Angiotensin receptors in the eyes of arterial hypertensive rats
Abstract.
Purpose: The aim of the present study was to determine whether the eye tissues of arterial hypertensive rats evince expression of angiotensin receptors (AT1 and AT2) as well as the novel Mas receptor, whose endogenous ligand is vasorelaxing Angiotensin (1–7) [Ang (1–7)].
Methods: Enucleated eyes from spontaneously hypertensive rats (SHR) and double transgenic rats harbouring human renin and angiotensinogen genes (dTGR) and their normotensive controls were used. Half of the rats were pretreated orally with an Angiotensin II (Ang II) type 1 receptor blocker (ARB). The eyes were snap-frozen in isopentane at −40° and stored at −70° for subsequent reverse transcriptase polymerase chain reaction (RT-PCR) analysis or in vitro autoradiography.
Results: The mRNA expression of AT1a and AT 2 as well as the novel Mas receptor was detected in all rat groups, being markedly higher in the retina than in the ciliary body. dTGR had significantly more receptors than SHR, but no direct relation to blood pressure level was seen. According to the autoradiography, treatment with ARB blocked a part of AT1 receptors but had no clear effect on AT2 receptors.
Conclusion: The novel Mas receptor was found by RT-PCR in eye tissue for the first time. Its specific ligand, Ang (1–7), may be involved in the regulation of intraocular pressure – as recently demonstrated by us – and in the pathogenesis of retinal diseases as a counter-regulatory component for the vascular and proliferative actions of Ang II. The results suggest that the density of AT1 receptors in the eye is independent of the blood pressure level of the animal.
Introduction
The renin-angiotensin system (RAS) is well known to be involved in regulating systemic blood pressure via the control of sodium balance, body fluid volumes and vascular tone (Hall 2003; Jackson 2006). In addition to the circulating RAS, there is a specific tissue-localized RAS (Kramkowski et al. 2006; Paul et al. 2006) that regulates long-term changes in various organs (Deschepper et al. 1986; Derkx et al. 1987) including the eye (Sramek et al. 1992; Danser et al. 1994; van Haeringen 1996; Wagner et al. 1996; Savaskan et al. 2004).
Drugs involved in RAS have been shown to be able to reduce intraocular pressure (IOP) in animal (Watkins et al. 1987; Giardina et al. 1990; Shah et al. 2000; Inoue et al. 2001; Wang et al. 2005) and human studies (Costagliola et al. 1995, 2000). Our study group has very recently found that intravitreally administered Angiotensin (1–7) [Ang (1–7)], a breakdown product of Angiotensin II (Ang II), reduces IOP even in the normotensive rabbit eye (Vaajanen et al. 2008a). In addition to these IOP-lowering effects, drugs involved in RAS have been reported to protect against diabetic retinopathy (EUCLIC Study Group 1997; Sjølie & Chaturvedi 2002).
The effects of angiotensins are exerted through specific heptahelical G-protein-coupled receptors (de Gasparo et al. 2000; Jackson 2006). Ang II receptors in the cardiovascular system are divided into three main subtypes: Ang II type 1 (AT1), type 2 (AT2) and type 4 (AT4) receptors. In addition to these three classical receptor types, there is the recently discovered Mas receptor. It is also a G-protein-coupled receptor encoded by the Mas protooncogene. It mediates a number of the positive cardiovascular effects of Ang (1–7) – e.g. vasodilatation, antiproliferation, antifibrosis – and it has a counter-regulatory role in fluid volume homeostasis for Ang II and AT1 activation (Santos et al. 2000, 2003).
The main aim of the present study was to establish the presence of the novel Mas receptor for vasorelaxing and antiproliferative peptide Ang (1–7) by using reverse transcriptase polymerase chain reaction (RT-PCR). The other aim was to quantify the expression profile of the AT1 and AT2 receptors in the ocular tissues of two types of arterial hypertensive rats and their respective controls with or without antihypertensive treatment by using an autoradiography method.
Materials and Methods
Animals
The eyes used here were obtained from animals involved in our previous study (Vaajanen et al. 2008b), in which the relationship between the development of hypertension and IOP was investigated using two arterial hypertensive rat strains and their normotensive control animals. The hypertensive animals were: male spontaneously hypertensive rats (SHRs) purchased from Charles-River (Charles River Wiga GmbH, Sulzfeld, Germany) aged 15 weeks and weighing 308 ± 5 g [mean ± standard error of the mean (SEM), n = 8]; and double transgenic rats harbouring human renin and human angiotensinogen genes (dTGR; Biotechnology and Animal Breeding Division, Füllingsdorf, Switzerland), aged 7 weeks and weighing 236 ± 6 g (mean ± SEM, n = 8). Their respective age-matched arterial normotensive controls were Wistar Kyoto (WKY; Charles River Wiga GmbH) weighing 350 ± 13 g (mean ± SEM, n = 8) and Sprague Dawley rats (SD; Taconic Europe A/S, Ry, Denmark) weighing 286 ± 5 g (mean ± SEM, n = 8). Half of each group was treated with oral AT1 receptor antagonist: olmesartan medoximil 10 mg/kg/day (Olmetec®; Leiras, Turku, Finland) for SHR and their WKY controls and valsartan 30 mg/kg/day (Diovan®; Novartis, Basel, Switzerland) for dTGR and their SD controls. Two different ‘sartans’ were used because dTGR and their controls were obtained from another, concomitant (cardiovascular) study carried out by our research group in addition to the original study of SHR and WKY rats, and treated according to a study protocol of their own. Tablets were ground and mixed in the food. At the end of the treatment (10 weeks for SHR and WKY and 3 weeks for dTGR and SD) the rats were killed with CO2/O2 (AGA, Riihimäki, Finland) and decapitated. The short lifespan and early death of dTGR because of severe hypertension and its complications were the reasons for the difference in ages and duration of antihypertensive treatment.
In our previous in vivo study with these same rats, a slight positive relationship (p ≤ 0.05) between developing blood pressure and IOP in SHR was seen during 8 weeks’ follow-up, while the high baseline IOP in arterial hypertensive dTGR fell drastically in their short follow-up period. At the end of that in vivo study the eyes were enucleated immediately after death and snap-frozen in isopentane at −40° and stored at −70° for RT-PCR analysis and in vitro autoradiography. Blood pressure and IOP in each rat group prior to taking eye samples are shown in Fig. 1.
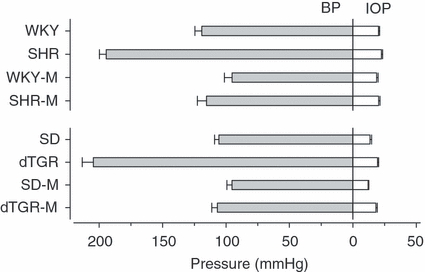
Blood pressure (BP) and intraocular pressure (IOP) in hypertensive rats and their controls in the original in vivo study prior to taking the eye samples for the present study. SHR, spontaneously hypertensive rats; WKY, normotensive Wistar Kyoto rats; dTGR, hypertensive rats harbouring human renin and angiotensinogen genes; SD, normotensive Sprague Dawley rats; M, medicated with oral angiotensin II receptor type 1 blocker. Mean ± standard error of the mean (SEM), n = 8 (SHR/SHR-M and WKY/WKY-M), n = 15 (dTGR), n = 14 (dTGR-M), n = 5 (SD), n = 6 (SD-M).
Ethics
All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals and the study protocol was approved by the National Animal Ethics Committee in Finland.
Real-time quantitative RT-PCR
Enucleated whole frozen eyes were embedded in OCT™ compound (Tissue-Tec®, Sakura, Japan) cornea upwards and 150 μm vertical sections of the whole eye were cut using a cryostat (−19°). The presence of mRNA of AT1a, AT2 and Mas receptors was determined at the level of the ciliary body and the level of the anterior retina without ciliary tissue (Fig. 2).
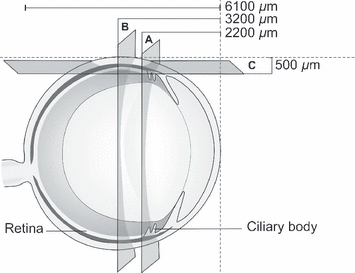
A schematic illustration of the rat eye. Two vertical sections were used for reverse transcriptase polymerase chain reaction analysis: (A) level of ciliary body and (B) level of anterior retina. Horizontal sections were used for quantitative in vitro autoradiography analysis (C).
RNA extraction and cDNA synthesis from tissue sections were performed as described previously (Lakkisto et al. 2002). The expression of AT1a, AT2 and Mas receptor mRNAs and housekeeping control 18S were studied by real-time quantitative RT-PCR using the ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, California, USA). The following primer sequences were used: AT1a forward 5′-GGCAGCCTCTGACTAAATGGC-3′ and reverse 5′-ACGGCTTTGCTTGGTTACTCC-3′; AT2 forward 5′-TGTCTGTCCTCATTGCCAACA-3′, reverse 5′-TTCATTAAGGCAATCCCAGCA-3′ and probe 5′FAM-TCAGAACCATTGAATACTT-MGB; and Mas forward 5′-TCATGTGTATTGACAGCGGAGAA-3′ and reverse 5′-CACTAACATGAGCGGAGTGAAGA-3′. PCR reactions for AT1a and Mas receptors were performed in duplicate in a 25-μl final volume containing 1X SYBR Green Master mix (Applied Biosystems) and 300 nm of primers. PCR reactions for AT2 receptor and 18S were performed in duplicate in a 25-μl final volume containing 1X TaqMan Master mix (Applied Biosystems) and 300 nm of AT2 receptor primers and 150 nm of AT2 probe, or 1X 18S TaqMan Gene Expression Assay primer and probe mix (Hs999999_s1, Applied Biosystems). PCR cycling conditions were 10 min at +95° and 40 cycles of 20 seconds at +95° and 1 min at +60°. Data were analysed using the absolute standard curve method, as described elsewhere (Lakkisto et al. 2002). 18S was used for normalization of results.
In vitro autoradiography
Enucleated whole frozen eyes were embedded in OCT™ compound (Tissue-Tec®), the cornea facing forward. Horizontal sections (20 μm) were cut through the ciliary body with a cryostat (−19°). In vitro autoradiographs of AT1 and AT 2 receptors were taken in the retina and in the ciliary body with the radioligand 125I-[Sar1-Ile8]Ang II, as described elsewhere (Zhuo et al. 1999; Stewen et al. 2003) (Fig. 2). Optical densities were quantified by the AIDA computer image analysis system (AIDA 2D densitometry) coupled to a FUJIFILM BAS-5000 photoimager (Tamro Oy, Vantaa, Finland). Specific binding was calculated as total binding minus non-specific binding. The effect of oral antihypertensive treatment with ARB on receptor density was evaluated.
Statistics
The results are reported as mean ± SEM. For statistical analyses, t-tests (SigmaPlot version 10.0; Systat Software Inc., San Jose, California, USA) were used. Differences in receptor expression/densities between ciliaris and retina (paired t-test) and between rat groups (unpaired t-test) were considered significant at p ≤ 0.05.
Results
RT-PCR
Overall, the mRNA expression of AT1a, AT2 and Mas receptors was markedly higher in the retina than in the ciliary body in every rat group (Fig. 3). There was no clear difference in AT1a receptor expression between different rat strains, but hypertensive dTGR had higher receptor expression compared to SHR. The expression of the novel Mas receptor was lower than that of AT1a, but higher than that of AT2 receptors in all groups except WKY rats.
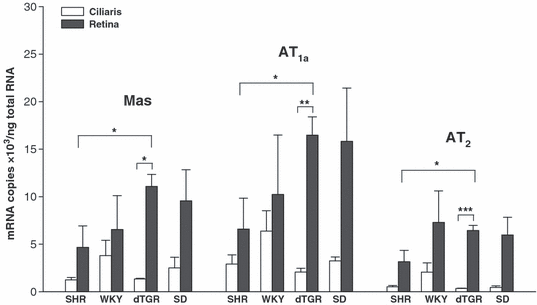
Expression of Mas, AT1a and AT2 receptors (mRNA) in the ciliary body and the retina of different rat strains (four animals in each group) measured by reverse transcriptase polymerase chain reaction. Abbreviations as per Fig.1. Mean ± standard error of the mean (SEM). *p ≤ 0.05, **p < 0.01, ***p < 0.001, retina versus ciliary body and SHR versus dTGR.
Because treatment with ARB did not systematically influence the expression of mRNA for Mas receptors or that of AT1a or AT2 receptors, the results from non-treated and treated rat groups were combined for RT-PCR analysis. Only treated SHR animals evinced slightly but not significantly lowered expression of AT1a receptors in both tissues evaluated.
Autoradiography
The densities of AT1 and AT1 receptors in the retina were overall higher compared to the respective ciliaris in every rat group, in accordance with the results of the RT-PCR analysis (Fig. 4A). There was no clear difference in AT1 receptor densities between different rat strains, but ARB-medicated animals had lower AT1 density, especially in hypertensive dTGR. The density of AT2 receptors was overall smaller than that of AT1 receptors in every rat group (Fig. 4B). An example from the autoradiography analysis is presented in Fig. 5.
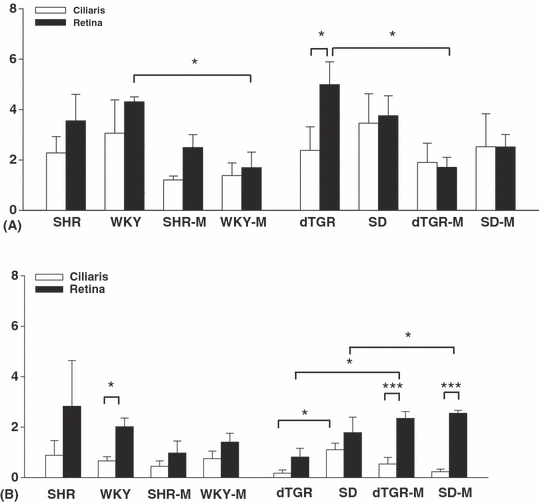
Densities in arbitary units of AT1 (A) and AT2 (B) receptors in the retina and the ciliary body (from four animals in each group) measured by quantitative in vitro autoradiography. Mean ± standard error of the mean (SEM). M, medicated with oral angiotensin II receptor type 1 blocker. *p < 0.05, ***p < 0.001, retina versus ciliary body and non-medicated versus medicated rat groups.
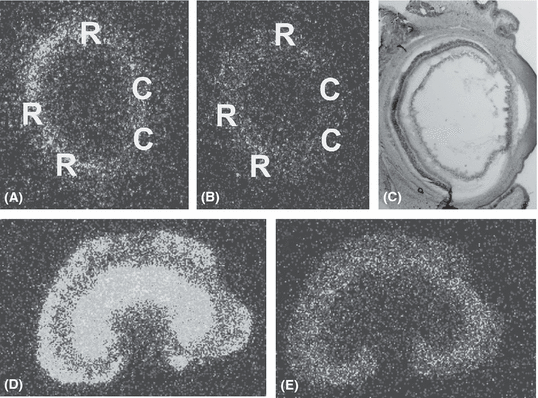
Quantitative in vitro autoradiography analysis in the eye of a non-treated spontaneously hypertensive rat. (A) Density of AT1 receptors, (B) density of AT2 receptors, (C) toluene blue staining of the respective eye section, 25 × magnification. C, ciliary body; R, retina. Densities of AT1 (D) and AT2 (E) receptors in the respective kidney sample.
A difference was seen between ARB-treated and non-treated animals in this analysis. The apparent density of AT1 receptors in the retina in treated animals was approximately 30% lower in SHR and SD rats and about 60% lower in dTGR and WKY compared with non-treated animals. This tendency was also seen in the ciliary body. Densities of AT1 and AT2 receptors and the respective blood pressure values in individual animals are presented in Fig. 6. The results suggest that the density of AT1 receptors in the eye is independent of the blood pressure level of the animals, but more dependent on oral ARB treatment. Medicated dTGR and WKY had significantly lower retinal AT1 density compared to the non-treated animals. Medication with ARB did not systematically influence the density of AT2 receptors but medicated dTGR had more AT2 receptors, especially in the retina (p < 0.05).
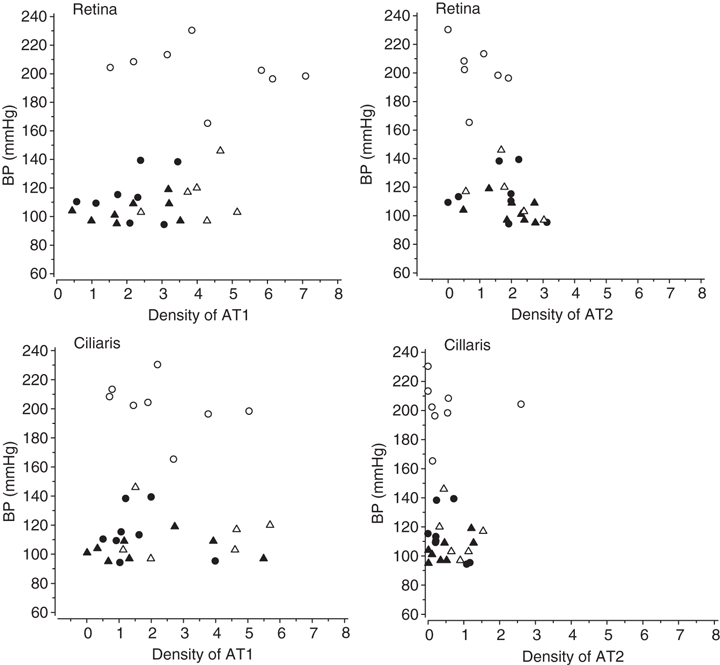
Blood pressure (BP) and angiotensin receptor densities in individual animals. Open circle, non-treated hypertensive rat; filled circle, hypertensive rat treated with Angiotensin II (Ang II) type 1 receptor blocker (ARB); open triangle, non-treated normotensive control; filled triangle, ARB-treated normotensive control.
Discussion
The present study focuses on angiotensin receptors in the eyes of arterial hypertensive animals. Abnormal blood pressure is a known risk factor for some ocular diseases such as glaucoma (Tielsch et al. 1995; Bonomi et al. 2000) and retinopathy (Luo & Brown 2004; Walsh et al. 2006), although the exact pathophysiological mechanism underlying these disorders is unclear. Hypertension has been reported to be related to high IOP (Dielemans et al. 1995), although observations to the contrary have also been reported (Leske et al. 1995). According to the vascular theory, blood pressure and especially low perfusion pressure are linked to the progression of glaucoma damage (Flammer et al. 2002). High blood pressure can cause a number of direct vascular changes in the retina, leading to poor vision (Walsh et al. 2006).
The RT-PCR results of this study indicate for the first time the presence of the novel Mas receptor in eye tissue. The Mas receptor and AT1a and AT2 receptors were found in all rat strains, being more pronounced at the level of the retina than that of the ciliary body. mRNA of these receptors was determined from slices cut from a whole eyeball at the level of the ciliary body and at the level of the retina without ciliary tissue. It was thus not possible to establish more specifically the type of cells in which each receptor was localized. Interestingly, the retinal mRNA expression of all angiotensin receptors was higher in dTGR than in SHR, while the arterial normotensive control animals also yielded high values of mRNA of AT1 receptors, indicating a strain-dependent variation of as yet unclear significance. No clear effect of oral antihypertensive treatment on mRNA expression of receptors was observed in RT-PCR analysis. According to other investigators, e.g. Zhou et al. (2005, 2006), treatment with AT1 receptor antagonists blocked (cerebrovascular) AT1 receptors in SHR with no significant effect on receptor protein and mRNA expression. These data support our decision to combine the results of untreated and treated animals in RT-PCR analysis.
The autoradiography results suggest that the receptor densities in the eye were independent of the blood pressure level of the animals, but more dependent on oral ARB treatment. The AT1 receptor blockade in treated animals was most obvious in the retina of dTGR, but the same tendency was also seen in other medicated rats. ARB treatment did not influence AT2 receptor density systematically but medicated dTGR had higher receptor levels, especially in the retina. Observations consistent with this (Zhou et al. 2006) but also contrary (Nishida et al. 2008) have been reported. There was a big difference between results of mRNA expression and ‘true’ receptor density. We have no clear explanation for this, but the RT-PCR method measures mRNA for receptors in the tissues so the ‘tools for receptors’ were found in all rat strains in retina and ciliaris (although in the case of receptor density the differences were less obvious). The density of the novel Mas receptor could not be examined by in vitro autoradiography because of a lacking methodology.
Ang II receptors are classically divided into three main subtypes. Most known biological effects of vasoactive Ang II are mediated by the AT1 receptors in cardiovascular and other target cells, which are specifically blocked by AT1 receptor antagonists, widely used as antihypertensive drugs –‘sartans’ (de Gasparo et al. 2000; Burnier 2001). In rodents, AT1 receptors are further divided into AT1a and AT1b receptors, having similarities in their ligand binding and activation properties but differences in their tissue distribution (Kakar et al. 1992; de Gasparo et al. 2000). There is previous evidence regarding the expression of AT1a in rat ocular tissue (Murata et al. 1997; Wheeler-Schilling et al. 1999; Mizoue et al. 2006). AT2 receptors are characterized less well than AT1 receptors, but they are considered as cardiovascular-protective receptors that antagonize the effects of Ang II mediated via AT1 receptors. They have been identified in eyes in only a few studies (Senanayake et al. 2007). AT4 receptors are not determined in detail, but they are known to be involved in cardiovascular pathology (Ruiz-Ortega et al. 2007). In addition to these three classical receptor types there is the recently discovered Mas receptor, which has now been determined in ocular tissue for the first time.
The Mas receptor was found a couple of years ago, first in the mouse kidney and subsequently in other organs (e.g. heart, brain and vasculature) (Santos et al. 2003; Iwata et al. 2005). In vivo Mas receptor acts as an antagonist to the AT1 receptor, and in addition Mas can hetero-oligomerize with the AT1 receptor and by doing so inhibit the actions of Ang II (Kostenis et al. 2005). There are no previous reports concerning Mas receptor expression in ocular tissues, although our recent functional study using Mas receptor agonist, Ang (1–7), and antagonist suggested its existence (Vaajanen et al. 2008a, 2008c). Ang (1–7), a heptapeptide formed from Ang II (Welches et al. 1993; Brosnihan et al. 1996; Muthalif et al. 1998; Santos et al.2000; Kucharewicz et al. 2002), is an endogenous ligand for this receptor type (Santos et al. 2003). It has been found very recently in the human retina (Senanayake et al. 2007).
To summarize, we have previously found a functional Mas receptor when intravitreally administered Ang (1–7) lowered IOP in rabbits. This effect was abolished by A-779 (=Mas receptor antagonist) (Silva et al. 2007) and partially by PD123,319 (=AT2 receptor antagonist) (Ford et al. 1996), but the AT1 antagonist (olmesartan) did not antagonize it. In most situations, Ang (1–7) and Ang II exert opposing actions, suggesting a primary role for Ang (1–7) as a counter-regulatory component for the vascular and proliferative actions of Ang II (Iwata et al. 2005; Kostenis et al. 2005). The release of nitric oxide and prostaglandins (Seyedi et al.1995; Brosnihan et al. 1996;Santos et al. 2000) might explain the lowering of IOP by Ang (1–7). Based on such and the present findings, it would seem justifiable to assume that the RAS with its different components not only regulates blood pressure but is also involved locally in the regulation of IOP and may have a role in retinal vascular diseases.
Conclusion
To the authors’ knowledge, the present findings and our previous observation of an oculohypotensive effect of Ang (1–7) constitute the first evidence that Mas receptors are expressed in eye tissue. Vasorelaxing Ang (1–7) is considered to be an endogenous ligand for the Mas receptor, which is distinct from the classical AT1 and AT2 receptors. The receptor density of AT1 and AT2 measured by autoradiography was not related to the systemic hypertension but more to the ARB medication.
Acknowledgements
This study was supported by the following Finnish grants: The Päivikki and Sakari Sohlberg Foundation, The Hilda and Evald Nissi Foundation, The Eye Foundation, The Sigrid Jusèlius Foundation and The Medical Research Funds of Helsinki University Hospital. The authors thank Prof. Eero Mervaala from Helsinki University and Dr Dominik Müller from Berlin for providing dTGR rats for this study, M.Sc. Sole Lätti for help in illustrations, Ms Hanna Wennäkoski, Ms Anne Reijula, Ms Anneli von Behr, Ms Marja Mali, Ms Jaana Tuure, Ms Riina Hatakka, M.Sc. Riikka Kosonen and Mr Jarkko Lakkisto for skilful technical assistance.
The main findings of this study were presented as poster presentations at the International Endothelium, Vasoactive Factors and Inflammation Symposium, Tampere, Finland on 24–27 June 2008 and at the World Ophthalmology Congress, Hong Kong, 28 June–7 July 2008.




