Dystrophia Smolandiensis: a novel morphological picture of recurrent corneal erosions
Abstract.
Purpose: The aim of this study was to describe morphological changes in Dystrophia Smolandiensis, a corneal disease that is characterized by recurrent corneal erosive episodes and the formation of central corneal keloid-like opacities in approximately half of those affected.
Methods: The corneas of seven affected individuals were examined using in-vivo confocal microscopy. Specimens of one primary corneal graft, one regraft and one biopsied keloid-like region – all obtained from members of a large family with the disease – were re-examined with a light microscope. Sections were stained with Congo red and analysed immunohistochemically for fibronectin and S100A4.
Results: Light microscopic examination revealed epithelial hyperplasia, absence of Bowman’s layer and subepithelial fibrosis. Fibronectin was expressed in the area of subepithelial fibrosis, and the keratocytes in this area generally expressed S100A4. The biopsy specimen stained positive for Congo red, suggesting an amyloid deposit. In-vivo confocal microscopy confirmed epithelial abnormalities, loss of Bowman’s layer and significant alterations of the subbasal nerve plexus in affected individuals.
Conclusion: The morphological picture in Dystrophia Smolandiensis is novel for a condition dominated by recurrent corneal erosions at the clinical level. Although no single morphological feature unique to the disease could be found, the general morphological pattern of pathology (true keloid formation, absence of Bowman’s layer, subepithelial fibrosis and abnormal subbasal nerves) probably reflects a novel phenotypic expression of the healing response to recurrent erosion of the corneal epithelium. However, the pathogenesis of Dystrophia Smolandiensis remains to be elucidated fully.
Introduction
Recurrent corneal erosion (RCE) is a commonly encountered ophthalmic disorder that is accompanied by symptoms of incapacitating pain, redness, watering and photophobia (Ramamurthi et al. 2006). Abnormality of the adhesional complexes at the corneal epithelial basement membrane – responsible for anchoring the epithelium to the underlying Bowman’s layer – results in defective adhesion and recurrent erosive episodes of the epithelium (Goldman et al. 1969; Tripathi & Bron 1972; Brown & Bron 1976; Hammar et al. 2008a, 2008b). However, the pathogenesis of the condition is not understood fully. Morphological signs of RCE include loosely adherent and elevated epithelium, epithelial microcysts, corneal epithelial defects (such as thinning and reduplication), and stromal infiltrates and opacities (Dursun et al. 2001). In-vivo confocal microscopy in corneas affected by RCE shows intracellular deposits in the basal epithelium, subbasal microfolds and streaks, altered subbasal nerves or abnormal morphology of the anterior stroma (Rosenberg et al. 2000).
RCE can be caused by previous trauma, it may be idiopathic in nature or it may occur secondary to corneal dystrophies or inherited systemic diseases, such as Epidermolysis Bullosa (Bron & Burgess 1981). However, reports of inherited, primary RCE are rare (Franceschetti 1928; Wales 1955; Remler 1959; Legrand 1963; Shindo 1968; Brown & Bron 1976; Bron & Burgess 1981). Previously, our group has reported on a corneal disorder with autosomal-dominant inheritance and RCE episodes as the primary clinical symptom (Hammar et al. 2008a). Until the genetic origin of this disorder can be traced, it has been given the name Dystrophia Smolandiensis. It is characterized by RCE and the frequent development of subepithelial opacities in the central cornea (Fig. 1). Both phenotypic and genotypic studies support the notion of a previously unknown disorder (Hammar et al. 2008a). Although knowledge of inherited corneal diseases has advanced significantly in recent years (Aldave & Sonmez 2007), inherited conditions that have not been described or classified previously are still being discovered. For such conditions, detailed documentation of the clinical and morphological characteristics is essential: this will aid in the identification of the condition and its differential diagnoses, as well as in its future genetic classification. Our previous study (Hammar et al. 2008a) described the clinical features of this disorder. Accordingly, the aim of this study was to describe the morphological characteristics of Dystrophia Smolandiensis through histopathological examination and immunohistochemistry of excised tissue samples, as well as examination of affected corneas in real time using in-vivo confocal microscopy.
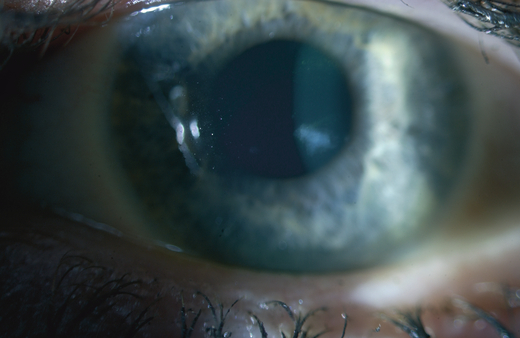
A central corneal opacity found in a 41-year-old woman.
Materials and Methods
We studied a six-generation family with 44 affected individuals (26 female, 18 male). Affected individuals had been examined previously using slit-lamp biomicroscopy, which was performed by experienced ophthalmologists (Hammar et al. 2008a). Following penetrating keratoplasty (PKP) and diagnostic biopsy, corneal tissue samples were obtained from three affected individuals and were subsequently processed for routine histology and immunohistochemistry. Additionally, seven affected and two unaffected individuals were examined by in-vivo confocal microscopy. All study participants were treated in accordance with the tenets of the Declaration of Helsinki, and approval was obtained from the local ethics committees at the University of Linköping and the Karolinska Institute. Informed consent was obtained from all patients.
Histopathology
Out of the nine affected individuals who had undergone PKP because of vision-impairing subepithelial corneal opacities, four specimens from three individuals were available for histopathological review. Excised material from the remaining transplanted individuals was discarded because of original clinical diagnoses of Meesmann’s dystrophy, lattice-type dystrophies or corneal dystrophy of Bowman’s layer type I (CDBI) (previously known as Reis–Bückler’s corneal dystrophy). The first specimen available was a corneal button from an individual who underwent PKP at the age of 20 years. The second specimen was a biopsy sample from the same individual, who had a corneal keloid excised in the fellow eye at 35 years of age because of impaired vision. The third specimen was a corneal button from a second individual who underwent PKP at the age of 40 years. The fourth specimen was a corneal button from a third individual who underwent PKP at the age of 20 years. All specimens were originally fixed in 4% formaldehyde and embedded in paraffin. Sections (4 μm thick) were cut from paraffin blocks and routinely stained with haematoxylin and eosin, the van Gieson stain for collagen, and the periodic acid-Schiff (PAS) stain to mark carbohydrates (e.g. glycogen, glycoprotein, proteoglycans such as those found in basement membranes, keratocytes and Goblet cells). Additional sections were stained with Congo red for amyloid, with antibodies against S100A4 (a calcium-binding protein expressed in keratocyte, fibroblast and myofibroblast phenotypes during corneal wound healing) and with fibronectin, as outlined below.
Immunohistochemistry
Polyclonal affinity-purified rabbit anti-human antibodies against S100A4 (A 5114) and fibronectin (A 245) were obtained from DakoCytomation (Glostrup, Denmark).
Slides to be stained for fibronectin were pretreated with pronase. After blocking of endogenous peroxidase, slides were incubated for 1 hr at room temperature with the respective primary antibody diluted to 1 : 250 in Tris buffered saline (TBS) containing 10% normal bovine serum. The slides were then incubated for 30 min at room temperature with biotinylated goat anti-rabbit immunoglobulin G (IgG) (Vector Laboratories, Burlingame, California, USA) diluted 1 : 200 in 10% normal goat serum and TBS; they were further incubated with the avidin–biotin complex (Vector Laboratories) according to the manufacturer’s instructions. Detection was performed with 3-amino-9-ethylcarbazole (Sigma-Aldrich, St Louis, Missouri, USA), after which the slides were counterstained with haematoxylin.
In-vivo confocal microscopy
Four affected and two healthy individuals were examined using a slit-scanning system (ConfoScan P4; Tomey Corporation, Erlangen, Germany) fitted with an Achroplan 40×/0.75 water immersion objective (Zeiss, Jena, Germany). Optical section thickness for this instrument was 8 μm. Analogue video images were captured and subsequently digitized. The three remaining affected individuals were examined using a laser-scanning system [Heidelberg Retina Tomograph III Rostock Corneal Module (HRT3-RCM); Heidelberg Engineering GmbH, Heidelberg, Germany] fitted with a 63×/0.95 water immersion objective (Zeiss). Prior to examination, one drop of topical anaesthetic (0.4% oxybuprocaine hydrochloride; Chauvin Pharmaceuticals, Kingston-upon-Thames, Surrey, UK) was given; tear gel (2 mg/g carbomer, Novartis, Täby, Sweden) was used for optical coupling between the cornea and the microscope objective. Several scans through the full corneal thickness were taken in both eyes of each individual. With the HRT3, each scan consisted of 100 digital image frames that were stored during examination and could be retrieved for later analysis. Each digital frame from the HRT3 represented a corneal area of 400 × 400 μm with an axial resolution and optical section thickness of 2 and 4 μm, respectively. The slit-scanning confocal images obtained were of poorer quality, and therefore only the general features of these examinations are described. However, the high-resolution laser-scanning confocal images were suitable for detailed analysis and are presented here.
Results
Slit-lamp examination in an earlier study revealed permanent subepithelial opacities in 52% of the affected individuals (Hammar et al. 2008a). The youngest individual found to have a corneal opacity was 7 years old. Only a single or a few dense, keloid-like subepithelial opacities developed in any given cornea, and the opacities were located centrally. There seemed to be no correlation between the opacities and gender. Opacities were observed over a wide age range, although they were found more frequently in the elderly. When opacities were found on the visual axis, visual acuity was affected.
The appearance of the opaque regions varied from subepithelial fibrosis to protruding keloid-like formations (Fig. 1). In corneas with more protruding opacities, an epithelial iron line frequently surrounded the base of the nodule. Significantly thickened stromal nerves were observed in two patients under illumination during slit-lamp examination. No changes consistent with epithelial basement membrane dystrophy or any other previously described corneal dystrophy were observed.
Histopathology and immunohistochemistry
The central and mid-peripheral regions of two of the three excised corneal buttons (from the first and third specimens) showed epithelial hyperplasia, subepithelial fibrosis and an absence of Bowman’s layer; the deeper stroma, Descemet’s membrane and the endothelium appeared normal (Fig. 2A). The third excised corneal button (the fourth specimen, not shown) exhibited a mixed picture with epithelial hyperplasia, thinned epithelium and absence of Bowman’s layer centrally; peripherally, the corneal epithelium and stroma appeared normal. In all excised buttons, areas of the cornea without characteristic epithelial and subepithelial changes disclosed histopathologically normal corneal epithelium overlying an intact Bowman’s layer without signs of pathology.
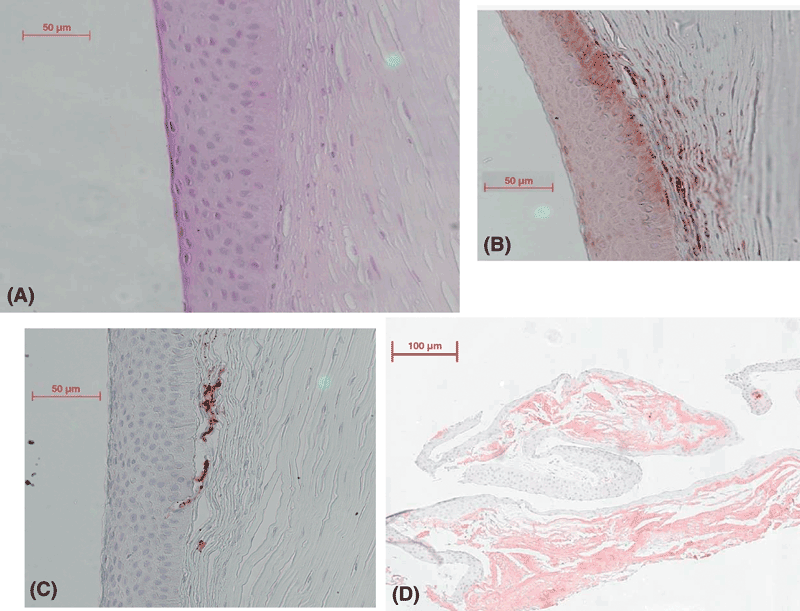
Four light micrographs of histopathology and immunohistochemistry. (A) Light micrograph from an excised cornea showing epithelial hyperplasia, resorption of Bowman’s layer and subepithelial fibrosis. [Stained with haematoxylin and eosin, the van Gieson stain and the periodic acid-Schiff (PAS) stain.] Bar = 50 μm. (B) Light micrograph from an excised cornea showing the expression of S100A4 in several of the keratocytes constituting the subepithelial fibrosis. Bar = 50 μm. (C) Light micrograph from an excised grafted cornea with recurrence of the disease. Fibronectin is expressed in parts of the subepithelial fibrosis, suggesting recent wound-healing events in this area. Bar = 50 μm. (D) Light micrograph showing the biopsied part of a keloid-like structure staining positive with Congo red and thus amyloid. This suggests strongly that the specimen is a true keloid. Bar = 100 μm.
Abundant fibronectin was present in the central subepithelial stroma and colocalized with areas of subepithelial fibrosis (Fig. 2B). Keratocytes in these areas featured distinct immunoreactivity for S100A4 (Fig. 2C). No morphological evidence of corneal neovascularization, inflammatory cells or macrophages could be detected.
The biopsy sample had an overlying epithelium exhibiting mild hyperplasia. The stroma contained an acellular amorphous deposit positive for the Congo red stain (Fig. 2D) and exhibited a characteristic green birefringence. The deposit stained negative for both fibronectin and S100A4.
In-vivo confocal microscopy
Examination of four affected individuals (aged 20, 32, 51 and 72 years) with the slit-scanning confocal microscope revealed abnormalities in the nerve layer at the subbasal epithelium. The subbasal nerves comprising this layer took a tortuous path and were distributed sparsely in certain regions of the cornea, whereas the normal long, straight, parallel orientation of these nerves was observed in other regions. Regions in which subbasal nerves exhibited abnormal morphology were distributed in a seemingly random manner across the cornea.
Three additional affected individuals were examined with laser-scanning confocal microscopy. The first two were sisters aged 19 and 23 years who had not undergone any surgical treatment. In the elder sister, abnormal epithelial cell morphology, epithelial opacity, an absence of Bowman’s layer and a subbasal epithelium devoid of normal-appearing subbasal nerves were all noted in the central cornea of the right eye (Fig. 3). The left eye had a normal-appearing epithelium without opacities; however, Bowman’s layer was absent in the central to mid-peripheral region, and the central subbasal nerve plexus was only sparsely innervated, with dendritic cells also present (Fig. 3). In the younger sister, abnormal epithelial cells, epithelial and subepithelial opacities, absent Bowman’s layer both centrally and peripherally, and a subbasal region devoid of nerves were observed bilaterally (Fig. 4). In addition, the corneal epithelium in both eyes was abnormally thin (central thickness 30–35 μm). In both sisters, only anterior corneal abnormalities could be detected, while the stroma and endothelium appeared normal.

In-vivo confocal microscope images taken from the corneas of a 23-year-old woman. All images 400 × 400 μm, scale bar = 100 μm for non-oblique sections. Depths given are from the epithelial surface and all images are from the central cornea unless otherwise indicated. In the right eye (top row, left to right): superficial epithelial cells are abnormally small and dense (10 μm); abnormally highly reflective cytoplasm and nuclei of epithelial wing cells (16 μm); basal epithelial cells with abnormally reflective nuclei and short, sparse subbasal nerve strands (33 μm); absent Bowman’s layer evidenced by the presence of basal epithelial cells, subepithelial nerve bundles and anterior stromal keratocyte nuclei in the same confocal plane (52 μm). In the left eye (bottom row, left to right): normal superficial epithelial cells (0 μm); epithelial wing-cell layer with bright microcysts (arrows) (21 μm); subbasal nerve plexus with sparse, abnormally tortuous nerves and small dendritic cell bodies (32 μm); oblique section in the mid-peripheral cornea revealing absent Bowman’s layer (keratocyte nuclei extend to the basal epithelium).
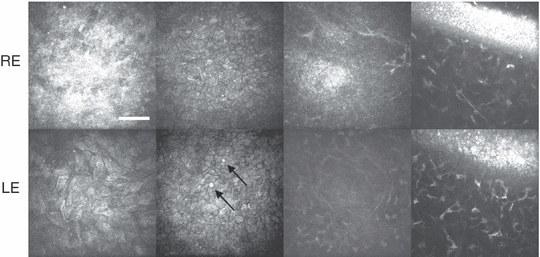
In-vivo confocal microscope images taken from the corneas of a 19-year-old woman. All images 400 × 400 μm, scale bar = 100 μm for non-oblique sections. In the right eye (top row, left to right): opacity just below the superficial epithelium (9 μm); epithelial wing cells with abnormally reflective cytoplasm (12 μm); absent Bowman’s membrane and subbasal nerves, with basal epithelial opacity, subepithelial nerve bundles and anterior stromal keratocyte nuclei visible in the same confocal plane (27 μm); oblique section in the mid-peripheral cornea revealing absent Bowman’s membrane. In the left eye (bottom row, left to right): abnormally shaped superficial epithelial cells (0 μm); abnormally reflective cytoplasm in wing cells with microcysts (arrows) additionally present (10 μm); absence of subbasal nerves and Bowman’s membrane centrally (35 μm); oblique section of absent Bowman’s membrane mid-peripherally.
The third individual examined with the confocal microscope was a 33-year-old woman who underwent bilateral phototherapeutic keratectomy (PTK) 2 and 7 years earlier in the left and right eyes, respectively, as a treatment for RCE. Confocal microscopy in the left eye revealed epithelial microcysts, regenerated subbasal nerves, patch-like subepithelial opacities and evidence of anterior stromal keratocyte necrosis (Fig. 5). In the right eye, a greater number of microcysts, more pronounced subbasal and subepithelial opacity, and abnormally tortuous and highly branched regenerated subbasal nerves were observed (Fig. 5). The superficial epithelium in both corneas was normal to slightly hyperplastic, while the epithelium in both corneas was abnormally thin (central thickness 30–35 μm). Confocal microscopy images confirmed the complete absence of Bowman’s layer by the PTK procedure.
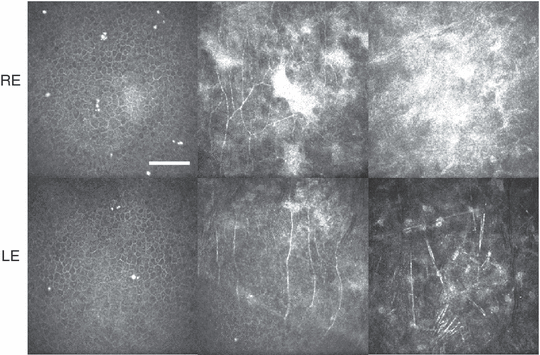
In-vivo confocal microscope images taken from the corneas of a 33-year-old woman. All images 400 × 400 μm, from the central cornea. Scale bar = 100 μm. In the right eye, 7 years after PTK treatment (top row, left to right): epithelial microcysts and abnormally reflective wing cell nuclei (18 μm); patch-like opacities and irregularly branched subbasal nerves (39 μm); anterior stromal opacity (63 μm). In the left eye, 2 years after phototherapeutic keratectomy treatment (bottom row, left to right): epithelial microcysts and abnormally reflective wing-cell nuclei (22 μm); subbasal nerves and patch-like anterior stromal opacities (44 μm); anterior stromal keratocytes and needle-shaped structures that may represent necrotic or activated keratocytes (120 μm).
Discussion
Dystrophia Smolandiensis would usually be classified as a Franceschetti-type corneal dystrophy (Bron & Burgess 1981), but with the important distinction that in half of those affected vision-impairing, centrally located keloid-like structures occupied the superficial stroma and sometimes necessitated corneal grafting. All grafted patients had recurrences of opacities, usually located in the graft periphery. The recurrences appeared as subepithelial fibrosis combined with a tendency to form keloid-like structures. We postulate that the keloid-like structures and the subepithelial fibrosis were formed as a reaction to the recurrent erosive events, although with a greater intensity compared to regular recurrent erosions.
Among the histopathological findings in the examined corneal buttons, a total loss of the Bowman’s layer in affected areas appeared a characteristic finding of this disease (Fig. 2A). Another prominent finding was local variations in epithelial thickness (presumed as the result of epithelial hyperplasia), which is a feature seen frequently after superficial corneal trauma and has the function of maintaining ocular surface shape (Cameron 2005).
Subepithelial fibrosis, where present, was widespread and located in the superficial stroma whereas the deeper stroma appeared normal. Moreover, the thickness of the fibrotic layer varied over the corneal surface. These findings are in general accordance with a non-specific corneal response to pathological processes associated with corneal opacity and other disturbances in transparency (Cameron 2005).
Immunohistochemical results showed that fibronectin was expressed in the area of subepithelial fibrosis and the superficial keratocytes in this area generally stained positive for S100A4. The expression of S100A4 suggests activation and myofibroblastic differentiation typical of corneal wound healing (Ryan et al. 2003); meanwhile, fibronectin expression has been considered to be a sign of recent healing activity of the corneal epithelium (Filenius et al. 2003). Recurrent erosive events in the present family are concordant with these findings.
The biopsy sample of a keloid-like formation in a non-grafted cornea stained positive for amyloid using the Congo red stain and is strongly suggestive of actual keloid material. The deposit was lined by hyperplastic epithelium and stained negative for fibronectin and S100A4, indicating quiescence of the surrounding stroma. The characteristic Congo red staining has been used for many decades as an assay for the presence of amyloid proteins and has been considered the hallmark for diagnosis (Bély & Apáthy 2000).
Amyloid is a designation used to describe a family of proteins identified by distinctive histological qualities like homogeneous eosinophilia with haematoxylineosin stain, ultraviolet fluorescence with thioflavine T and red staining with Congo red, monitored by characteristic green birefringence. Dysfunctional protein metabolism leads to extracellular deposition of a characteristic eosinophilic hyaline material (Kenyon et al. 2005). Amyloid accumulates in the cornea in a wide variety of disorders, both localized and systemic, many of which are genetically determined. Such inherited diseases include the inherited lattice corneal dystrophies and familial subepithelial corneal amyloidosis (Rosenberg et al. 2001). With the exception of type II lattice corneal dystrophy, amyloid deposition occurs principally within the corneal tissue. Amyloid is also often found in the cornea in numerous non-specific long-standing ocular disorders, including keratoconus, trachoma, uveitis, infections, neoplasms, connective tissue disorders, phlyctenular keratoconjunctivitis, contact lens wear and trichiasis (Kenyon et al. 2005).
Keloid-like formations are thought to be the result of reactivity to a stimulus, mostly of a chronic kind. A similar reactivity can be found in Lowe’s syndrome (Cibis et al. 1982), in CDBI, in Salzmann’s nodular degeneration (Waring et al. 1978), in the form of nebulae at keratoconus (Moodaley et al. 1991) and in surgical scars. Chronic insults have also been noted to result in a different morphological expression in the cornea, notably in epithelial basement membrane dystrophy (EBMD, also known as map-dot-fingerprint dystrophy), where the epithelial basal lamina assumes an intraepithelial location and gives the cornea a specific appearance. This appearance is believed not to be a specific entity of itself but rather the result of recurrent epithelial erosions (Labbé et al. 2006). Similarly to Dystrophia Smolandiensis, cases of EBMD are often idiopathic and sometimes inherited with an autosomal-dominant mode of inheritance (Laibson & Krachmer 1975).
Confocal microscopy of the non-surgically altered cornea in two affected individuals revealed a pathological epithelium and absence of Bowman’s layer in affected areas, confirming histopathological findings. Opacities were localized to intraepithelial regions and were characterized microscopically by increased cytoplasmic reflectivity in wing and basal cells and possible additional deposition of reflective extracellular material. Intracellular accumulation and increased reflectivity of epithelial cells have also been observed via in-vivo confocal microscopy in cases of EBMD and primary RCE (Hernández-Quintela et al. 1998; Rosenberg et al. 2000); this may represent the general morphology of an epithelium prone to recurrent erosion. Notably, the nerves comprising the subbasal plexus were either totally absent or sparsely distributed, with dendritic cell bodies present – also echoing earlier findings in association with recurrent erosions (Hernández-Quintela et al. 1998; Rosenberg et al. 2000). Given the importance of corneal nerves in maintaining epithelial integrity (Garcia-Hirschfeld et al. 1994; Müller et al. 2003), it is reasonable to expect that recurrent erosions and corneal nerve status are closely linked (Rosenberg et al. 2001), although the precise nature of this relationship remains to be fully elucidated. Epithelial microcysts observed in-vivo bore resemblance to the dot-like opacities of EBMD thought to represent degenerated or necrotic cellular material (Waring et al. 1978); microcysts have also been observed in cases of RCE (Hernández-Quintela et al. 1998). Neither the epithelial and nerve abnormalities nor the thinned epithelium nor the absence of Bowman’s layer observed by in-vivo confocal microscopy appear to be unique to Dystrophia Smolandiensis.
In the affected individual who underwent PTK treatment, epithelial microcysts and abnormally reflective wing-cell nuclei were observed in-vivo. However, such findings are common in patients with recurrent erosions after treatment with PTK (Lagali & Fagerholm, unpublished) and are not believed to be specific to Dystrophia Smolandiensis. Similarly, stromal opacity and necrotic stromal material may also have been the result of PTK. It is of interest to note that a substantial regeneration of subbasal nerves occurred following PTK. Their continued presence years after treatment indicates that therapeutic laser ablation of the superficial cornea may be effective in halting erosive episodes in some individuals affected by this disease. However, further clinical data are required to establish this hypothesis.
Conclusion
The corneas of individuals affected by Dystrophia Smolandiensis generally exhibited morphological abnormality in areas with frequent recurrent erosive episodes. Epithelial hyperplasia, subepithelial fibrosis, loss of Bowman’s layer and altered subbasal nerves were observed. An active corneal wound healing response was confirmed by the expression of S100A4 and fibronectin and was probably the result of corneal repair following erosive events. About half of the patients developed central subepithelial opacities and keloid-like formations in the cornea that stained positive for Congo red, suggesting that they were amyloid in composition. The morphological picture in Dystrophia Smolandiensis is novel for a condition dominated by RCEs at the clinical level.
Acknowledgements
This project was supported by grants from the Swedish Medical Research Council, Kronprinsessan Margaretas Arbetsnämnd, Thyselius Foundation, David and Beth Dahlins Foundation and the County Council of Östergötland.




