Retinal arterioles have impaired reactivity to hyperoxia in type 1 diabetes
Abstract.
Purpose: Diabetes has adverse effects on the retinal microvasculature. The purpose of this study was to compare the effects of inhalation of hypoxic, hyperoxic and normoxic–hypercapnic gas mixtures on retinal vessel diameter in people with and without diabetes.
Methods: Sixty-one participants (aged 24–50 years) 29 with (male : female ratio 2.6 : 1) and 32 without (male : female ratio 0.7 : 1) diabetes, inhaled hypoxic, hyperoxic and normoxic–hypercapnic gas mixtures for 3–5 mins. The diameters of arterioles and venules were measured using digital retinal images taken before and after gas inhalation.
Results: There was no significant difference in the diameters of arterioles and venules prior to gas inhalation in people with and without diabetes. Inhalation of the hyperoxic gas mixture caused a statistically significant decrease in arteriolar and venular diameters without altering mean arterial pressure significantly. Arteriolar vasoconstriction in response to the hyperoxic gas mixture was significantly reduced in people with diabetes (3.95% versus 7.75%; p = 0.04), but venular vasoconstriction did not differ significantly. A hypoxic gas mixture caused increased arteriolar and venular diameter and a normoxic–hypercapnic gas mixture had no significant effect on vessel diameter. Responses to hypoxic and normoxic–hypercapnic gas did not differ significantly between diabetes and non-diabetes subjects.
Conclusions: Type 1 diabetes impairs retinal arteriolar responses to hyperoxia. Abnormalities in retinal arteriolar reactivity in response to oxygen may play a role in the development of diabetic retinopathy and this technique may represent a simple means of identifying early abnormalities in the reactivity of retinal arterioles in diabetes.
Introduction
Diabetes is a common cause of blindness and visual loss in the working-age population in the Western world (Klein & Klein 1983; Sjølie & Green 1987). With time, 80% of patients with type 1 diabetes develop retinopathy and up to 40% of these suffer from severe sight-threatening retinopathy (Klein et al. 1984; Diabetes Control and Complications Trial Research Group 1993; Sjølie et al. 1997). However, the underlying molecular mechanisms linking diabetes to the development of retinopathy remain unclear (Frank 2004).
Under normal conditions, the autoregulation of the retinal circulation serves to maintain a relatively constant blood flow to the retina despite changes in perfusion pressure (Riva et al. 1986; Robinson et al. 1986). Autoregulation is mediated by compensatory changes in arterial and arteriolar diameter, a response that also protects the microvasculature from blood pressure-induced damage. Diabetes impairs retinal autoregulation (Sinclair et al. 1982; Dumskyj & Kohner 1999; Ciulla et al. 2002) and this may account for the detrimental effects of the combination of high blood pressure and hyperglycaemia in the development of retinopathy. Other factors including arterial pO2, pCO2, endothelin-1 and nitric oxide may also influence retinal arteriolar tone and blood flow (Dallinger et al. 2000; Chan et al. 2000; Chapman et al. 2000) and responses to some of these stimuli may be affected by diabetes (Chan et al. 2000). Studies in dogs with diabetes (Gardiner et al. 1994) have also shown that, along with pericyte destruction in capillaries, there is a loss of smooth muscle cells in retinal arterioles and this may result in impaired vasomotor responses.
The reactivity of retinal blood vessels can be tested by a number of methods (Grunwald et al. 1984; Dumskyj & Kohner 1999; Chapman et al. 2000; Jeppesen et al. 2004). We used inhalation of gases (hypoxic, hyperoxic and normoxic–hypercapnic mixtures) as a non-invasive means of testing if the reactivity of retinal arterioles and venules was impaired by type 1 diabetes.
Materials and Methods
Participants
The Danish Twin Register was established on the back of the Danish Civil Registration System (Kyvik et al. 1995). The ‘new’ part of the register includes all twin pairs (20 888) born between 1 January 1953 and 31 December 1982. From these, one or both individuals from 102 pairs were identified as having type 1 diabetes, based on World Health Organization (WHO 1980) criteria and fasting C-peptide. Of these, 47 individuals from the twin pairs agreed to participate in this project and an additional 14 healthy, age-matched volunteers were recruited from among hospital staff and medical students. In total, 61 persons participated, 29 of whom had diabetes (all were twins) and 32 of whom did not (18 twins and 14 volunteers).
Procedure
Three different test gases (Table 1) were inhaled by participants in a fixed sequence of hypoxia, normoxic hypercapnia and hyperoxia. Hypoxic and hyperoxic gas mixtures were administered for 5 mins and the normoxic–hypercapnic gas mixture for 3 mins. There was a 10-min washout period between the administrations of different gases. The optimal durations of gas exposure and washout period were established in preliminary studies (data not shown) to minimize possible interaction between gas exposures and carryover effects.
| Composition | Gas 1 (hypoxia) | Gas 2 (hypercapnia) | Gas 3 (hyperoxia) |
|---|---|---|---|
| Oxygen, % | 15 | 21 | 100 |
| Nitrogen, % | 85 | 74 | 0 |
| Carbon dioxide, % | 0 | 5 | 0 |
| Exposure time, mins | 5 | 3 | 5 |
Gas mixtures were administered through a one-way mouthpiece connected to a 100 l Douglas bag, pre-filled with the appropriate gas mixture. A nose clip was used to allow only oral breathing. Before and during each gas inhalation, sitting heart rate, blood pressure, CO2 and O2 levels and blood O2 saturation were measured. Heart rate (HR) and blood pressure were measured in the left arm using an automated device (Omron M4, HEM-722C1-E; Omron Healthcare Europe BV, Hoofddorp, the Netherlands). Mean arterial pressure (MAP) was calculated using the equation: MAP = ([2 × diastolic blood pressure] + systolic blood pressure)/3. CO2 and O2 levels were measured using a capnograph (Capnomac®; Datex-Ohmeda, Inc., Madison, WI, USA). Oxygen saturation was measured by a pulse oximeter (Ohmeda Biox 3700e; Datex-Ohmeda, Inc.) attached to the middle finger of the left hand.
Retinal images and geometric analysis
Digital red-free, disc-centred, retinal images (1024 × 1024 pixels) taken in the right eye were acquired using a 30-degree field of view, before and after each gas inhalation period using a Canon CF-60 UVi fundus camera connected to a monochrome digital camera (Basler Vision Technologies, Basler AG, Germany).
Vessel diameters were measured using operator-directed image analysis software purpose-written in Matlab 5.2 (MathWorks Inc., Natick, MA, USA) and based on the sliding linear regression filter (SLRF) technique (Chapman et al. 2001). Diameters were measured in pixels (absolute diameters were not estimated on the basis of the optical characteristics of the eye because relative change was the principal outcome measure, but 1 pixel is equivalent to ∼ 5 μm). The vascular trees for analysis were identified as arteriolar or venular by the operator and measurements were made on selected vessel segments over a length that corresponded to approximately one-sixth of the disc diameter (Fig. 1). Vessel segments were chosen using a concentric grid (Fig. 1) to ensure that repeated measures were made at similar locations in the vascular tree. Sites of vessel branching, overlap or poor resolution of vessel edges were avoided. Between 15 and 25 cross-sectional measurements of diameter were taken across each segment and the mean diameter calculated. All major retinal vessels were measured. The diameter was measured and the percent change (Δ) in the diameter of the vessels was calculated for each inhaled gas.
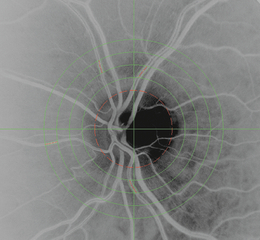
A digital, red-free, disc-centred retinal image highlighting some arterioles used for analysis.
Reproducibility
Six normal individuals were examined on two different occasions with intervals of 7–22 days between them. Bland–Altman plots with limits of agreement (LOA) were used to evaluate the reproducibility (Bland & Altman 1986). The mean difference was 0.11 pixel (LOA − 0.98 to 1.2), showing that the method has a high reproducibility.
Statistical analysis
Demographic data are expressed as medians (range). As the majority of the participants were twins, robust regression was used for the analysis to allow for clustering (Rogers 1993) and results are presented as means (95% confidence intervals) derived from robust regression. A p-value < 0.05 was considered statistically significant. Statistical analysis was performed using stata Version 9 (StataCorp LP, College Station, TX, USA).
Ethics
The study was approved by the local research ethics committee. Fully informed written consent was obtained from each individual and the research was conducted in accordance with the principles of the Declaration of Helsinki.
Results
The two groups, consisting, respectively, of people with and without diabetes, did not differ significantly in terms of age, sex or blood pressure (Table 2). Further, the arteriolar and venular diameters measured at baseline did not differ significantly between diabetes and non-diabetes subjects (Table 3). The hypoxic gas caused an increase in arteriolar and venular diameters (Table 3). Vasodilatation tended to be greater in non-diabetes participants, but the difference was not statistically significant. The normoxic–hypercapnic gas had no significant effect on arteriolar or venular diameters in either group (Table 3). The hyperoxic gas caused arteriolar and venular vasoconstriction. Arteriolar vasoconstriction was significantly less in diabetes subjects compared with non-diabetes subjects (Table 3), but venular responses did not differ by diabetes status.
| Diabetes subjects (n = 29) | Non-diabetes subjects (n = 32) | p-value | |
|---|---|---|---|
| Age, years | 37 (24–50) | 33 (24–49) | 0.09 |
| Males, n (%) | 11 (38) | 13 (41) | 0.3 |
| Duration of diabetes, years | 25 (10–40) | – | |
| HbA1c, % | 8.4 (5.9–10.6) | – | |
| MAP, mmHg | 99 (78–119) | 91 (74–116) | 0.07 |
- HbA1c = glycosylated haemoglobin; MAP = mean arterial pressure.
| Diabetes subjects (n = 29) | p-value (versus baseline) | Non-diabetes subjects (n = 32) | p-value (versus baseline) | p-value (diabetes versus non-diabetes subjects) | |
|---|---|---|---|---|---|
| Arterioles | |||||
| Baseline diameter, pixels | 14.9 (14.2, 15.6) | – | 14.5 (13.8, 15.2) | – | 0.38 |
| Δ Gas 1 (hypoxia), % | 1.7 (0.1, 3.3) | 0.04 | 2.9 (1.9, 4.0) | 0.00 | 0.25 |
| Δ Gas 2 (hypercapnia), % | 0.2 (− 1.3, 1.6) | 0.81 | − 0.7 (− 1.9, 0.4) | 0.20 | 0.31 |
| Δ Gas 3 (hyperoxia), % | − 4.0 (− 7.1, − 0.8) | 0.02 | − 7.8 (− 9.5, − 6.0) | 0.00 | 0.04 |
| Venules | |||||
| Baseline diameter, pixels | 19.2 (18.2, 20.1) | – | 18.4 (17.7, 19.0) | – | 0.16 |
| Δ Gas 1 (hypoxia), % | 1.5 (0.1, 3.0) | 0.04 | 2.9 (2.0, 3.8) | 0.00 | 0.12 |
| Δ Gas 2 (hypercapnia), % | 1.3 (− 1.1, 3.6) | 0.30 | 0.0 (− 0.7, 0.8) | 0.07 | 0.29 |
| Δ Gas 3 (hyperoxia), % | − 9.5 (− 11.4, − 7.7) | 0.00 | − 11.4 (− 13.8, − 9.1) | 0.00 | 0.21 |
- Δ = percentage change in diameter of arterioles and venules when breathing the test gases.
The effects of breathing the three test gases on MAP and HR are shown in Table 4. Mean arterial pressure did not change significantly in subjects breathing the hypoxic or hyperoxic mixture, regardless of diabetes status, and increased significantly during exposure to the hypercapnic mixture only in non-diabetes subjects. Heart rate increased significantly when breathing the hypoxic mixture in both groups, and decreased significantly during exposure to the hyperoxic mixture in diabetes subjects, but did not change significantly in non-diabetes subjects. The hypercapnic mixture caused a significant increase in HR in diabetes subjects but decreased HR in non-diabetes subjects.
| Diabetes subjects | Non-diabetes subjects | p-value (diabetes versus non-diabetes subjects) | |||
|---|---|---|---|---|---|
| Δ MAP | p-value (versus baseline) | Δ MAP | p-value (versus baseline) | ||
| Hypoxia | 0.8 (− 1.1, 2.6) | 0.40 | − 0.9 (1.5, − 3.3) | 0.42 | 0.06 |
| Hypercapnia | 4.0 (− 5.0, 3.1) | 0.37 | 7.3 (3.8, 10.9) | 0.00 | 0.60 |
| Hyperoxia | 1.9 (− 0.9, 4.0) | 0.06 | − 4.3 (−16.1, 7.6) | 0.46 | 0.14 |
| Δ Heart rate | p-value | Δ Heart rate | p-value | ||
|---|---|---|---|---|---|
| Hypoxia | 3.0 (1.2, 4.8) | 0.02 | 3.1 (0.1, 6.0) | 0.04 | 0.14 |
| Hypercapnia | 2.72 (0.2, 5.2) | 0.03 | − 1.9 (− 3.5, − 0.3) | 0.02 | 0.88 |
| Hyperoxia | − 3.07 (− 4.7, − 1.4) | 0.01 | − 1.1 (− 5.0, 2.8) | 0.56 | 0.33 |
Discussion
Our study shows that the arteriolar vasoconstrictor response to breathing 100% O2 is reduced in people with type 1 diabetes compared with non-diabetes subjects of similar age. Venular responses to 100% O2 and responses to hypoxic and hypercapnic gas mixtures did not differ by diabetes status. Arterial blood pressure did not change significantly after breathing 100% O2 and thus the changes in diameter can be attributed to vasoconstriction. These data indicate a defect in the reactivity of retinal arterioles in type 1 diabetes, which is consistent with previous suggestions based on laser Doppler studies of changes in retinal flow velocity in response to exercise (Dumskyj & Kohner 1999).
Other researchers (Hickam & Sieker 1960; Grunwald et al. 1984) have also reported a difference in reactivity to hyperoxia between individuals with and without diabetes. Hickam & Sieker (1960) measured the diameters of larger retinal arteries and veins from fundus photographs by microscopy. The participants, 50 people with diabetes and a control group of 47 non-diabetes subjects, inhaled 100% O2. The authors reported reduced arteriolar vasoconstriction in response to 100% O2 in the diabetes subjects, in accordance with our findings. However, venular vasoconstriction in response to hyperoxia was only significantly reduced in diabetes subjects with retinopathy or diabetes subjects with co-existing hypertension. Grunwald et al. (1984) examined 11 normal volunteers and 16 insulin-treated diabetes subjects with background retinopathy. Participants inhaled 100% O2 and only veins were measured. They found a significantly smaller reduction in vessel diameter in the diabetes subjects. Interestingly, a recent study using magnetic resonance imaging retinal oximetry (Trick et al. 2006) reported that patients with diabetes show an exaggerated rise in retinal pO2 following exposure to hyperoxia, suggesting that abnormal regulation of retinal oxygenation, perhaps secondary to impaired vascular reactivity, may be an early feature of diabetes that contributes to development of retinopathy.
The mechanism by which diabetes affects retinal arteriolar reactivity to 100% O2 is unknown. Acute hyperglycaemia can impair endothelium-dependent vasodilation (Williams et al. 1998), but both short-term postprandial hyperglycaemia (Sullivan et al. 1991) and acute hyperglycaemia (Gilmore et al. 2007) have been shown to have no or very limited influence on resting retinal blood flow and in our study measurements of HbA1c (glycosylated haemoglobin) did not indicate severe chronic hyperglycaemia in the diabetes subjects. However, recent work (Gilmore et al. 2008) has shown that a combination of isocapnic hyperoxia and glucose provocation causes comparable vasoconstriction in individuals with and without diabetes and that hyperglycaemia reduces vasoconstriction in response to isocapnic hyperoxia in non-diabetes subjects, thereby suggesting a role for hyperglycaemia in the impaired response to hyperoxia in diabetes.
Responses to other gases did not differ significantly between individuals with and without diabetes in our study. This may indicate that the adverse effect of diabetes is specific for the arteriolar vasoconstriction evoked by hyperoxia; however, it should be noted that the responses to hypoxic and hypercapnic gas mixtures were smaller than the response to hyperoxia, and possibly too small to allow differences related to diabetes status to be detected. Indeed, the hypercapnic gas mixture failed to cause any significant effect on retinal vascular diameter in either diabetes or non-diabetes individuals. This is consistent with some previous studies which have failed to observe retinal vasodilation in response to hypercapnic gas mixtures (Deutsch et al. 1983; Harris et al. 1996; Luksch et al. 2002), although other studies have reported modest vasodilation in response to hypercapnia (Pakola & Grunwald 1993; Dorner et al. 2002). Inconsistencies in response to hypercapnic stimuli may reflect variable effects of different gas combinations on end-tidal CO2 and O2 (Gilmore et al. 2004), although the changes in HR seen in response to the hypercapnic gases mixture suggests that some change in pCO2 was probably achieved in our study. An additional limitation of this study is that the order of the test gases was not randomized. This was because hyperoxia has relatively prolonged effects and, consequently, this gas mixture was administered last. However, using a fixed order of administration risks introducing a confounding time or order effect. We used a washout period of 10 mins between each gas and, as preliminary studies indicated that vessel diameters returned to close to baseline before the next test gas was administered, we believe our findings are unlikely to have been confounded by the fixed order of administration. Other limitations of our study include its relatively small number of participants and the lack of concurrent measurements of glucose, arterial blood gases, arterial pH and frequency of respiration. The strengths of the study include its use of a non-invasive method that is readily applied in a clinical setting and which generates precise quantitative measures using a semi-automated, computer-based technique with excellent reproducibility.
In summary, we have shown that type 1 diabetes impairs the vascular reactivity response to hyperoxia. Our approach may be useful in the early identification of abnormalities in retinal arteriolar reactivity and could also be employed for mechanistic and pharmacological studies of retinal microvascular function.
Acknowledgements
This study was supported by the Danish Eye Association (Værn om Synet), the Diabetes Association, the Gerda and Aage Haensch Foundation, the A. P. Møller and C. M. McKinney-Møller Foundation, the Synoptik Foundation, the Danish Eye Research Foundation and the Velux Foundation. NC, SAT and ADH received support from the UK National Institute for Health Research Biomedical Research Centre Funding Scheme.




