Generalized Fatal Cowpox virus Infection in a Cat with Transmission to a Human Contact Case
Summary
A 4-month-old female domestic shorthair cat was infected by a virus of the Poxvirus family. The animal developed a severe pneumonia and generalized ulcerating lesions of the skin. Histologically, typical eosinophilic intracytoplasmic inclusion bodies indicative of an Orthopoxvirus (OPV) infection were present. The lung showed grey-white to haemorrhagic nodular lesions with a central zone of complete necrosis of alveolar and bronchial tissue. Electron microscopy from skin and lung nodules revealed typical square-shaped OPV particles. Cultivation of the virus on chorio-allantoic membranes of embryonated chicken eggs resulted in haemorrhagic plaques. Restriction enzyme analysis, PCR and sequencing of the D8L gene identified the OPV isolate as a typical Cowpox virus. It was transmitted by the cat to a human contact person who developed a local nodular dermatitis at the inoculation site in association with signs of general infection and had an increase of OPV-specific neutralizing antibodies in paired serum samples.
Introduction
Poxvirus infections in cats were first described by Thomsett et al. (1978). Several virus strains have been isolated and well characterized (Bennett et al., 1989; Naidoo et al., 1992; Czerny et al., 1997). They all are members of the Orthopoxvirus (OPV) genus and belong to the species Cowpox virus (CPXV). The disease is rare but endemic to Europe and Western Asia. Skin alterations are characterized by typical pox-viral lesions, which normally heal spontaneously (Bennett and Baxby, 1995). There is good evidence that wildlife rodents are the reservoir in nature (Marennikova et al., 1977; Baxby et al., 1994; Czerny et al., 1996; Hazel et al., 2000). Severe illness or even lethal outcome due to CPXV-induced pneumonia occurs rarely in domestic cats (von Bomhard et al., 1989; Hinrichs et al., 1999), but is well known in non-domestic cats from zoological parks, especially cheetahs (Naidoo et al., 1992). Human infections usually result from direct skin affections causing single lesions at the inoculation site (Baxby, 1988; Baxby et al., 1994). However, generalized involvement of the skin and even death can occur in immunocompromised individuals or in association with systemic corticosteroid treatment for underlying allergic diseases (Czerny et al., 1991; Bennett and Baxby, 1995).
The case reported here demonstrates a lethal poxvirus infection in a 4-month-old cat with severe pneumonia and generalized skin lesions. The pathogen was also transmitted from the cat to the 18-year-old unvaccinated owner, who developed an ulcerative dermatitis at the inoculation site and signs of general infection. The virus was isolated from the cat's lung and skin nodules and could be clearly allocated to the species CPXV by PCR and DNA sequencing.
Materials and Methods
Pathology, sample preparation, histology, immunohistochemistry and electron microscopy
A 4-month-old cat which developed a generalized fatal skin infection was killed and examined by a pathological gross section. At necropsy, representative tissues were fixed by immersion in 10% neutral-buffered formalin, embedded in paraffin wax, cut at 4-μm thickness and stained with haematoxylin and eosin. Immunohistochemical examination was carried out with a bovine vaccinia antiserum known to cross react with CPXV and the streptavidin–biotin-complex technique according to standard protocols (Hinrichs et al., 1999). Negative contrast transmission electron microscopy was performed according to standard protocols with unfixed material from skin and lung nodules, cultured cells and plaques on chicken embryo chorio-allantoic membranes (Czerny and Mahnel, 1990; Czerny et al., 1994, 1997).
Cells and viruses
Chorio-allantoic membranes of 11-day-old chicken embryos and the permanent African green monkey kidney cell line MA-104 (microbiology associated) cultured in minimal essential medium and supplemented with 2% fetal calf serum were used to propagate the feline OPV isolate of the presented cat (TGD99 OPV FF). For virus isolation unfixed material from skin and lung nodules was grinded with ca. 2.0-ml phosphate-buffered saline and sterile sea sand. The homogenates were freeze thawed (−80/25°C) three times, transferred to a 12-ml Falcon tube (BD Biosciences, Heidelberg, Germany) and sonicated by a Branson sonifier for a few seconds. After low-speed centrifugation at 800-g for 30 s, the supernatant was passed through a 0.45-μm sterile filter. MA-104 cells were also used for propagation of the CPXV reference strain KR2 Brighton, vaccinia virus (VACV) Elstree, VACV Munich 1 and the human cowpox virus isolate TGD99 OPV Le (derived from J. T. van Oirschot, Lelystad, the Netherlands) which were used as controls.
Plaque-reduction test
Paired serum samples were taken from the 18-year-old son of the cat's owners within an interval of 14 days. After heat inactivation (56°C, 30 min), neutralizing antibody titres were examined by plaque-reduction tests (PIRT) performed by standard methods (Mayr et al., 1977; Czerny and Mahnel, 1990; Czerny et al., 1994). A 50% reduction of 100 plaque-forming units of VACV Munich 1 was considered positive and indicative of an OPV infection.
Species-specific differentiation by antigen-capture ELISA
Orthopoxviruses were differentiated by a monoclonal species-specific antigen-capture ELISA (Czerny et al., 1996). A combination of eight monoclonal antibodies (mAbs) derived against modified VACV Ankara (MVA), CPXV KR2 Brighton, monkeypox virus (MPXV) Copenhagen and ectromelia virus (ECTV) Munich 1 (Czerny and Mahnel, 1990; Czerny et al., 1994, 1997) was used as capture antibodies. They differentiated orthopoxviruses by species-specific variations of their antigenic sites localized on the 14 kDa fusion protein, the 32 kDa adsorption protein and the A-type inclusion body protein (ATIP) encoded by the open reading frames ORF A27L, D8L and A25L.
Extraction of viral DNA
The extraction of OPV-DNA from 500-μl cell culture aliquots was performed by standard procedures as outlined elsewhere (Meyer et al., 1994, 1997; Czerny et al., 2001). The DNA was stored at −20°C until use.
Polymerase chain reaction (PCR)
The amplification of the D8L gene encoding the 32-kDa adsorption protein in various OPV species was carried out with genus-specific primers (D8L-up-1: 5′-ATG CCG CAA CAA CTA TCT CCT-3′, D8L-low-1: 5′-CTA GTT TTG TTT TTC TCG CGA A-3′) binding to the highly conserved 5′- and 3′-termini of the D8L gene (Czerny et al., 2001). The PCR was performed using the ExpandTM High Fidelity PCR System (Roche Diagnostics, Mannheim, Germany). After incubation for 3 min at 95°C, amplification was achieved by 35 PCR cycles. Each cycle included a denaturation step at 95°C for 30 s, an annealing step at 55°C for 1 min and an extension step at 72°C for 1 min. The final elongation step was prolonged to 5 min to ensure complete extension of the amplification products. The PCR of the ATIP-gene fragment was carried out with the forward primer ATIP-up-1 (5′-AAT ACA AGG AGG ATC T-3′) and the reverse primer ATIP-low-1 (5′-CTT ACC TTT TTC TTT CTC-3′) according to previously published data of Meyer et al. (1994). Five microlitres of the PCR products was analysed by electrophoresis in a 1% SeaKem LE agarose gel (FMC BioProducts, Rockland, MA, USA). The gel was photographed and digitalized on a Gel Doc 1000 image analysing system (Bio-Rad, Munich, Germany).
Restriction enzyme analysis
The amplicons of the ATIP-PCR were digested with 10-U BglII or XbaI (New England BioLabs, Frankfurt, Germany) at 37°C for 8 h followed by electrophoresis in 3.5% NuSieve 3 : 1 agarose (FMC BioProducts, Rockland, MA, USA).
Cycle sequencing of the D8L-gene
PCR products were sequenced on the ALFexpress automatic sequencer (Amersham Biosciences, Freiburg, Germany) by cycle sequencing. A preceding purification of the amplicons with the Wizard® PCR Preps DNA Purification System (Promega, Mannheim, Germany) was necessary. Purified and 100-fold diluted template DNA was sequenced in both directions with Cy5-labelled primers using the ‘Thermo sequenase fluorescent labelled primer cycle sequencing kit with 7-deaza-dGTP’ (Amersham Biosciences, Freiburg, Germany) according to the manufacturer's instruction. The sequence determined was aligned to sequences of OPV reference strains and other mammalian poxvirus isolates by the dna star lasergene computer program (GATC, Konstanz, Germany).
Results
Clinical findings
A 4-month-old, female domestic shorthair cat was presented to the clinician with signs of acute upper respiratory infection and conjunctivitis. A few edematous papular and nodular lesions – which later ulcerated – were present on the paws of the forelimbs, nose and ears. Systemic involvement of the skin, ulcerated plaques on the tongue and dyspnoea were observed on days 5 and 6, when the cat was killed.
Human contact case
During the first 2 days after the presentation, the cat remained with their owners. On one occasion, the 18-year-old unvaccinated son was scratched at the inner site of the left forearm and subsequently developed a nodular to ulcerative dermatitis of approximately 1 by 2 cm diameter at the inoculation site. Signs of general infection (fever, nausea, anorexia, painful regional lymph nodes) were present and the lesion progrediated over the next 4 weeks before demarcation and healing started. Paired serum samples taken 1 and 5 weeks after the development of the primary lesion were tested in a PIRT for OPV-specific antibodies. The serum titre increased from 1 : 4 to 1 : 64 significantly indicative of an OPV infection.
Pathology of the cat
At necropsy, the skin showed generalized, roughly circular, ulcerated, nodular lesions, occasionally with wheel formation around a necrotic centre covered by a thick crust. Histologically, there was a full thickness necrosis of the epidermis variably extending into the superficial and deep dermis and involving the hair infundibula and adnexal glands. Formation of syncytial cells within the epidermis and infundibula was a constant feature but often obscured by necrosis. Only a few epidermal cells at the hyperplastic margins of the lesions contained eosinophilic intracytoplasmic inclusion bodies. The necrotic lesions were demarcated by marked perivascular to diffuse infiltrations of predominantly lymphocytes and eosinophilic granulocytes. Primary skin lesions were sparse and histologically characterized by severe ballooning degeneration of akanthocytes containing a single to multiple eosinophilic intracytoplasmic inclusion bodies (Fig. 1). Multiple ulcerated plaques were present throughout the oral cavity with necrosis of the oral mucosa extending deep into the underlying skeletal muscle. Primary lesions were sparse and consisted of ballooning degeneration of oral mucosal cells containing eosinophilic intracytoplasmic inclusion bodies. The nasal cavity was completely filled with mucopurulent exudate. Histologically, there was diffuse full thickness necrosis of the nasal mucosa extending to the underlying bone. Necrotic mucosal and glandular epithelial cells were preserved as intensively eosinophilic cell shadows. Only very small areas of the mucosa remained intact. The lung showed multiple grey-white to haemorrhagic, prominent, 3- to 9-mm round nodular lesions with a central zone of complete necrosis of alveolar and bronchiolar tissue surrounded by a zone with severe mixed inflammatory infiltration and multiple syncytial cells containing eosinophilic intracytoplasmic inclusion bodies. Adjacent to superficial lung nodules, there was hypertrophy and hyperplasia of serosal cells with single cell necrosis and eosinophilic intracytoplasmic inclusion bodies in a few cells.

Cat, skin, acute lesion, ballooning degeneration of akanthocytes containing eosinophilic intracytoplasmic inclusion bodies characteristic of poxviral infections. Haematoxylin and eosin stain.
Negative contrast transmission electron microscopy using unfixed tissues from skin and lung nodules revealed typical square-shaped OPV particles.
Immunohistochemically, strongly positive intracytoplasmic staining was observed in epithelial and glandular cells of the epidermis, hair infundibula, nasal and bronchiolar mucosa as well as pneumocytes, syncytial cells in the lung and pleural serosa.
Virus isolation
Specimens from the lung and skin nodules were used for virus isolation in MA-104 cells and on chorio-allantoic membranes of 11-day-old chicken embryos. Within 48 h after inoculation, plaques developed in the cell culture. Virus could be isolated from the lungs and skin lesions. The latter was used for further examinations. The chorio-allantoic membranes showed haemorrhagic plaques within 4 days after inoculation indicative of the presence of CPXV.
Characterization of TGD99 OPV FF based on species-specific antigenic sites
The feline isolate TGD99 OPV FF was compared with several OPV reference strains and virus isolates using an antigen-capture ELISA based on eight genus- and species-specific mAbs reactive with epitopes on the 14 kDa fusion-, the 32 kDa adsorption- and the A-type inclusion body protein. TGD99 OPV FF had an identical reaction pattern as a group, which contained VACV and CPXV together. Because of cross-specificity of the corresponding capture mAbs, this group cannot be further differentiated by antigenic sites alone. In this case, further molecular biological investigations are necessary to distinguish between vaccinia and cowpox virus isolates. For this reason, we used the D8L-PCR and DNA sequencing of amplicon. As an additional molecular biological genus-specific differentiation method between vaccinia and cowpox viruses, we used the ATIP-PCR and subsequent restriction enzyme analysis.
Characterization of TGD99 OPV FF by D8L PCR and DNA sequencing
The D8L genes of the cell culture adapted feline isolate TGD99 OPV FF were amplified by genus-specific primers. Gel electrophoretic analysis of the PCR products revealed a fragment size of 915 bp, identical to those of the corresponding reference strains CPXV KR2 Brighton and VACV Elstree (Fig. 2) and of the human isolate TGD99 OPV Le. To identify species-specific relationships, a phylogenetic tree based on the amino acid sequences of the 32 kDa adsorption proteins of orthopoxviruses was deduced (data not shown). The feline virus isolate TGD99 OPV FF was identified as a typical CPXV isolate when its D8L protein sequence was aligned to published D8L sequences and those obtained by our group in a comparative study with 29 reference strains and isolates from humans, cats, elephants, pigs, foxes, tapirs, etc. The D8L gene reference sequence data (carbonic anhydrase alpha) of VACV Copenhagen (NCBI GenBank accession no. NP_063770) were used in both studies for comparisons, as well as the corresponding gene homologues of CPXV KR2 Brighton ‘red’ (CPX125 gene; NCBI GenBank accession no. NP619909), ECTV (EVM097 gene; NCBI GenBank accession no. NP_671615), MPXV Zaire_96-I-16 (E8L gene; NCBI GenBank accession no. NP_536532), CMLV M96 (CMLV111 gene; NCBI GenBank accession no. NP_570501), and VARV major India – 1967 (F8L gene; NCBI GenBank accession no. NP_042142).
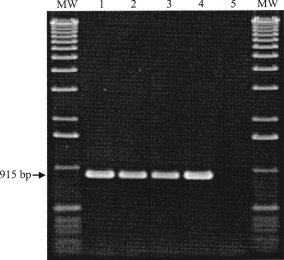
Identification of orthopoxviruses by electrophoresis of PCR products of the D8L gene; MW, molecular weight marker; lane 1: VACV Elstree; lane 2: CPXV KR2 Brighton; lane 3: human CPXV isolate TGD99 OPV Le; lane 4: TGD99 OPV FF; lane 5: water (negative PCR control).
Characterization of TGD99 OPV FF by A-type inclusion protein (ATIP) PCR and restriction enzyme analysis
PCR experiments with the primer pair ATIP-up-1 and ATIP-low-1 and template DNA derived from VACV Elstree and CPXV KR2 Brighton produced amplicons of the expected sizes of 1596 bp and 1672 bp, respectively (Meyer et al., 1994, 1997). The amplicons observed after amplification of DNA of the feline isolate TGD99 OPV FF-DNA as well as the recently isolated human CPXV isolate TGD99 OPV Le were of an identical size as that of VACV Elstree (Fig. 3). In order to differentiate between CPXV and VACV, the ATIP- gene fragments had to be further characterized by subsequent restriction enzyme analysis. The restriction endonuclease pattern of the amplified ATIP-gene fragments of TGD99 OPV FF and TGD99 OPV Le with BglII was similar to CPXV KR2 Brighton. They could be distinguished from VACV Elstree by the appearance of the two 522- and 466- bp fragments. VACV Elstree offered in this molecular weight range two bands of 470 and 444 bp (Fig. 4a). The only difference of the isolates TGD99 OPV FF and TGD99 OPV Le to CPXV KR2 Brighton was the loss of a hardly visible small fragment of ca. 70 bp (data not shown). This difference could better be recognized by the digestion of the ATIP-gene amplification products with XbaI (Fig. 4b). With one exception, the three cowpox viruses offered identical patterns. A fragment of 520 bp in CPXV KR2 Brighton revealed an approximately 70 bp smaller size of only 450 bp in TGD99 OPV FF and TGD99 OPV Le. These data clearly indicated that the feline isolate TGD99 OPV FF was a CPXV. Based on the data published for the ATIP-PCR differentiation (Meyer et al., 1994, 1997), it belonged to a CPXV subgroup distinct from the reference strain CPXV KR2 Brighton.
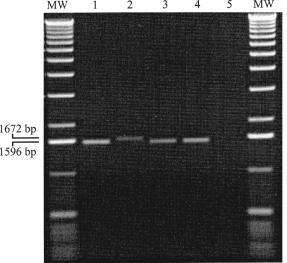
Identification of orthopoxviruses by electrophoresis of PCR products of the A-type inclusion protein gene fragment; MW, molecular weight marker; lane 1: VACV Elstree; lane 2: CPXV KR2 Brighton; lane 3: human CPXV isolate TGD99 OPV Le; lane 4: feline CPXV isolate TGD99 OPV FF; lane 5: water (negative PCR control).
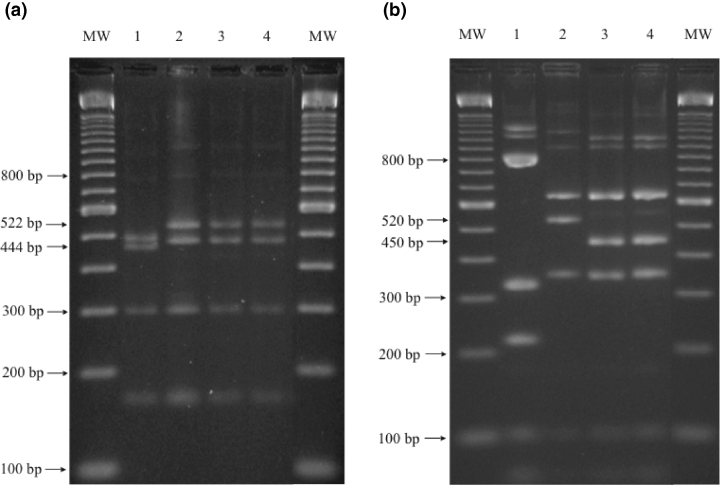
Identification and differentiation of vaccinia virus and cowpox virus strains by electrophoresis of BglII (a) and XbaI (b) digests of PCR products of the A-type inclusion body gene fragment; MW, molecular weight marker; lane 1: VACV Elstree; lane 2: CPXV KR2 Brighton; lane 3: human CPXV isolate TGD99 OPV Le; lane 4: feline CPXV isolate TGD99 OPV FF.
Discussion
The case described here represented a rather untypical example of a fatal cowpox virus infection in a domestic shorthair cat because of the simultaneous occurrence of severe dermatitis, necrotizing rhinitis and pneumonia. In general, CPXV-induced pneumonia in domestic cats is mild (Bennett and Baxby, 1995) and only a few cases of fatal CPXV-pneumonia have been reported so far (Mahnel et al., 1989; Hinrichs et al., 1999). As in this case, CPXV forms a potential risk to human health especially after the eradication of the smallpox virus in 1979 (World Health Organisation, 1980) and ceasing the vaccination programmes afterwards. Infection is usually mild and transient in healthy humans, but severe and even lethal infections can occur in immunocompromised individuals (Czerny et al., 1991).
The natural reservoir for CPXV has not been conclusively identified, although virus isolations and several seroepidemiological investigations conducted in Russia, Poland, Great Britain and Norway led to a wide acceptance that small rodents may serve as a natural reservoir (Marennikova et al., 1977; Kaplan et al., 1980; Sandvik et al., 1998; Hazel et al., 2000). The hunting behaviour of cats makes exposure to this reservoir probable (Baxby, 1977; Bennett et al., 1986). In an epidemiological study in Germany, a collection of 2173 feline serum samples without poxvirus history were examined for antibodies against OPV. This investigation revealed in 2% of the sera positive antibody titres (Czerny et al., 1996).
After isolation of the the feline OPV isolate TGD99 OPV FF from the case presented here, a species-specific distinction between vaccinia and cowpox virus strains was not completely possible by the differentiation of the antigenic sites in a rapid antigen-capture ELISA (Czerny and Mahnel, 1990; Czerny et al., 1994, 1997). For a final differentiation between CPXV and VACV, further molecular biological analyses were necessary. For this reason, the D8L and ATIP-gene sequences were amplified by PCR from the cell culture-adapted virus isolate. PCRs were not performed directly from homogenized clinical specimens, because of the limited material available from the pathological examination, it was necessary to save the virus isolate by tissue culture propagation. Interestingly, the amplified ATIP-gene fragment of TGD99 OPV FF had an identical length to that of VACV Elstree, distinct from the CPXV reference strain KR2 Brighton. This deviation is shared by numerous other recently isolated CPXVs, including isolates derived from a tapir and a pig (data not shown) as well as from a human OPV isolate TGD99 OPV Le used as a control in this study. Prior alignments of the ATIP–gene sequence of several OPV reference strains like CPXV KR2 Brighton or VACV Western Reserve resulted in ATIP-genes of varying length (Meyer et al., 1994). This was caused by a variable DNA region containing deletions of different sizes compared with CPXV KR2 Brighton. The variable region is flanked by conserved sequences, in which the primers were chosen. Different sized fragments could be amplified, allowing a differentiation between OPV species. The PCR primers ATIP-up-1 and ATIP-low-1 spanned a fragment of 1672 bp in CPXV KR2 Brighton, whereas in the case of the feline isolate TGD99 OPV FF, the human isolate TGD99 OPV Le and VACV Elstree fragments of 1596-bp were amplified. These 1596/1672 bp fragments were further characterized by restriction enzyme digestion. After cleavage with the restriction enzyme BglII, CPXV and VACV could be differentiated according to the two largest BglII fragments. Their sizes were 522 and 466 bp in the case of CPXV and 470 and 444 bp in the case of VACV (Meyer et al., 1994, 1997 ). When the ATIP amplicons were digested with the restriction enzyme XbaI, it was confirmed that CPXV TGD99 OPV FF and TD99 OPV Le were missing a fragment of ca. 70 bp. of the ATIP-gene. The absence of a fragment of 72 nucleotides is also known from other recent CPXV isolates and led to a classification of cowpox viruses in the subtypes A and B (Meyer et al., 1994). Subtype B CPXV-like TGD99 OPV FF and TGD99 OPB Le contained an XbaI fragment of an estimated size of 450 bp in contrast to subtype A isolates like CPXV KR2 Brighton where this fragment offered a length of 520 bp (Meyer et al., 1994).
The analysis of the D8L gene and protein sequences of the feline isolate TGD99 OPV FF confirmed its classification as a CPXV. The 915 bp ORF D8L codes for the 32 kDa adsorption protein, which is one of the most important proteins involved in early and late virus/host interactions (Maa et al., 1990; Rodriguez et al., 1992; Czerny et al., 1994, 1997). It is highly conserved among the genus Orthopoxvirus. The amino acid sequence of the 32 kDa adsorption protein of the feline isolate TGD99 OPV FF was aligned to the corresponding sequences of 29 OPV reference strains and recent isolates available from a comparative study of our group. A homology of 93–99% was found and a deduced phylogenetic tree indicated that variations in the 32 kDa adsorption protein sequences of different OPV were conserved in a species-specific dependence. On the basis of these data, the isolate TGD99 OPV FF was clearly identified as a CPXV, as it formed a group with further well-known CPXV reference strains and CPXV isolates.
Acknowledgements
The authors would like to thank F. van Mil, Department of Pathology, Faculty of Veterinary Medicine, Utrecht University, the Netherlands for immunohistochemical staining of paraffin-embedded tissues.




