Stemming the flood
Coverage on: Quintana et al. (2008). Efficient tumour formation by single human melanoma cells. Nature 456: 593–598.
The cancer stem cell debate over the last 10 months has been an exciting time for melanoma research. At the outset of 2008, Markus Frank’s laboratory published evidence that only a tiny subpopulation of melanoma cells (approximately one cell in a million) has the capacity to maintain tumour progression (Schatton et al., 2008). By the standards of the day, it was an extremely important work, linking together cancer stem cells, disease progression and therapeutic resistance, and it seemed that a great leap forward had been made in the fight against metastatic disease. However, at the close of the year Sean Morrison’s group raised the standards and presented evidence opposing the idea of a tiny subpopulation of melanoma cells which are critical for tumour progression, finding instead that as much as a quarter of all melanoma cells within any given lesion are tumorigenic (Quintana et al., 2008).
The cancer stem cell (CSC) hypothesis rests on the premise that there are a small number of specialized cancer cells which combine the stem cell-like properties of limitless renewal and asymmetric division with the capacity to invade and populate regions distal from the original transformative event. The attractions of this hypothesis include that these cells may explain both the cellular heterogeneity of tumours and resistance of metastatic disease to current therapeutic approaches. That melanoma metastases are composed of different populations of malignant cells is now well accepted; however, the explanation for this heterogeneity has not yet been established. Asymmetric division of melanoma stem cells to yield differently committed progeny is a good model for persistent heterogeneity throughout disease progression. Likewise, resistance to therapy may occur because tumour cells are targeted via characteristics which, although widely expressed in melanoma tissue, may not be expressed by a truly tumorigenic subpopulation. The possibility that melanoma stem cells underlie heterogeneity and therapeutic failure is plausible and the seminal work by Schatton and co-workers appeared to bear this out.
Challenges to the CSC hypothesis, include concerns about whether or not the models used to evaluate CSCs are valid. Xenograft models take advantage of compromised immune mechanisms to provide an essentially ‘black box’ environment in which cancer cell proliferation may be experimentally monitored. A typical CSC experiment separates cells into fractions characterized by surface markers known to be expressed by normal stem cells, subcutaneously injects them into immunocompromised mice, and evaluates their capacity to form tumours. It has been suggested that xenografting underestimates the number of cells truly capable of driving tumourigenesis, because non-human environments may proliferatively select, in a non-informative fashion, some cancer cells over others. In their study, Quintana et al. compared xenograft viability in the NOD/SCID mouse used by Schatton et al. with that of a more severely immunocompromised model lacking natural-killer cell activity (NOD/SCID Il2rg−/−). They were able to largely replicate the results of the earlier study, finding that about 800 000 cells are required to initiate xenograft growth in NOD/SCID mice. On the other hand, limiting dilution analyses of xenografts using the NOD/SCID Il2rg−/− model, where as few as five unsorted melanoma cells were injected subcutaneously, showed that approximately one cell in nine is capable of forming a tumour. To ensure that their model was not somehow enriching for tumorigenic cells, serial xenograft experiments performed to monitor for increased tumorigenicity showed none for either of the NOD/SCID or NOD/SCID Il2rg−/− models. These experiments, performed with unsorted melanoma cell populations sourced from a dozen different patients, showed unequivocal evidence that tumorigenic cells are common in melanoma and that less immunocompromised mouse models greatly underestimated this. Most importantly, Quintana et al. xenografted single unsorted melanoma cells into NOD/SCID Il2rg−/− mice, succeeding in more than a quarter of their attempts. This is the first time that individual cells have been shown to form tumours in an experimental setting. A critical facet of this is that the authors did not deliberately select cells for specific characteristics. Indeed, when they did sort melanoma populations according to a variety of markers and inject them into NOD/SCID Il2rg−/− mice, Quintana et al. demonstrated that there was no substantial difference in xenograft success rate between marker-positive and marker-negative cells. If these results truly reflect the situation in human patients and that there is no specialized subpopulation of cells responsible for maintaining tumourigenesis, how does melanoma maintain a heterogeneous cell population capable of evading therapy and driving metastatic progression? The answer may be related to why only some single melanoma cells initiate tumours and not others. One possibility which Quintana et al. discuss is that during the process of isolation or injection some cells may be stressed enough to activate cell-death mechanisms (e.g. via p53 induction). However, there may be additional reasons for tumorigenic selectivity which are determined by the melanoma cells themselves. This must be true, because of cell subpopulations deliberately fractionated from tumour tissue, some ‘take’ to xenografting (even in NOD/SCID models) more rapidly than others. Recent studies conducted on unsorted cell cultures may shed some light on this.
Typically, melanoma cell cultures are derived from relatively small fragments of biopsied melanoma tissue. Until recently, it was assumed that the melanoma cells within a given melanoma are homogenous and that a cell culture derived from it would be a reasonable in vitro representation. However, melanoma tissue is not homogenous, but rather is composed of different melanoma cells with different characteristics and expressing various markers. Experiments with unsorted cell cultures show that they can be phenotyped according to gene expression profiling and observed characteristics of proliferation and invasion. These different phenotypes of melanoma cultures, obtained from cellularly heterogeneous metastases, are analogous to the different subpopulations which may be deliberately fractioned from a single heterogeneous metastasis. For example, while Schatton et al. separate out ABCB5-positive and ABCB5-negative cell populations from individual metastases, DNA microarray data available online reveals that different melanoma cultures may strongly express ABCB5 mRNA or strongly down-regulate it (Figure 1). Critically, as they demonstrate that ABCB5-positive cells are more tumorigenic than ABCB5-negative cells, this is also true for ABCB5 mRNA positive and negative cell cultures (Hoek et al., 2008). This suggests that the act of culturing cells from tumour tissue is an essentially accidental process of selection from heterogeneous collections of tumour cells to yield in vitro populations which roughly resemble fractions obtained by deliberate sorting. How it helps us to understand persistent heterogeneity, therapy evasion and metastatic melanoma progression is explained by what is known about the different melanoma culture phenotypes and their interrelationships.
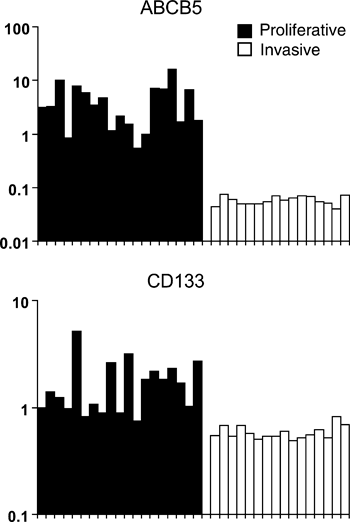
Putative stem cell marker expression in unfractionated melanoma cell lines. Normalized gene expression data for putative melanoma stem cell markers with statistically significant phenotype-specific gene expression patterns. Each marker shows a significant difference in expression between proliferative (black) and invasive (white) expression signature phenotypes. The Mannheim melanoma cell line dataset was extracted from NCBIs publically accessible Gene Expression Omnibus database (accession number GSE4843).
Every library of melanoma cell cultures is comprised of two distinct cell phenotypes, termed proliferative and invasive, which represent separate programs of metastatic potential. Because ABCB5 and other proposed melanoma stem cell markers are often up-regulated in proliferative phenotype cells (Figure 1), the xenograft growth kinetics presented by CSC-hunting laboratories who fractionate tumours based on the expression of such markers make perfect sense. Indeed, recent models propose that melanoma progression is driven by melanoma cells switching between phenotypes of proliferation and invasion, and that these phenotypes underlie both tumour heterogeneity and therapy evasion (Hoek et al., 2008) (Carreira et al., 2006). Consistent with this, irrespective of whether cells were positive for a particular marker or not, and however long it took for a xenograft lesion to form, the lesion itself contained evidence for both proliferative and invasive cells (Hoek et al., 2008). Satisfyingly, in support of the phenotype switching model, Quintana et al. found essentially the same result; regardless of whether they seeded xenografts with CD133-positive or CD133-negative melanoma cells, the resulting tumours were always comprised of both types – indicating that either cell type could give rise to the other in vivo.
The heterogeneity of melanoma tissue may explain why Quintana et al. found that of their single cell dilutions only a fraction could readily initiate xenograft growth. Their cells were extracted directly from patient tissue, which we and others have shown are composed of melanoma cells which express melanocytic markers (proliferative phenotype) and melanoma cells which do not (invasive). Whether the lone melanoma cell in Quintana’s final dilution had a proliferative phenotype or an invasive one would strongly influence the readiness with which it would ‘take’. They also point out that some melanoma cells undergo senescence and that this might also account for the failure of some single-cell xenografts. Invasive phenotype cells express several senescence markers (e.g. Ris1 and PAI-1), and that while a lesion may produce many invasive cells fated to seed metastases elsewhere, most are likely to succumb to senescence before they can effect the ‘switch’ of the phenotype switching hypothesis. Thus, part of why only a quarter of cells are successful may be due to the likelihood that the final cell was often the invasive phenotype, which proliferates slowly and may be susceptible to senescent death.
Quintana et al. elegantly demonstrate that a large and heterogeneous fraction of individual melanoma cells within any given tumour have the potential to seed additional tumours is a timely intervention. After the earlier work of Schatton et al., it is likely that many research groups around the world diverted resources towards the search for melanoma-specific CSCs. Perhaps they should reconsider.