Looping tumor suppression
Coverage on: Acosta et al. (2008). Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018.
Kuilman et al. (2008). Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133: 1019–1031.
Wajapeyee et al. (2008). Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132: 363–374.
New regulatory networks driven by feedforward and feedback loops are unveiling the complex nature of intrinsic mechanisms of tumor control. Identifying the on–off rheostats of these regulatory cascades may provide new targets for therapeutic intervention.
Mammalian cells are endowed with multiple built-in barriers to oncogenic transformation. p53, RB, PTEN and the products of the INK4a/ARF locus have long been at the center stage in cancer research. This has been in part related to the direct ability of these factors to blunt the accumulation of potentially dangerous cells (for example, by promoting premature senescence or by eliciting programmed cell death). However, the current understanding of how pro-tumorigenic signals are sensed by these surveillance mechanisms is still under intense investigation. Traditionally, tumor suppressors are considered to be activated in response to a dysregulated cellular state, which globally has been referred to as ‘oncogenic stress’. Aberrant mitosis, DNA damage and reactive oxygen species are some of the stressors that emanate ‘within’ cells acquiring pro-oncogenic defects (Collado et al., 2007). Two recent reports by the groups of Daniel Peeper and Jesús Gil have provided new insights on regulatory loops engaged as part of a secretory program, and thus, involving previously unanticipated (‘in-out’) paracrine and autocrine signaling cascades (Kuilman et al., 2008; Acosta et al., 2008; Figure 1).
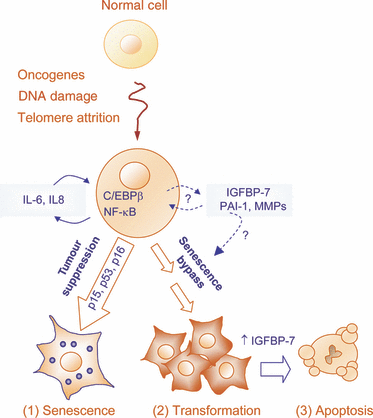
Tumor suppressor networks. A complex network of cytokines, chemokines and other secreted factors is emerging as a novel layer of control of oncogenic transformation, acting in a concerted manner (and in a complex series of feedback loops) with classical tumor suppressors such as p15INK4B, p16INK4a or p53. The genetic background and the relative amount of the specific cytokines expressed will determine whether hyperactivation of transforming oncogenes will ultimately drive cells into senescence (Collado et al., 2007), proliferation (Kuilman et al., 2008) or apoptosis (Acosta et al., 2008). Therefore, understanding the interplay between oncogenes and the secretome holds promise not only to unravel new aspects of tumor inhibition, but also in the development of molecularly targeted therapies.
Both Kuilman et al. (2008) and Acosta et al. (2008) arrived to the secretome as a key switch in oncogene driven senescence (OIS), but using two independent approaches. Kuilman et al. (2008) aimed to identify genes that respond differentially to activated BRAF. They tested the BRAFV600E mutant as the prototype of melanoma-associated pro-oncogenes, able to promote cellular senescence in vitro and in vivo (Michaloglou et al., 2005). The initial working model was telomerase-expressing human fibroblasts that are either competent or deficient for OIS (i.e. with wild type or hyperactivated Cdc42V12, respectively). Acosta et al. (2008), in turn, used a retroviral-mediated shRNA library, in a functional screen for extension of life span of human fibroblasts cultured at high passage number. The two laboratories analyzed also different cell types and finally addressed and validated their hypothesis in human specimens (preneoplastic lesions).
cDNA arrays are notorious for providing a large number of positive hits. Kuilman et al. (2008) exploited bioinformatic tools (gene ontology, GO; ingenuity pathway analysis, IPA; and gene set enrichment analysis, GSEA) to narrow down on the key modulators of OIS. These analyses rendered a cytokine and chemokine footprint induced specifically in senescent but not in quiescent cells. Using blocking antibodies and combination of genetic approaches (shRNA, and constitutive overexpression), they demonstrated an autocrine role of IL-6 as an essential (although not sufficient) factor in the induction and maintenance of BRAFV600E-driven senescence. IL-6 was subsequently shown to be under the transcriptional control of C/EBPβ, which was also critical for OIS. IL-6 shRNA downregulated C/EBPβ expression, and vice versa, revealing interconnecting feedforward loops among the two genes (Kuilman et al., 2008).
Although the precise sequence of events downstream of IL-6 still to be defined, Kuilman et al. showed at BRAFV600E-driven senescence is least in part dependent on p15INK4b. Importantly, p16INK4a, which is a prototype of senescence associated phenotypes in a variety of cell types, is dispensable for the response of fibroblasts BRAFV600E. Instead, they propose that IL-8 may be a further element in the secretome that respond to aberrant oncogenic signaling. The implication of IL-8 in tumor control was supported by the visualization of high levels of this protein correlating with proliferative silent adenocarcinomas. An interesting pending question is the extent to which IL-6 and IL-8 contribute to p16INK4a-independent senescence programs in benign neoplasias driven by BRAF or RAS mutations (e.g. nevi). This is particularly intriguing because immune responses (e.g. driven by IL-6 or IL-8) can serve as a potent mechanism of clearance of senescent cells, and yet, nevus cells can remain in place for decades.
The putative impact of a secretory network was also shown by Acosta et al. (2008). In this case, the central mediator was CXCR2, a receptor for multiple CXC chemokines, including L-8 and CXCL1-7. Downregulation of CXCR2 by shRNAs delayed senescence driven by telomere shortening, oncogenes (activated MEK) and DNA damage (X-radiation). Intriguingly, p53 (but not p21, p16INK4a or p19ARF) was required as a downstream effector of CXCR2-driven cell cycle arrest. C/EBPβ was also found to contribute to oncogene driven senescence by modulating CXCR2 ligands. Cooperating signals were also driven by NF-κB and the p38 pathway. Thus, the RelA protein can directly bind the IL-8 promoter. Similarly, I-κB or p38 inhibitors blunted the induction of IL-8. Next, Acosta et al. demonstrated high, although heterogeneous, expression of CXCR2 in benign lesions in mice (chemically induced papillomas) and in humans (prostate intraepithelial neoplasia). Importantly, Acosta et al. identified a loss of function mutation in CXCR2 in a lung adenocarcinoma cell line. While this mutation may be anecdotal, it serves as the proof of principle for CXCR2 in tumor control. In contrast to classical tumor suppressors which are inactivated or downregulated in during tumorigenesis, neither IL-6, IL-8 nor CXCR2 expression is lost in advanced tumors. These observations suggest a fundamentally different wiring of the secretome in normal and tumor cells.
It should be noted that IL-6, IL-8 and C/EBPβ had been previously linked to oncogene driven senescence. Moreover, these factors are not the only secretory loops controlling premature senescence. The group of Michael Green has recently reported 17 new factors that contribute to oncogene driven senescence in human fibroblasts and melanocytes (Wajapeyee et al. 2008). Key among these factors is the Insulin-like growth factor binding protein 7 (IGFPB-7), also a secreted protein. IGFBP-7 was in fact sufficient to drive premature senescence if expressed at sufficiently high levels (Wajapeyee et al., 2008). These results add to the notion that senescent cells are not metabolically inactive, and can secrete a variety of growth factors, matrix metalloproteinases, protease inhibitors and other cytokines, which can have pleiotropic effects on the stroma and surrounding cells, and ultimately, on tumor development (see Figure 1). Importantly, some of these secreted proteins are induced 30 to 100 times over their normal levels, which may saturate the folding capacity of the endoplasmic reticulum. This overexpression can account for the activation of an Unfolded Protein Response (UPR) to drive senescence as described in melanocytic systems (Denoyelle et al., 2006).
The significance of the recent data of the Peeper, Gil and Green laboratories relies on the basic and translational implications of their observations. First, altogether these groups identified more than 20 different proteins whose inactivation can blunt oncogene-driven senescence. These results emphasize the rich nature of tumor suppression, but also illustrate multiple putative points of failure of these checkpoints. Second, the durability of premature senescence may not be simply determined by the endogenous genetic makeup of affected cells. As suggested by Kuilman et al., changes in the microenvironment affecting discrete nodes in the cytokine network, (e.g. IL-6 or C/EBPβ) may be sufficient to revert senescence into a proliferative state, without the need for additional genetic alterations (see Figure 1). Still, there is experimental evidence for the possibility of an adroit interference with secretome: exogenous administration of IGFBP-7 can blunt the growth of aggressive melanomas in vitro and in mouse xenograft models (Wajapeyee et al., 2008). To which extent even a transient dysbalance of pro-inflammatory cytokines can be achieved in a selective and effective manner in the clinic has yet to be demonstrated. In any case, it is clear that our understanding of the ‘senescence secretome’ and how to tackle it therapeutically will not longer come in the shape of linear one-way pathways. The Pandora’s box of cellular senescence is now populated with unexpected loops and turns.
Acknowledgements
The author apologizes to many researchers in the field of tumor suppression whose work could not be cited because of space limitations. Work in the group of M. Soengas is supported by grant NIH R01 CA107237 (M.S.S) and an institutional grant from the Spanish National Cancer Research Centre.