Sensitive Detection of PrPSc by Western Blot Assay Based on Streptomycin Sulphate Precipitation
Summary
Transmissible spongiform encephalopathies, also termed prion diseases, are fatal neurodegenerative disorders that affect both humans and animals, which are characterized by presences of protease-resistance disease-associated prion protein (PrPSc) in brains. In the present study, we optimized the Western blot assay for PrPSc with a precipitation procedure of streptomycin sulphate. After incubated with suitable amount of streptomycin sulphate, the detective sensitivity for PrPSc was remarkably improved. The precipitation of PrPSc was obviously influenced by pH value in the solution. Employs of PrPSc stock sample into various mimic specimens, including normal hamster brain homogenate, human cerebrospinal fluid and urine, demonstrated that streptomycin precipitation markedly increased the detective sensitivity of PrPSc, regardless in low concentration or in large volume. In addition, the PrPSc from a human brain tissue of familiar Creutzfeldt–Jakob disease (fCJD) was efficiently precipitated with streptomycin sulphate. As a sensitive, specific, rapid and flexible protocol for PrPSc, the protocol in this study has the potential, alone or combined with other techniques, to detect low levels of PrPSc in the specimens not only from central nerve system, but also from peripheral organs or fluids.
Introduction
Transmissible spongiform encephalopathies (TSEs), also termed prion diseases, are rare and fatal neurodegenerative disorders characterized by accumulation of an abnormal isoform of prion protein (PrPSc) in central nervous system (Prusiner, 1998). The appearance of a new variant of Creutzfeldt–Jakob disease (vCJD) in the United Kingdom, which is caused by the same TSE agent as bovine spongiform encephalopathy (BSE) in cattle (Will et al., 1996; Bruce et al., 1997), has raised great concerns worldwide. Infection with the BSE agent may induce a permanent carrier status, with affected individuals being clinically healthy but having the potential to transmit the infectious agent to others (Collinge, 1999). Therefore, development of simple, sensitive, and/or non-invasive diagnostic tests that are able to identify TSE-infected animals and humans before the onset of clinical symptoms is of the utmost importance.
Most of the diagnostic tests for TSEs are based on an immunological approach using appropriate PrP-specific antibodies after treatment of brain tissue samples with proteinase K (PK), utilizing the fact that PrPSc, but not PrPC, is partially protease resistant (Oesch et al., 1985). The brain tissues were collected by postmortem or biopsy after onset of clinical manifestations. Some techniques that aimed on the pre-symptomatic cases have been reported, but still in the stage close to the clinical phase (Grassi et al., 2001). Soto et al. (2005) reported a sensitive detection method for PrPSc in pre-symptomatic hamsters with protein misfolding cyclic amplification technology. Combining the usage of antigen–antibody competition and capillary electrophoresis, PrPSc has been detected in the blood of sheep with scrapie and elk with chronic wasting disease (Schmerr et al., 1999). However, these results have not been reproduced with the blood samples from human CJD case and CJD agent-infected chimpanzees (Cervenakova et al., 2003). Some publications also described methodologies for non-nerve specimen, for example a dual-colour fluorescence correlation spectroscopy for PrPSc in cerebrospinal fluid (CSF) from TSE-affected humans (Cervenakova et al., 2003), an ultracentrifugation and dialysis technique for PrPSc in urine (Shaked et al., 2001). More recently, it has been reported that normal PrP can be detected in urine specimens with an ion-capture-based, solid-phase extraction (Narang et al., 2005).
During the pathogenesis of TSE, the infectious agents mainly restrict in the tissues of the central nerve system. The presence of PrPSc in brains is much earlier than the appearances of clinical manifestations (Gao et al., 2004). It is known that the surgical instruments or blood products can act as vectors for transmission of TSEs (Wadsworth et al., 2001). In sporadic and genetic TSEs, PrPSc sometimes could be detected in peripheral tissues or biological fluid before the onset of the disease (Ingrosso et al., 2002). However, the content of PrPSc in the tissues out of central nerve system is often very rare both in natively occurred or experimental infected cases. Therefore, enrichment and concentration of PrPSc in the tested specimens, especially in easily accessible tissues or body fluids, are the key step for the development of early diagnostic methods.
The PrP shows characteristics to interact with high-molecular-weight mass (e.g. some neuron proteins) and small one (e.g. glycans). Both the biochemical features and the biological functions may change through interacting with others. Moussa et al. (2006) firstly reported on the ability of streptomycin sulphate to combine and precipitate PrPSc from brain homogenates. It is most likely to occur via electrostatic interaction between streptomycin sulphate possessing two guanidine groups and/or two ammonium and different amino acids from PrP molecules. When PrP molecules are cross-linked by such very small molecule proportionally, reticulation is expected, leading to the formation of flocculated aggregates in liquid solutions. Further confirming the features, optimizing the conditions for PrPSc precipitation with streptomycin sulphate and especially observing efficiency of recovery of low level of PrPSc from tissues or body fluid helps to evaluate the possibility of streptomycin precipitation to be an effective diagnostic tool of TSEs.
In this study, we further investigated the method of concentrating with streptomycin sulphate by analysing its effect on PrPC, undigested PrPSc and digested PrPSc as well as the concentrating effect from large volumes using lysis buffer, CSF or urine.
Materials and Methods
Preparations of brain homogenates
Normal and scrapie-adapted (i.e. 263K prion) hamsters’ brain tissues (Gao et al., 2004) and human brain tissue from a patient with familiar CJD (fCJD) (Wang et al., 2007) were homogenized in nine volumes (10% w/v) of lysis buffer (100 mm NaCl, 10 mM ethylenediaminetetraacetic acid, 0.5% Nonidet P-40, 0.5% Na deoxycholate in 10 mm Tris–HCl, pH 7.4), and then brain homogenates were dispersed by sonication. Scrapie-adapted hamster’s and human’s brain homogenates were centrifuged at 10 000 g for 30 min, and the supernatants were used as PrPSc stock sample for further streptomycin sulphate precipitation and Western blotting. Normal hamster brain homogenate was centrifuged at 10 000 g for 30 min in a microfuge, and the supernatant was collected as PrPC stock sample for the subsequent experiments.
Urine and CSF samples
Urine samples were collected from healthy individuals. The CSF specimens were collected from the patients with other CNS diseases, but excluding the diagnosis of CJD. Samples were centrifuged at 300 g for 10 min to remove the occasional debris.
Optimizing streptomycin sulphate precipitation
The PrPSc stock sample was spiked into different volumes of lysis buffer under various conditions of pH values. Various amounts of streptomycin sulphate were employed into the preparations and incubated at 37°C for 1 h and subsequently centrifuged at 10 000 g for 5 min. The supernatants were carefully separated and the pellets were resuspended in 20 μl of 2× sample loading buffer (SDS 4%, mercaptoethanol 2%, glycine 192 mm, Tris 25 mm and 5% sucrose denaturing buffer) containing 6 m guanidine hydrochloride (GdnHCl) or urea. After vigorously vortex stirring for 5 min, the preparations were heated at 80°C in the preparation with GdnHCl or 100°C in that with urea for 10 min. The supernatants that corresponded to re-solubilized materials from the pellets were gathered by centrifugation at 10 000 g for 5 min.
Spiking experiments
To prepare the mimic tissue samples that contained PrPSc, various amounts of PrPSc stock samples were spiked into the different volumes of brain homogenate, CSF and urine samples. Treatment of the preparations with streptomycin sulphate at a final concentration of 60 mm was conducted as described above.
Gel electrophoresis and Western blotting
Samples were separated in 12% polyacrylamide gel and electronically transferred to nitrocellulose membranes. For immunoblotting, the membrane was blocked with 5% non-fat dried milk and incubated with 1 : 4000 diluted PrP monoclonal antibody 3F4 (Dako, Glostrup, Denmark) in 0.5% non-fat dried milk in 135 mm NaCl, 1.3 mm KCl, 3.2 mm Na2HPO4, 0.5 mm KH2PO4, 0.05% Tween 20, pH 7.4 (PBST) at room temperature for 2 h. After washing twice in PBST, the membrane was immerged in a 1 : 5000 diluted peroxidase conjugate anti-mouse IgG (Boehringer, Mannheim, Germany) in PBST and incubated at room temperature for 1 h. Detection of signal was performed with an enhanced chemiluminescence detection kit (Amersham-Pharmacia Biotech, Piscataway, NJ, USA). The image of immunoblot was scanned with Typhoon (Amersham-Pharmacia Biotech) and digitalized, saved as TIF format. The immunoreactive bands were quantified by densitometry using computer-assisted software Image TotalTech (Pharmacia).
Results
Validation and optimization of streptomycin sulphate precipitation for detection of PrPSc from 263K-infected hamster brain
To address the potential influences of streptomycin sulphate on the distribution of PrPSc in solution, 10 μl PK-digested PrPSc stock sample from scrapie 263K-infected hamster brains (equal to 1 × 10−3 g brain tissue) was mixed with 60 mm streptomycin sulphate. Western blot assay revealed that streptomycin sulphate induced the precipitation of PrPSc (Fig. 1). Consistent with the previous study (Moussa et al., 2006), the molecular weights of streptomycin sulphate-precipitated PrPSc molecules moved at the positions from Mr. 25 000–43 000 in SDS-PAGE, which were significantly higher than the native PrPSc (Fig. 1), highlighting obviously molecular interaction between streptomycin sulphate and PrPSc, regardless of di-, mono- and non-glycosal isoforms. To improve the electrophorestic patterns of streptomycin sulphate-precipitated PrPSc, the precipitated pellets were resuspended in 2× sample loading buffer containing 6 m GdnHCl. Compared with the preparation without GdnHCl (Fig. 1, lane 1), three more distinguishing PrPSc bands appeared in the preparation of GdnHCl (lane 2).
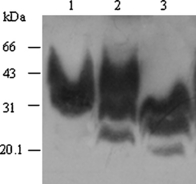
An increase in the molecular weight of PrPSc after treated with streptomycin sulphate. Ten microlitre proteinase K (PK)-digested hamster PrPSc stock samples were treated with streptomycin sulphate at a final concentration of 60 mm. Following streptomycin precipitation, the pellet was mixed with the loading buffer containing with 6 m GdnHCl (lane 2) or without GdnHCl (lane 1). Same amount of PK-digested PrPSc stock sample without treatment of streptomycin sulphate was directly loaded as the control (Lane 3).
To probe the optimal working concentration of streptomycin sulphate, different quantities of streptomycin sulphate ranging from 1.88 to 120 mm at final concentration were subjected into the preparations containing 10 μl PrPSc stock sample in 100 μl volume. Western blot analyses identified a clearly streptomycin sulphate dose-dependent manner of PrPSc distribution in fractions of the supernatants and pellets after shortly spin (Fig. 2). Compared with the preparation without streptomycin sulphate (Fig. 2, lane 8) that had no PrP signal in the pellet, small amounts of streptomycin sulphate (1.88 mm) started to induce PrPSc precipitation. Maximal PrPSc recovery in the pellet was achieved by the addition of streptomycin sulphate at a final concentration of 60 mm (Fig. 2, lane 2).
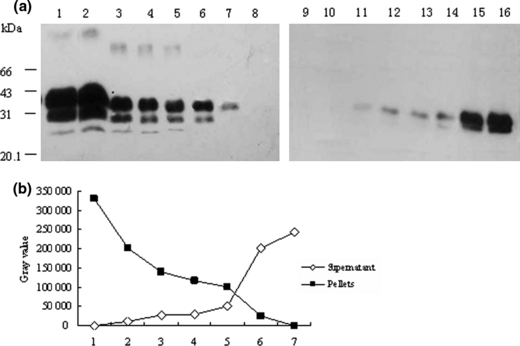
Western blot analyses for precipitation of PrPSc by various amounts of streptomycin sulphate. Ten microlitre hamster PrPSc stock sample was spiked into lysis buffer to 100 μl in each preparation. (a) Proteinase K (PK)-digested products were treated with streptomycin sulphate at a final concentration of 120 (lane 1 and 9), 60 (lane 2 and 10), 30 (lane 3 and 11), 15 (lane 4 and 12), 7.5 (lane 5 and 13), 3.75 (lane 6 and 14), 1.88 (lane 7 and 15) and 0 mm (lane 8 and 16). Lanes 1 to 8 are the pellets and lanes 9 to16 are the supernatants respectively. (b) Changes of the grey values of PrP-immunoreactive bands in the fractions of pellets and supernatants evaluated by a densitometry.
To address whether the precipitation of streptomycin sulphate on PrP had conformational specificity, 10 μl PrPC or PrPSc stock sample was spiked into lysis buffer to 200 μl containing 60 mm streptomycin sulphate respectively. The pellet of each reaction was separately recovered by centrifugation and subjected into Western blots. The results identified that both PrPSc (Fig. 3, lane 3) and PrPC (Fig. 3, lane 7 and 8) were efficiently precipitated by streptomycin sulphate.
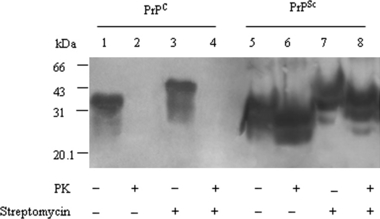
Detection for PrPC and PrPSc in Western blots after streptomycin sulphate precipitation. Ten microlitre hamsters’ PrPC and PrPSc stock samples treated without (lanes 1 and 5) or with (lanes 2 and 6) proteinase K (PK) were directly loaded as controls. Ten microlitre hamsters’ PrPC and PrPSc stock samples were spiked into lysis buffer to 200 μl in each preparation. Afte precipitated with streptomycin sulphate at a final concentration of 60 mm, individual pellet was resuspended and treated without (lanes 3 and 7) or with (lanes 4 and 8) PK.
To investigate the possible influence of streptomycin on the activity of PK digestion on PrPSc, 10 μl PrPSc stock samples were comparatively employed into lysis buffer in the final volume of 200 μl, in which PK digestion was conducted firstly and streptomycin precipitation subsequently, or streptomycin precipitation firstly and PK digestion latterly, or PK digestion and streptomycin precipitation simultaneously. Western blots of the pellets from each preparation did not reveal clear difference in the intensity of PrP-specific signal (Fig. 4, lane 5, 6 and 7). It indicated that PK activity on PrP was not influenced in the presence of 60 mm streptomycin sulphate.
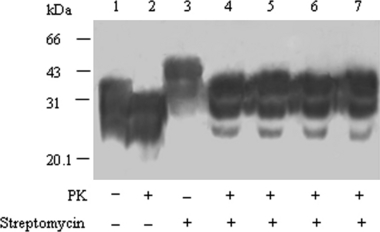
Comparative evaluation of the possible influence of streptomycin in the efficacy of proteinase K (PK) digestion. Ten microlitre hamsters’ PrPSc stock samples treated without (lane 1) and with (lane 2) PK were directly loaded as control. Ten microlitre hamsters’ PrPSc stock samples (lane 3, 5–8) or 10 μl PK-digested PrPSc stock sample (lane 4) were spiked into lysis buffer to 200 μl. Lane 3 and 4: directly employed into streptomycin precipitation. Lane 5: PK digestion performed before streptomycin precipitation. Lane 6: PK digestion and streptomycin precipitation performed simultaneously. Lane 7: streptomycin precipitation performed in advance and the pellet resuspended with 10 μl H2O and subsequently digested with PK.
To see the influence of pH value on the effectiveness of streptomycin precipitation for PrPSc, 10 μl hamsters’ PrPSc stock samples were spiked into lysis buffer to 200 μl in each preparation. The preparations were incubated with streptomycin sulphate at a final concentration of 60 mm under various pH values (from 5.0 to 11.0). After shortly spinning, the pellets were resuspended with H2O and subsequently digested with PK, then subjected into SDS-PAGE. Western blot identified that the input PrPSc were efficiently enriched after streptomycin precipitation in the preparations whose pH values were from 5.0 to 8.0 (Fig. 5a, lane 1–4), while the PrPSc signals in the reactions with pH 9.0, 10.0 and 11.0 decreased gradually (lanes 5–7). Analysis of grey values of PrP-positive signals after scanning showed an obvious pH value-dependent decrease manner under alkaline condition (Fig. 5b). These results indicated that the precipitating activity of streptomycin for PrPSc was quite efficient and stable under the neutral or weakly acidic condition, but dropped down along with the increase of pH value.
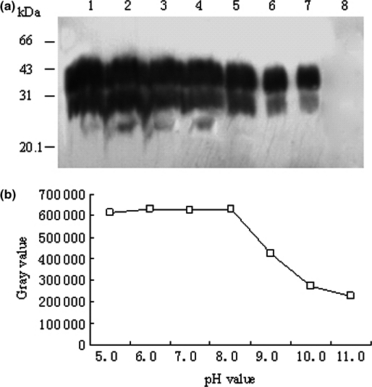
The influence of pH values on the efficacy of streptomycin precipitation. Ten microlitre hamsters’ PrPSc stock samples were spiked into lysis buffer to 200 μl in each preparation. Following streptomycin precipitation, the pellet was resuspended with 10 μl H2O and subsequently digested with proteinase K (PK). (a) Western blot of PK-digested products subjected into streptomycin precipitation under various conditions of pH values. Lanes 1 to 7 show pH value of the preparations 5.0, 6.0, 7.0, 8.0, 9.0, 10.0 and 11.0 respectively. Lane 8 shows the preparation without streptomycin sulphate. (b) Changes of the grey values of PrP-immunoreactive bands under various conditions of pH values evaluated by a densitometry.
Treatment of PrPSc with streptomycin sulphate increased the identifying sensitivity in lysis buffer and various mimic tissue samples
To seek whether treatment of PrPSc with streptomycin sulphate increased the identifying sensitivity in Western blots, different amounts of PK-digested hamster PrPSc stock sample from 0.08 to 10 μl were incubated with 60 mm streptomycin sulphate in 1 ml lysis buffer. After centrifugation, the pellets were resuspended for Western blotting. In parallel, same amounts of PK-digested PrPSc stock sample were directly loaded in SDS-PAGE used as controls. Analyses revealed that the lowest detectable amount of PK-resistant PrP signal in the control group was the preparation containing 0.60 μl PrPSc stock sample (Fig. 6a, lane 5), whereas that was 0.15 μl in the streptomycin sulphate group (Fig. 6b, lane 7). Clear di- and mono-glycosal PrPSc signals could be distinguished in the preparation containing 2.5 μl PrPSc stock sample when directly loaded (Fig. 6a, lane 3) and 0.6 μl PrPSc stock sample after streptomycin sulphate treatment (Fig. 6b, lane 5).
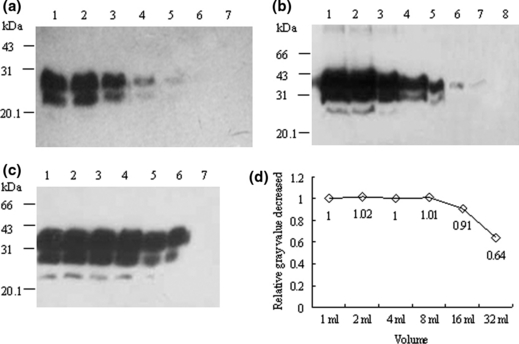
Detection limititations for PrPSc in Western blots before and after streptomycin sulphate precipitation in lysis buffer. (a) Proteinase K (PK)-digested hamster PrPSc stock samples directly loaded. Serial twofold dilutions of PrPSc stock samples in a final volume of 10 μl with lysis buffer were digested with PK and loaded directly in SDS-PAGE. Lanes 1–7 show 10, 5, 2.5, 1.25, 0.60, 0.30 and 0.15 μl PK-digested PrPSc stock samples respectively. (b) Serially twofold diluted PrPSc precipitated with streptomycin sulphate. After digested with PK, the serially twofold diluted hamster PrPSc stock samples were subjected into 1 ml lysis buffer containing 60 mm streptomycin sulphate. Lanes 1 to 8 represent 10, 5, 2.5, 1.25, 0.60, 0.30, 0.15 and 0.08 μl PK-digested PrPSc stock samples respectively. (c) PrPSc in twofold serially increased volumes of lysis buffer precipitated with streptomycin sulphate. Ten microlitre PK-digested PrPSc stock samples were employed into twofold serially increased volumes of lysis buffer containing 60 mm streptomycin sulphate. Lanes 1 to 6 represent 1, 2, 4, 8 16 and 32 ml lysis buffer respectively. Lane 7 shows the preparation of 10 μl PK-digested PrPSc stock samples in 1 ml lysis buffer without streptomycin sulphate as control. (d) Relative decreased recovery activity of PrPSc by streptomycin sulphate precipitation along with the increases of the volumes. The grey value of each PrP-immunoreactive band was evaluated by a densitometry. The relative grey value decreased of each preparation was calculated by division of the individual grey value with that of the preparation of 1 ml.
To evaluate the precipitating capacity of streptomycin sulphate on PrPSc, 10 μl PrPSc stock samples was transferred into serial enlarged volumes (from 1 to 32 ml) of lysis buffer containing 60 mm streptomycin sulphate, whereas the same amount of PrPSc stock sample was added into 1 ml lysis buffer without streptomycin sulphate as control. The PrPSc signals were detected in all preparations containing streptomycin sulphate (Fig. 6c, lane 1–6), but not in the preparation without streptomycin sulphate (lane 7). Compared with the grey value of PrPSc signal in the volume of 1 ml, incubation of streptomycin sulphate induced the same precipitating effects on PrPSc in the volumes of 2, 4 and 8 ml, and 91% and 64% decreased in the volumes of 16 and 32 ml (Fig. 6d).
To address the precipitating activities of streptomycin sulphate on PrPSc in brain tissue or body fluids, 10 μl hamster PrPSc stock sample was spiked into 1 ml hamster’s PrPC stock sample, 1 ml CSF and 10 ml urine respectively. Following streptomycin sulphate precipitation, the pellets were subjected to PK digestion and Western blotting. Contrast to the preparations without streptomycin sulphate that no positive signal was observed (Fig. 7a,b,c, lane 1), the specific PrPSc reactive signals were obviously detected in all preparations (lane 3). Comparisons of the intensities of the PrP-immunoreactive bands in the streptomycin-precipitated preparation with that produced by the same amount of directly loaded PrPSc demonstrated similar grey values, indicating satisfactory recovery efficiency.
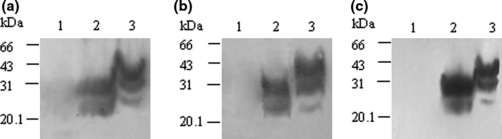
Streptomycin sulphate precipitation of PrPSc in the mimic tissue samples. Ten microlitre hamster PrPSc stock sample was spiked into 1 ml hamster PrPC stock sample (a), 1 ml human CSF (b) and 10 ml human urine (c) respectively. Following streptomycin precipitation, the pellet was resuspended with 10 μl H2O and subsequently digested with proteinase K (PK). Lane 1 in (a), (b) and (c) represents the preparations treated without streptomycin sulphate; lane 2 shows the 10 μl PK-digested hamster PrPSc stock sample directly loaded as control; lane 3 shows the preparations treated with 60 mm streptomycin sulphate.
Efficient precipitation of PrPSc from fCJD brain with streptomycin sulphate
To evaluate whether streptomycin sulphate could combine and precipitate the PrPSc from human brain homogenate, 20 μl human PrPSc stock samples (equal to 2 × 10−3 g brain tissue) prepared from a fCJD patient with seven extra octapeptide repeats insertion (Wang et al., 2007) were mixed with streptomycin sulphate and the pellets treated with or without PK were subjected to Western blotting. Distinct PrP-specific reactive singles were observed both in the preparations treated without and with PK (Fig. 8, lane 3 and 4) Compared with the PrP-immunoreactive bands produced by directly loading same amount of human PrPSc stock samples (lane 1 and 2), the PrPSc reactive signals in the streptomycin-precipitated preparations were remarkably enhanced.
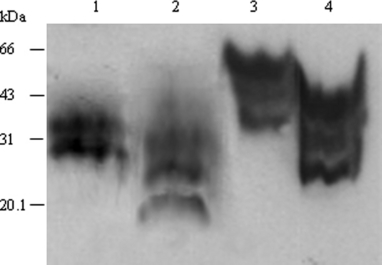
Streptomycin sulphate precipitation of PrPSc from human familiar Creutzfeldt–Jakob disease (fCJD) brain homogenate. Twenty microlitre human PrPSc stock samples digested without (lane 3) or with (lane 4) proteinase K (PK) were treated with streptomycin sulphate at a final concentration of 60 mm. Same amount of human PrPSc stock sample treated without (lane 1) or with (lane 2) PK were directly loaded as control.
Discussion
Protease-resistance disease-associated prion protein is the best-known biological marker for TSEs, hereby demonstration of PrPSc in human or animal tissues is essential for TSEs diagnosis (Prusiner, 1998; Ingrosso et al., 2002; Soto, 2004). One important limitation to this approach is the sensitivity of the detection system, because amounts of PrPSc high enough to be revealed by conventional methods are only present in the brain at the late stages of the disease. However, animal transmission studies show that infectivity is present at a relatively early stage of the incubation period and gradually increases in quantity as the disease progresses (Kimberlin and Walker, 1988). Here, we demonstrate that through the established streptomycin sulphate precipitation procedure of PrPSc, the detective sensitivity of PrP-specific Western blot is remarkably increased. It would be interesting to address whether this methodology could be useful to detect low levels of PrPSc in brain, peripheral tissues or biological fluid before onset of the disease. It may lead to developing an early, sensitive or non-invasive biochemical diagnosis technique for TSEs.
Among the presently used methods for PrPSc detection, Western blotting is the widely validated method, which offers the advantage of recognizing different forms of PrPSc through the analysis of the molecular mass and the relative abundance of di-, mono- and non-glycosylated bands. These parameters characterize the so-called PrP glycotype, a kind of ‘PrP signature’, which varies among different forms of TSEs (Collinge et al., 1996; Parchi et al., 1996; Cardone et al., 1999; Kuczius and Groschup, 1999). Although exposure of PrPSc to streptomycin sulphate consistently results in an apparent increase in the molecular weight of PrPSc, it seems not to change the ratios of PrPSc glycosylating patterns. Same as the directly loaded PrPSc, streptomycin-precipitated PrPSc show three major bands in SDS-PAGE, in which the di-glycosylated PrPSc is predominant and followed by mono-glycosylated one. Additionally, the electrophoresis pictures of streptomycin-precipitated PrPSc seem to be greatly improved. The non-glycosylated PrPSc from 263K-infected hamster brain, which is usually very weak or even unobservable in Western blot, become easily and repeatedly identifiable. Comparing the PrPSc electrophoresis patterns before and after streptomycin precipitation using more animal and human TSE strains will help to address the possibility for PrPSc typing with this technique.
Simply resuspending the streptomycin-precipitates with 2× loading buffer as Moussa et al. (2006) reported previously seems not be able to get satisfactory electrophoresis picture, as the three bands of PrPSc tended to migrate altogether in a smear-like distribution in the following Western blot initially. After we resuspended the pellets with 2×sample loading buffer containing 6 m GdnHCl or urea, the smear PrPSc reactive signals changed to much sharper three bands. From this observation, it suggests that application of GdnHCl or urea may disperse the streptomycin-induced PrPSc aggregation, leading to different glycosylated PrPSc molecules being easily separated in SDS-PAGE.
The binding of streptomycin sulphate with PrPSc seems quite stable, as apparently higher molecular mass of PrPSc present always in Western blotting, even after treated with 2×sample loading buffer containing 6 m GdnHCl or urea. The possible mechanism of interactions between streptomycin sulphate and prion protein is supposed to be through a hydrogen bond transfer between the guanidinium groups present on the streptomycin molecules and negatively charged amino acids present on the surface of the prion protein (Bencsik et al., 2006; Moussa et al., 2006). The precipitation of streptomycin sulphate on PrP seems not to be conformational specific, as our experiment identified that PrPC can be also precipitated in the presence of streptomycin sulphate. Although binding of streptomycin sulphate to PrPSc leads to a change in the sedimentation coefficient of PrPSc, the immunological reactivity of PrPSc with its specific antibody 3F4 is fully maintained. Additionally, streptomycin sulphate does not influence the effectiveness of PK digestion on PrP, which allows conducting precipitation and enrichment of PrPSc before, after or simultaneously with the procedure of PK digestion.
The pH value shows great impact on the streptomycin precipitating efficiency for PrPSc, thus when conducting the precipitation procedure, keeping suitable pH value is essential. As the pH value of the solution may influence the characteristics of PrPSc, it has been reported that under weakly acidic condition PrPSc is more likely to aggregate each other (Ma et al., 2007). Our data indicated that the precipitating activity of streptomycin for PrPSc was quite efficient and stable under neutral or weakly acidic condition. Relatively lager range of pH value (from pH 5.0 to 8.0) suitable for precipitation of PrPSc with streptomycin makes this methodology easy to be carried out.
The sensitivity and flexibility of this assay is an important advantage for the detection of low levels of PrPSc in brain, peripheral tissues or biological fluid. Our analyses revealed that the sensitivity of PrPSc detection by streptomycin sulphate precipitation is about fourfold higher than that by the direct Western blotting in a serial twofold dilutions of PrPSc stock sample from scrapie-infected hamsters, which is similar to Moussa et al. (2006) observation of about 4- to 16-folds more sensitive for PrPSc detection in a final volume of 100 μl serially diluted bovine BSE brain samples (Moussa et al., 2006). Subsequently, we further evaluated the detection limit of PrPSc from scrapie-infected hamsters in various volumes. Our data indicated that PrPSc may be detected by this protocol at concentrations which are approximately 100- to 1000-fold lower than that by the direct Western blotting. The simple protocol presented in the present study effectively extracted PrPSc from various samples into small quantities of pellets, thus providing a convenient way for the enrichment of PrPSc from large volumes of sample.
Sensitive methods are always required for TSE diagnosis, especially for the peripheral samples. The data obtained in this study indicate a brilliant usage for identifying PrPSc that may have, so far, evaded detection due to its low concentration. In fact, some special subtype of CJD, for example in patients with fatal familial insomnia, the amount of PrPSc is often 5- to 10-fold lower than that in sporadic CJD (Parchi et al., 1998). Tonsil, spleen, and lymph node tissues of vCJD, which are usually PrPSc positive are believed to contain much less PrPSc in the range of 0.1–15% of those found in brain (Wadsworth et al., 2001). Therefore, the sensitivity of diagnostic methods and the procedures to concentrate PrPSc become crucial. Concentration of PrPSc can be realized by some substances, such as Sarkosyl which can help precipitation of insoluble PrPSc by ultra-centrifugation, sodium phosphotungstic acid, trichloroacetic acid and a newly discovered plasminogen which can also precipitate PrPSc. However, most of those methods are time consuming or complicated. Nevertheless, the features of streptomycin binding with high affinity to PrPSc and high efficient precipitation for PrPSc by low-speed centrifugation might boost new hopes for preclinical diagnosis of TSEs. Western blot assay based on streptomycin sulphate precipitation, as a sensitive, specific, rapid and flexible protocol for PrPSc has the potential, alone or combined with other techniques, to detect low levels of PrPSc in the specimens not only from central nervous system, but also from peripheral organs or fluids.
Acknowledgements
This work was supported by Chinese National Natural Science Foundation Grants 30130070, 30571672 and 30500018, National Science and Technology Task Force Project (2003BA712A04-02) and EU Project QLRT 2000 01441.




