophobic quaternary ammonium cations tetraethylammonium and cetyltrimethylammonium, and a moderate inhibition was observed by a polyamine, spermine. The absorption rate of methochlorpromazine markedly depended on temperature. The Arrhenius plot of the apparent transfer rate constant revealed high activation energy for the transport. The findings suggested that methochlorpromazine bound to the relatively hydrophobic region of small intestinal epithelial cells and transferred by passing through a high energy barrier.[96] Quinidine increased the bioavailability of etoposide by inhibiting P-gp mediated efflux.[97]
The everted sac model may be useful to understand metal ion interaction. Molybdate and tungstate mutually and competitively inhibited the transport of each other, while sulphate competitively inhibited the transport of both.[98] In a metal ion interaction study, rats were fed with milk supplemented with an iron source (ferrous sulphate), the in-vitro intestinal transport of manganese was suppressed by the iron.[99] Absorption interference between three amino acids was confirmed by the everted sac model, isoleucine and valine inhibited the absorption rate of leucine, and leucine inhibited absorption of isoleucine and valine.[100] The absorption of drugs at lower concentrations takes place by active transport/partially-active and partially-passive transport, while at higher concentrations passive diffusion predominates.[92,93,101]
Screening the role of pharmaceutical excipients and formulations in transport modulation
Pharmaceutical excipients were expected to be pharmacologically inert; but current research has indicated that pharmaceutical excipients may alter the pharmacokinetics of drugs. The everted gut sac model has been used to investigate the pharmacokinetic interaction of drugs with excipients. Surfactants such as Cremophor EL and Polysorbate 80 modulate the P-gp pump to improve the bioavailability of poorly absorbed drugs.[102] Pluronic F68 enhanced celiprolol transport across the intestinal mucosa and also inhibited CYP3A4-catalysed formation of 1′-hydroxymidazolam.[103] Excipients, mostly surfactants, enhance the permeability of drugs in the intestine; the extent may vary from segment to segment and depends on the concentration of surfactant. Permeability of tanshinone IIA+2-hydroxypropyl-β-cyclodextrin inclusion complex across the intestinal epithelial membrane was different through different intestinal segments.[104] The state of the art has developed different microparticulate formulations to alter drug pharmacokinetics, and the pharmacokinetics parameters of microparticulates were investigated using the everted sac model.[105] The microparticulate carrier for oral delivery of poorly soluble drugs and efflux pump substrate can be screened through the everted sac model. Proliposomal formulations enhance the extent of dissolution and membrane transport of progesterone.[106] Safe and potential absorption enhancers for oral drug delivery can be screened for co-administration with drugs. The absorption enhancement efficacy, mechanism of action and toxicity of absorption enhancers (medium-chain fatty acids, cyclodextrins and bile salts) were evaluated using an in-vitro everted gut sac model. Light microscopy studies of gut sac incubated with absorption enhancer revealed morphological changes in mucosa. The order of toxicity was cyclodextrins > bile salts ≈ medium-chain fatty acids.[107] Investigations through everted rat colon sac have shown that triethyl chitosan could be used as a penetration enhancer for poorly absorbable compounds in the colon drug delivery system.[108] The different permeation enhancers affect the permeability of intestine at the same or different concentrations, for the same or different molecules. A penetration enhancer may enhance the absorption of one substance at a certain concentration but does not have significant effect on the permeability of another drug.[109] A number of surfactants/excipients have been shown to inhibit P-gp, and thus potentially enhance drug absorption. The everted gut sac technique can be used to screen excipients for their ability to enhance the absorption of drugs.[110] Evaluation of in-vitro mucoadhesion properties of oral bioadhesive microparticulates as well as multiparticulate formulations can be carried out using the everted sac model.[111,112] The everted sac model may assist in studying the interaction between surface charged nano/microdroplets and mucosal surface, and the effect of interaction over bioavailability and droplet integrity.[113] The absorption of drug from different formulations can be evaluated using the everted sac.[114]
Miscellaneous
To carry out permeability studies of drug candidates across the intestinal mucosa, a noneverted rat intestinal sac model can be used successfully. In this model, the drug solution is placed in noneverted intestinal sacs and then the sac is placed in an acceptor solution.[115]
The influence of endotoxins on the activity of transport modulators can be investigated through the everted sac model.[41] The everted sac model can be an excellent tool to understand the mechanism and factors involved in endotoxin absorption.[116]
Different analogues or derivatives of the same drug may have different rates, mechanisms, and sites of absorption. Tetracycline derivatives chlortetracycline, demethylchlortetracycline, and oxytetracycline had different rates of transfer across ileum, but tetracycline and oxytetracycline were transferred at the same rate.[117] Ampicillin-guaiacolsulfonate was better absorbed than ampicillin.[118] Tryptophan and its benzo[b]thiophene analogue tryptophan-S were actively transported across the intestine; while tryptophan-l-Me, its 1-methylindole analogue, was not actively transported.[119] In an everted sac investigation, cyclacillin (1-amino-cyclohexyl penicillin) was transported actively but the other amino penicillins (amoxicillin and ampicillin) were transported passively.[120] In an everted sac experiment, the pivaloyloxymethyl ester of ofloxacin was efficiently absorbed even in the presence of aluminium ions, whereas the absorption of ofloxacin was decreased significantly by the presence of aluminium ions; the findings were confirmed through in-vivo results.[121] The site and step of isomeric inversion can be investigated through the present model. In an everted sac experiment R-(-)- benoxaprofen was stereospecifically inverted to the S-(+)-enantiomer while passing through the gut wall.[122]
The ionic transport through active channels and implication of modulators can be studied by using the present model. Edelfosine enhanced the intestinal Ca2+ transport in the everted duodenal sac study.[123] The transport of metal ions in normal as well as in diseased conditions can also be studied using the everted sac model; along with the impact of metal ions on drug absorption.[124,125]
Various plant extracts and their effects on absorption, and metabolism of other substances can be screened through one of the in-vitro models, and can be confirmed by other models.[13] Some key references are summarized in Table 2.
| Author | Outcomes | Remark |
|---|---|---|
| Wilson and Wiseman[31] | Use of everted sac for studying transference of substances | Everted sac was suspended in well-oxygenated medium |
| Barthe et al.[32] | Developed tissue culture medium, and improve tissue vitality | Tissue vitality was improved |
| Kato et al.[17] | Glycerol absorbed through active as well as passive mechanism | Drug excipient interaction |
| Mizuma et al. [18] | Prodrug screening | Absorption clearance |
| Carreño-Gómez and Duncan[36] | Quantitation of P-glycoprotein (P-gp) mediated efflux of anticancer agents | Tool for investigating transport of P-gp substrate and role of P-gp modulators |
| Emoto et al.[44] | Use of everted sacs as an enzyme source to study first-pass metabolism | To study drug metabolism, transport and absorption |
| Chen et al. [73] Said et al. [74] | Transepithelial transport and surface area for absorption decreases in ageing | Ageing decreases transport or absorption |
| Gardiner and Barbul[53] | Sepsis depresses the expression of imino transporters | Impact of disease |
| Rivera-Calimlim[57] | Chronic oral treatment can modulate the mucosal transport | Impact of chronic therapy |
| Rajnarayana et al.[88] | Bioflavonoids decreased the transport of nitrendipine | Interaction of bioflavonoids with P-gp substrate in different segments |
| Wilkinson et al[89] | Lactase phlorizin hydrolase plays an important role in the metabolism of glycosylated phytochemicals | Enzymatic metabolism in intestine |
| Tian et al.[59] | Grapefruit juice and orange juice extracts inhibited the efflux of P-gp substrates | Interaction of fruit juices with P-gp substrates |
| Adler et al.[90] | Aluminium inhibits calcium absorption in the duodenum, but has no effect on ileal calcium absorption | Segment selective ion interaction |
| Ota et al. [91] | 2,4-Dinitrophenol inhibits the ileal uptake of bile acids, while no effect on jejunal uptake | Selective segmental uptake inhibition |
| Said and Redha[92] | At low concentrations biotin transport by a carrier-mediated process but at high concentrations it was simple diffusion | Different mechanism of absorption |
| Chan et al.[65] | Transport and uptake of manganese was less in presence of human milk than in bovine milk and infant formula | Media impact on in-vitro absorption |
| Hamaura et al[94] | Transfer and tissue uptake of sulfanilamide decreases with decreasing temperature | Temperature affects transfer and tissue uptake |
| Pento and Mousissian[69] | Time-dependent deterioration of the active transport in animals killed by cervical dislocation | Under anaesthesia excision of tissue can be done before death |
| Cornaire et al[102] | Cremophor EL and Polysorbate 80 modulate the P-gp | Excipients/ surfactants can modify the pharmacokinetics of P-gp substrates |
| Huang et al.[103] | Pluronic F68 is a potent in-vitro inhibitor of both P-gp and CYP3A4 | Excipients can modify the pharmacokinetics of P-gp and/ or CYP3A4 substrates |
| Younessi et al.[108] Sharma et al. [107] | TEC bearing +ve charge interact with the tight junctions of colon epithelia and increases the permeation of sodium fluorescein and brilliant blue through the tight junctions | Evaluating permeation or absorption enhancer |
| Cornaire et al.[110] | Softigen 767, TPGS, Imwitor 742 enhances the transport of celiprolol | Excipients/ surfactants can modify the pharmacokinetics of P-gp substrates |
| Santos et al. [112] Miyazaki et al. [111] | CAHN microbalance and everted sac experiments yield similar results when quantify bioadhesive strength | Bioadhesion assay of polymeric micro-particulates |
| Ruan et al.[115] | Oral absorption screening of new drug candidates | Screen the absorbed components of natural drug |
| Nolan et al.[116] | Quantitatively measure the mucosal to serosal flux of endotoxin | Kinetics of absorption in the isolated everted gut sac |
| Bosin et al.[119] | Tryptophan-S competitively inhibits active transport of tryptophan. | Analogue interaction |
| Dixon and Mizen[120] | Cyclacillin (1-amino-cyclohexyl penicillin) actively transported through intestine. Other penicillins diffuse passively. | Different mechanism of transport |
| Maeda et al.[121] | Prodrug, ofloxacin-PVM absorb better than parent compound | Investigate pro-drug absorption and its interaction |
| Simmonds et al. [122] | In rats R-(-)-benoxaprofen is stereospecifically inverted to the S-(+)-enantiomer while passing through gut wall | Site of transformation was confirmed by everted intestinal sac |
| Cardin and Mason[125] | Anion interaction | Effect of diet on transport |
| Krisanapun et al.[13] | Aqueous extract of Abutilon indicum Sweet inhibits glucose absorption in diabetic rats | Everted sac study supported in-vivo investigations |
Summary of applications
-
To confirm in-vivo pharmacokinetic findings.
-
To carry out drug permeation studies.
-
To investigate drug transport mechanism through intestine and to identify the carrier involved.
-
To evaluate the rate and extent of absorption through different segments of gastrointestinal tract.
-
To identify the absorption window of drug molecules.
-
To investigate drug–drug, and drug–food interaction.
-
To investigate intestinal metabolism of drug molecules, e.g. enzyme degradation.
-
To screen pharmaceutical excipients as well as formulations, for oral bioavailability enhancement.
-
To evaluate in-vitro mucoadhesive properties of oral bioadhesive microparticulate formulations.
-
To study the effect of disease state on absorption/expression of intestinal transporter.
-
To investigate site of isomeric conversion.
-
To study of intestinal conversion of prodrug into drug.
-
To investigate effect of chronic therapy and medical conditions on the absorption of ions, and drugs.
-
To screen the safe and effective efflux pump inhibitor for successful and effective chemotherapy.
Summary of limitations
-
Limited viability of intestine under in-vitro conditions.
-
Loss of enzymatic activity under in-vitro conditions.
-
Limited sampling points because of viability constraints.
-
Lack of nerve response through neurons after drug exposure.
Conclusions
The everted gut sac model has been used in various pharmacokinetic and pharmacodynamic studies such as drug absorption, metabolism, and mechanism of interaction. In absorption, the mode of drug absorption, identification of transporters, role of transporters, screening of penetration enhancers, identifying the absorption window, effect of media on absorption, absorption modulators, etc., have been studied successfully by using this model. The model has been utilized successfully for drug interaction studies. Pharmaceutical research is growing day by day and new challenges are ahead in drug pharmacokinetic studies, the everted gut sac model is expected to meet these challenges with time.
Declarations
Conflict of interest
The Author(s) declare(s) that they have no conflicts of interest to disclose.
Funding
This review received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.




