Comparative Effectiveness Analysis of Amlodipine/Renin-Angiotensin System Blocker Combinations
Abstract
J Clin Hypertens (Greenwich). 2012; 14:601–610. © 2012 Wiley Periodicals, Inc.
A comparative effectiveness analysis of antihypertensive therapy amlodipine (AML) and angiotensin receptor blocker (ARB) fixed- and loose-dose combinations (FDCs and LDCs) in achieving blood pressure (BP) reduction and Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7) goal attainment was made using retrospective electronic medical record (EMR) data. Treatment goal rates ranged from 35.0% for LDC AML/losartan to 45.7% for FDC AML/olmesartan (OM). FDC AML/OM achieved significantly greater reductions in systolic BP than FDC AML/benazepril (BEN), FDC AML/valsartan (VAL), and LDC AML/ARBs, respectively, and significantly greater reductions in diastolic BP than FDC AML/VAL and LDC therapy, respectively. Compared with patients treated with AML/OM, patients prescribed AML/VAL and LDC AML/ARB were significantly less likely to attain JNC 7 BP goal. Among subpopulations, AML/OM yielded higher rates of goal attainment among both African Americans and obese/overweight patients relative to AML/VAL and combined LDCs. Switchers from monotherapy with AML, OM, or VAL to AML/OM were significantly more likely to attain JNC 7 goals than those switching to AML/VAL or AML/BEN.
Nearly 33.5% of US adults have high blood pressure (BP),1 and the prevalence is expected to increase by 9.9% over the next 2 decades, reaching 37.3% by the year 2030.2 Untreated or undertreated hypertension is associated with significant cardiovascular morbidity and mortality1,3 and is a precursor to first stroke, first heart attack, or heart failure (HF) in approximately 75% of individuals.1
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7) recommends treatment to a BP <140/90 mm Hg for nondiabetic hypertensive persons and <130/80 mm Hg for those with diabetes or chronic kidney disease (CKD).4 However, despite recent improvements in BP control rates, hypertension remains inadequately managed in approximately 50% of all patients.5 About two thirds of the diagnosed hypertensive population will require combination therapy in order to achieve BP goals.6–8 Therapy using multiple antihypertensive agents from different therapeutic classes treats the disease through multiple physiologic pathways.7,9 JNC 7 guidelines recommend initial combination therapy for patients presenting with systolic BP (SBP) >20 mm Hg above goal or diastolic BP (DBP) >10 mm Hg above goal, since combination therapy increases the probability of achieving BP goals within a shorter period than single-drug therapy.4
A combination regimen of a calcium channel blocker (CCB) and angiotensin receptor blocker (ARB) benefits from the complementary mechanisms of action of these two classes. Dihydropyridine CCBs (DHP-CCBs) decrease the BP by promoting vasodilation, which may lead to activation of the sympathetic nervous system (SNS) and the renin angiotensin-aldosterone system (RAAS).10–12 This activation of SNS causes tachycardia and vasoconstriction, reducing the antihypertensive efficacy of the CCB; however, ARBs attenuate this counter-regulatory response.10–12 Furthermore, the addition of an ARB to a CCB significantly improves tolerability.11,12 CCBs produce arteriolar dilation, which leads to peripheral edema, a major side effect of CCB therapy,10–13 while ARBs cause venodilation, thereby decreasing the transcapillary gradient and ameliorating ankle edema.10–13 Moreover, the availability of ARB/CCB fixed-dose combinations (FDCs) lowers the pill burden to patients and is associated with improved adherence to therapy, and thus better treatment outcomes may be expected than observed with loose-dose combinations (LDCs).14,15 Two ARB/CCB FDCs, amlodipine/olmesartan (AML/OM) and amlodipine/valsartan (AML/VAL), are currently available in the US market.
The primary purpose of this study was to evaluate the comparative effectiveness of FDCs of AML/OM, AML/VAL, amlodipine/benazepril (AML/BEN), and LDCs of amlodipine and ARBs (olmesartan, valsartan, losartan [LOS], and irbesartan [IRB]) in real-world clinical practice. Secondary objectives included the evaluation of BP reduction among patient subgroups, including African Americans, diabetic patients, overweight and obese patients, and patients with chronic kidney disease (CKD).
Methods
Data Source
The datasource used in the study was the GE Centricity EMR database (GE Healthcare, Waukesha, WI). This database is populated at the point of care by clinical staff and all patient records are anonymized. Database content includes patient demographic information (such as age, sex, and race), as well as longitudinal clinical information (diagnoses, vital signs including BP measurements, body mass index [BMI], prescribed medications, clinical observations, and laboratory test results). As of December 2010, the database contained ambulatory electronic health record data for >18 million patients. These data were entered by >6000 physicians, 63% of whom were in primary care practice.
Study Population
Data for the time period of January 1, 2005, through December 31, 2010 were utilized for this study. Patient index date was defined as the earliest start date for one of the study medications on or after July 1, 2005, and prior to December 31, 2009, to ensure at least 6 months of baseline and 1 year of follow-up data for each patient. The FDC cohort included patients taking CCB/ARB FDCs AML/OM and AML/VAL, and CCB/ACE inhibitor FDC AML/BEN. The earliest instance of a prescription for an FDC was used for patients prescribed >1 FDC drug. Adult patients 18 years or older at the index date were eligible if they received a prescription for one of the study drugs during the timeframe defined above, with an available baseline BP that exceeded JNC 7 goal (≥140/90 mm Hg or ≥130/80 mm Hg for patients with diabetes or CKD).
Patients taking fixed-dose triple combination drugs such as AML/OM/hydrochlorothiazide (HCTZ) or AML/VAL/HCTZ were excluded, since the US launch dates for these drugs was too recent to allow for sufficient patient count based on the study identification period. Index date for LDC was based on the prescription date of the second drug in the sequence. LDC amlodipine and ARB combination cohorts were identified based on any of the following prescription permutations: ARB and AML, ARB/HCTZ and AML, AML and ARB, or AML and ARB/HCTZ without a prescription for any other study drug of interest in between the ARB and amlodipine.
The eligibility criteria for the study were as follows: age 18 years or older at index date and available baseline BP that exceeded the JNC 7 goal (≥140/90 mm Hg or ≥130/80 mm Hg for patients with diabetes or CKD).
No statistical adjustments were made to adjust for prior treatment status. However, the use of ARB or AML prior to the study index drug was captured as a variable for subgroup analyses. Based on International Statistical Classification of Diseases and Related Health Problems–Ninth Revision (ICD-9) codes, the presence of diabetes (ICD-9-Clinical Modification 250.xx), and/or CKD (ICD-9-Clinical Modification 585.xx) during the 6-month period preceding the index date was identified. The baseline BP on the study index date was obtained or, if missing, the last record within −30 days and +1 day of the index date was recorded. For each patient identified on the basis of the index study medication, all demographic information at baseline (age, race, sex, first and last activity dates, and BMI) was obtained. Pre-index and post-index BP data, records of prescribed antihypertensive medications, and comorbidity information based on selected ICD-9 codes were abstracted.
Outcome Measures
The primary study objective was to ascertain the effectiveness of AML/OM in BP reduction and JNC 7 BP goal attainment relative to the following: AML/VAL; AML/BEN; individual LDC AML+ARB combinations, namely AML+OM, AML+VAL, AML+IRB, and AML+LOS; and finally the AML/ARB LDC combined group. As randomized clinical trials have found that OM and IRB provide the greatest reduction in BP, followed by VAL and LOS,16,17 and previous retrospective, “real-world” studies have found improved BP lowering with OM as compared with IRB, LOS, and VAL,18,19 AML/OM was chosen as the reference group for all comparisons.
Primary outcomes of interest were systolic and diastolic BP change, calculated as the difference between the baseline value and the average follow-up value obtained over a 1-year period post-index. The follow-up value was calculated as an average of the quarterly post-index BP measures for each patient. Achievement of JNC 7 BP goal attainment was determined based on the average follow-up systolic and diastolic BP values.
Secondary objectives included the evaluation of goal attainment among the following 5 important subpopulations of interest: African Americans, diabetic patients, patients with BMI indicative of overweight (25 to <30) or obesity (≥30), and patients with CKD. Another objective was to compare goal attainment among patients who switched to AML/OM, AML/VAL, and AML/BEN from (1) monotherapy (OM, VAL, or AML) and (2) LDC AML and ARBs.
Statistical Methods
The longitudinal BP records of all eligible patients were included in the analysis. Daily average BP was calculated where multiple BP records existed in a single day. Day 6 to day 365 subsequent to the index date were used for follow-up BP data. The 1-year interval (day 6 to day 365) was divided into 4 equal quarters of 90 days each. Quarterly average BPs were calculated based on the average daily BPs. Finally, the average of the 4 quarterly BPs was used as the follow-up BP.
Treatment cohorts were compared on the basis of the difference in the mean change in SBP and DBP between baseline and follow-up, as well as on the basis of JNC 7 goal attainment using multivariate regression models. Analysis of covariance was used to estimate the difference in mean SBP and DBP change between cohorts. Logistic regression models were used to estimate the likelihood of attaining JNC 7 goal with the comparators relative to AML/OM. The following covariates were included in the regression models: age, sex, race, BMI, concomitant medication use (ie, number of non-ARB antihypertensive classes used at baseline), baseline comorbidities, year of index date, starting dose category (low, medium, high), baseline SBP and DBP, and propensity score. The assignment of dose categories was based on both contributing medications with the following logic: dose was considered low if both components of the combination were at a low dose (AML 2.5/5 mg, OM 20 mg, VAL 80/160 mg, LOS 50 mg, IRB 150 mg, BEN 10 mg); dose was considered high if both drugs were at a high dose (AML 10 mg, OM 40 mg, VAL 320 mg, LOS 100 mg, IRB 300 mg, BEN 40 mg); dose was considered medium if one component in the combination was high dose and the other was low dose, except in the case of benazepril (20-mg dose was considered medium).
The propensity score is the conditional probability of each patient receiving a particular treatment based on study baseline variables. Using the LOGISTIC procedure, propensity scores were calculated as the probability of being prescribed a treatment regimen based on the following patient baseline characteristics: sex, race, age group, existence of comorbidities (ischemic heart disease, nephritis, cardiovascular disease, diabetes mellitus, CKD, chronic HF [CHF]), body mass category, and baseline BP (SBP, DBP). Two propensity scores were calculated: (1) to reflect patients’ assignment to each of the seven treatment groups, and (2) to estimate the likelihood of treatment with AML/OM relative to the combined LDC group.
Results
Based on the study inclusion criteria, the final dataset consisted of 46,706 patients. The Figure 1 includes a consort diagram that outlines the study cohort identification algorithm. Table I includes the distribution of patients by treatment cohorts and associated baseline characteristics. The LDC study groups had more patients in the older age category, had higher rates of comorbidities, and had greater use of other concomitant antihypertensive medications compared with the FDC study groups. BMI and baseline BP values were similar across all study groups.
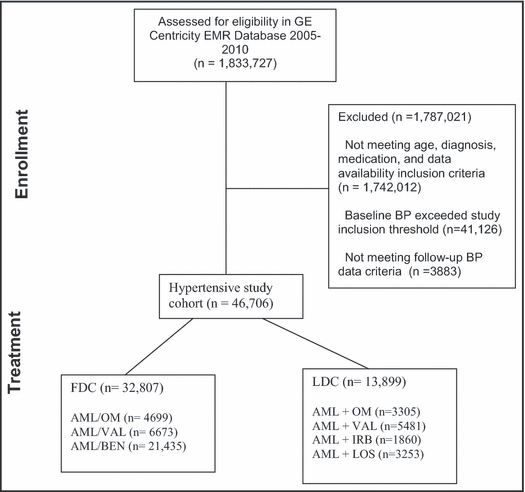
Study inclusion and exclusion criteria: consort diagram. See text for abbreviations.
| Fixed-Dose Combination Drugs | Loose-Dose Combination of ARB With AML | |||||||
|---|---|---|---|---|---|---|---|---|
| AML/OM (n=4699), No. (%) | AML/VAL (n=6673), No. (%) | AML/BEN (n=21,435), No. (%) | OM (n=3305), No. (%) | VAL (n=5481) No. (%) | IRB (n=1860), No. (%) | LOS (n=3253), No. (%) | Combined LDC (n=13,899), No. (%) | |
| ARB as single agent | 1926 (58.3) | 3268 (59.6) | 1178 (63.3) | 2126 (65.4) | 8498 (61.1) | |||
| ARB+HCTZ | 1379 (41.7) | 2213 (40.4) | 682 (36.7) | 1127 (34.6) | 5401 (38.9) | |||
| Age, y | ||||||||
| 18–40 | 339 (7.2) | 483 (7.2) | 1709 (8.0) | 117 (3.5) | 213 (3.9) | 52 (2.8) | 86 (2.6) | 468 (3.4) |
| 41–64 | 2382 (50.7) | 3314 (49.7) | 10,860 (50.7) | 1324 (40.1) | 2134 (38.9) | 712 (38.3) | 1247 (38.3) | 5417 (39.0) |
| 65+ | 1978 (42.1) | 2876 (43.1) | 8866 (41.4) | 1864 (56.4) | 3134 (57.2) | 1096 (58.9) | 1920 (59.0) | 8014 (57.7) |
| Sex | ||||||||
| Male | 1673 (35.6) | 2357 (35.3) | 7318 (34.1) | 1223 (37.0) | 2002 (36.5) | 754 (40.5) | 1222 (37.6) | 5201 (37.4) |
| Race | ||||||||
| White | 1673 (35.6) | 2357 (35.3) | 7318 (34.1) | 1198 (36.3) | 1976 (36.1) | 774 (41.6) | 1096 (33.7) | 5044 (36.3) |
| African American | 516 (11.0) | 862 (12.9) | 2513 (11.7) | 288 (8.7) | 710 (13.0) | 189 (10.2) | 435 (13.4) | 1622 (11.7) |
| Other | 112 (2.4) | 223 (3.3) | 575 (2.7) | 93 (2.8) | 195 (3.6) | 50 (2.7) | 125 (3.8) | 463 (3.3) |
| Missing | 2398 (51.0) | 3231 (48.4) | 11,029 (51.5) | 1726 (52.2) | 2600 (47.4) | 847 (45.5) | 1597 (49.1) | 6770 (48.7) |
| BMI | ||||||||
| Underweight (<18.5) | 22 (0.5) | 42 (0.6) | 128 (0.6) | 17 (0.5) | 31 (0.6) | 18 (1.0) | 19 (0.6) | 85 (0.6) |
| Normal (18.5–24.9) | 544 (11.6) | 813 (12.2) | 2714 (12.7) | 450 (13.6) | 690 (12.6) | 262 (14.1) | 434 (13.3) | 1836 (13.2) |
| Overweight (25–29.9) | 1218 (25.9) | 1674 (25.1) | 5544 (25.9) | 877 (26.5) | 1474 (26.9) | 490 (26.3) | 843 (25.9) | 3684 (26.5) |
| Obese (≥30) | 2358 (50.2) | 3409 (51.1) | 10,159 (47.4) | 1672 (50.6) | 2802 (51.1) | 926 (49.8) | 1699 (52.2) | 7099 (51.1) |
| Missing | 557 (11.9) | 735 (11.9) | 2890 (11.0) | 289 (8.7) | 484 (8.8) | 164 (8.8) | 258 (7.9) | 1195 (8.6) |
| Baseline BP | ||||||||
| SBP, mean (SD) | 158 (17.9) | 157 (17.6) | 156 (16.7) | 156 (17.2) | 156 (17.0) | 156 (17.2) | 156 (16.9) | 156 (17.0) |
| DBP, mean (SD) | 89 (13.3) | 89 (13.2) | 88 (13.0) | 85 (12.7) | 85 (12.7) | 84 (12.4) | 84 (12.9) | 85 (12.7) |
| Comorbiditiesa | ||||||||
| Ischemic heart disease | 617 (13.1) | 960 (14.4) | 2359 (11.0) | 577 (17.5) | 1015 (18.5) | 353 (19.0) | 634 (19.5) | 2579 (18.6) |
| Nephritis | 442 (9.4) | 767 (11.5) | 1661 (7.8) | 494 (15.0) | 857 (15.6) | 296 (15.9) | 577 (17.7) | 2224 (16.0) |
| Cardiovascular disease | 358 (7.6) | 575 (8.6) | 1511 (7.1) | 383 (11.6) | 653 (11.9) | 212 (11.4) | 400 (12.3) | 1648 (11.9) |
| Diabetes | 1406 (29.9) | 2172 (32.6) | 6279 (29.3) | 1218 (36.9) | 2151 (39.2) | 851 (45.8) | 1407 (43.3) | 5627 (40.5) |
| Chronic kidney disease | 293 (6.2) | 607 (9.1) | 1104 (5.2) | 322 (9.7) | 583 (10.6) | 206 (11.1) | 407 (12.5) | 1518 (10.9) |
| Heart failure | 168 (3.6) | 257 (3.9) | 611 (2.9) | 193 (5.8) | 346 (6.3) | 102 (5.5) | 231 (7.1) | 872 (6.3) |
- See text for abbreviations. aComorbid conditions defined by the presence of International Statistical Classification of Diseases and Related Health Problems–Ninth Revision–Clinical Modification codes during the 6-month period prior to study index date.
Overall, the proportion of patients taking concomitant medications tended to remain unchanged or slightly lower from baseline to follow-up. The proportion of patients who took a concomitant ACE inhibitor during the baseline period and the end of follow-up was lowest for AML/VAL (21.8% and 18.8%) followed by AML/OM (23.5% and 20.8%), the combined LDC cohort (24.6% and 20.1%), and AML/BEN cohort (31.9% and 29.3%). Concomitant diuretics declined slightly from baseline to follow-up and were most frequent in the LDC cohort (27.9–25.7%) followed by the AML/BEN cohort (24.0–22.1%), the AML/VAL cohort (22.4–20.9%), and the AML/OM cohort (22.4–20.7%). α-Blocker use was higher among patients taking ARB combinations at baseline and follow-up: LDC cohort (7.3% and 6.5%), AML/VAL (6.6% and 6.6%), and AML/OM cohort (5.8% and 5.8%), respectively. α-Blocker use in the AML/BEN cohort was 3.6% and 4.0% at baseline and follow-up, respectively. Concomitant direct rennin inhibitor use at baseline and follow-up was most common with the ARB FDCs, namely AML/VAL (3.8% and 4.1%) and AML/OM (2.9% and 3.1%) and was less frequent in the LDC cohort (1.1% and 1.0%) and among AML/BEN (0.3% and 0.6%).
Consistency in dosing was observed during the study follow-up period. Patients prescribed AML/OM, AML/VAL, or AML/BEN exhibited a high degree of consistency in that their initial dosage level (characterized as “low,”“medium,” or “high”) tended to remain unchanged among >70% of patients.
Table II details treatment comparisons of 1-year average BP changes, mean differences in SBP and DBP changes between comparators, and JNC 7 goal attainment rates for the entire sample. Patients in the AML/OM cohort experienced significantly greater regression-adjusted reductions in SBP compared with all other study groups. The mean regression-adjusted difference in SBP change from baseline to follow-up was significantly greater with AML/OM compared with AML/VAL (1.14 mm Hg, P<.001), AML/BEN (1.03 mm Hg, P=.0283), and combined LDC AML/ARB (1.73 mm Hg, P<.001). Similarly, regression-adjusted mean differences in DBP were significantly greater in the AML/OM cohort compared with all other study cohorts except AML/BEN. The mean regression-adjusted difference in DBP change from baseline to follow-up was significantly greater in the AML/OM cohort by 0.61 mm Hg (P<.001) vs AML/VAL, and by 0.68 mm Hg (P<.001) vs the combined LDC cohort. The absolute rate of JNC 7 BP goal attainment over the 1-year follow-up was the highest in the AML/OM cohort (45.7%). Attainment of JNC 7 BP goal was significantly less likely among patients treated with AML/VAL (odds ratio [OR]=0.86, P=.0004) and the combined LDC cohort (OR=0.76, P<.0001).
| Index ARB, No. | Mean BP Decrease From Baseline | 1-Y JNC 7 Goal Attainment | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | At Goal, % | Odds Ratio Relative to AML/OM | 95% CI | P Value | |||||||||||
| Unadjusted Mean (SD) | Regression-Adjusted | Unadjusted Mean (SD) | Regression-Adjusted | |||||||||||||
| Baseline | Mean Decrease | Decrease From Baseline | Diff in Change Relative to AML/OM | Baseline | Mean Decrease | Decrease From Baseline | Difference in Change Relative to AML/OM | |||||||||
| Mean | 95% CI | Mean | P Value | Mean | 95% CI | Mean | P Value | |||||||||
| AML/OM (4699) | 158.14 (17.9) | 20.15 (18.5) | 18.91 | 18.36–19.47 | – | – | 89.39 (13.3) | 9.78 (11.2) | 8.73 | 8.41–9.05 | – | – | 45.7 | – | – | – |
| AML/VAL (6673) | 157.49 (17.7) | 18.58 (18.4) | 17.77 | 17.32–18.21 | 1.14 | <.0001 | 88.76 (13.2) | 8.87 (10.6) | 8.12 | 7.86–8.37 | 0.61 | <.0001 | 41.6 | 0.86 | 0.79–0.93 | .0004 |
| AML/BEN (21,435) | 155.52 (16.7) | 17.83 (17.7) | 17.88 | 17.41–18.34 | 1.03 | .0283 | 88.22 (13.0) | 8.58 (10.7) | 8.24 | 7.97–8.51 | 0.49 | .0740 | 45.2 | 0.93 | 0.79–1.09 | .3655 |
| LDC OM+AML (3305) | 156.15 (17.2) | 16.76 (18.5) | 17.33 | 16.70–17.95 | 1.58 | <.0001 | 85.14 (12.7) | 7.11 (10.3) | 8.00 | 7.64–8.36 | 0.73 | <.0001 | 38.9 | 0.77 | 0.69–0.85 | <.0001 |
| LDC VAL+AML (5481) | 156.15 (17.0) | 16.42 (18.3) | 16.96 | 16.47–17.44 | 1.95 | <.0001 | 84.64 (12.7) | 6.98 (10.3) | 8.19 | 7.91–8.57 | 0.54 | .0014 | 38.1 | 0.78 | 0.70–0.86 | <.0001 |
| LDC IRB+AML (1860) | 156.08 (17.2) | 16.32 (18.2) | 17.21 | 16.44–17.99 | 1.70 | <.0001 | 83.85 (12.4) | 6.45 (9.9) | 8.05 | 7.60–8.50 | 0.68 | .0019 | 36.1 | 0.78 | 0.68–0.88 | .0001 |
| LDC LOS+AML (3253) | 155.63 (16.9) | 15.31 (17.7) | 16.48 | 15.86–17.09 | 2.43 | <.0001 | 84.05 (12.9) | 6.43 (10.1) | 7.92 | 7.57–8.28 | 0.81 | <.0001 | 35.0 | 0.72 | 0.64–0.80 | <.0001 |
| AML/OM (4699) | 158.14 (17.9) | 20.15 (18.5) | 18.51 | 18.07–18.91 | – | – | 89.39 (13.3) | 9.78 (11.2) | 8.07 | 7.83–8.32 | – | – | 45.7 | – | – | – |
| Combined LDC (13,899) | 156.02 (17.0) | 16.23 (18.2) | 16.78 | 16.55–17.01 | 1.73 | <.0001 | 84.52 (12.7) | 6.81 (10.2) | 7.39 | 7.26–7.52 | 0.68 | <.0001 | 37.3 | 0.82 | 0.75–0.89 | <.0001 |
- See text for abbreviations.
A general consistency in dosing was observed from index date to last follow-up date. Patients taking AML/OM, AML/VAL, and AML/BEN exhibited a high degree of consistency in that >70% of patients had their initial dosage level maintained. For patients taking LDC therapy, OM patients initially prescribed medium or high dosages tended to end the observation period at a lower dosage; 80% of LDC AML/OM patients who started at medium dosage finished at low dosage, and 43.6% who started at high dosage finished at medium dosage.
Table III presents mean differences in SBP and DBP changes between comparators and JNC 7 goal attainment rates for important subgroups. Among African Americans, the mean regression-adjusted difference in SBP change was significantly greater in the AML/OM cohort vs the AML/VAL cohort (1.33 mm Hg, P=.04). There were no statistically significant differences between FDC AML/OM and AML/BEN and LDC cohorts in terms of SBP change within this subgroup. Among African Americans, patients prescribed AML/VAL (OR=0.66, P<.01) and the combined LDC cohort (OR=0.72, P=.03) were significantly less likely to attain JNC 7 BP goal relative to patients prescribed AML/OM. Among diabetic patients, the mean regression-adjusted difference in SBP change was significantly greater for patients in the AML/OM cohort relative to the AML/VAL (1.23 mm Hg, P<.01) and combined LDC AML/ARB (0.94 mm Hg, P=.04) cohorts. The mean regression-adjusted difference in DBP change was significantly greater for patients in the AML/OM cohort relative to the AML/VAL (0.60 mm Hg, P<.01) cohort. The absolute rate of JNC 7 goal attainment was lower by nearly 50% among patients with diabetes relative to the overall sample. Diabetic patients in the combined LDC AML/ARB cohort (OR=0.78, P<.01) were less likely to attain JNC 7 BP goal relative to patients prescribed AML/OM.
| Race | Drug | Baseline Systolic | Regression-Adjusted Decrease From Baseline Systolic | Baseline Diastolic | Regression-Adjusted Decrease From Baseline Diastolic | Goal (%) | P Value Relative to AML/OM | 1-Y JNC 7 Goal Attainment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean | 95% CI | Mean (SD) | Mean | 95% CI | Odds Ratio Relative to AML/OM | P Value | ||||
| African American | AML/OM (516) | 158.98 (18.83) | 17.25 | 14.17–20.33 | 93.41 (13.15) | 7.39 | 5.61–9.18 | 35.27 | – | ||
| AML/VAL (862) | 157.76 (18.87) | 15.92 | 12.93–18.91 | 91.72 (12.92) | 7.20 | 5.46–8.93 | 28.42 | .0078 | 0.66 | <.01 | |
| AML/BEN (2513) | 155.91 (17.31) | 16.52 | 13.56–19.49 | 91.28 (12.65) | 7.49 | 5.77–9.21 | 33.78 | .5161 | |||
| Combined LDC | 156.70 (18.32) | 14.68 | 13.96–15.39 | 88.72 (13.01) | 7.49 | 7.09–7.89 | 26.02 | .82 | 0.72 | .033 | |
| Diabetes | AML/OM (1406) | 154.42 (18.75) | 18.32 | 15.37–21.26 | 84.63 (13.02) | 9.30 | 7.60–11.01 | 21.55 | – | ||
| AML/VAL (2712) | 154.12 (18.45) | 17.10 | 14.19–20.01 | 84.37 (12.69) | 8.70 | 7.01–10.38 | 19.89 | .2061 | |||
| AML/BEN (6279) | 151.71 (17.35) | 17.79 | 14.87–20.72 | 83.50 (12.21) | 9.28 | 7.59–10.98 | 21.79 | .846 | |||
| Combined LDC | 152.82 (17.52) | 13.60 | 13.26–13.95 | 81.07 (12.26) | 5.57 | 5.37–5.77 | 19.51 | .84 | 0.78 | <.01 | |
| Obese | AML/OM (2355) | 156.62 (17.73) | 19.04 | 16.12–21.96 | 90.18 (12.99) | 8.78 | 7.08–10.47 | 42.38 | – | ||
| AML/VAL (3408) | 155.91 (17.14) | 17.66 | 14.76–20.56 | 90.05 (13.04) | 8.18 | 6.50–9.86 | 38.85 | .0073 | 0.79 | .03 | |
| AML/BEN (10,145) | 154.08 (16.31) | 17.80 | 14.88–20.73 | 89.67 (12.68) | 8.23 | 6.54–9.93 | 41.79 | .6049 | 0.78 | .04 | |
| Combined LDC | 154.56 (16.61) | 15.36 | 15.06–15.67 | 85.67 (12.56) | 7.22 | 7.04–7.41 | 33.74 | .02 | 0.79 | <.01 | |
| Overweight | AML/OM (1221) | 158.98 (17.70) | 19.89 | 16.92–22.86 | 88.57 (13.44) | 9.65 | 7.92–11.37 | 50.45 | – | ||
| AML/VAL (1675) | 158.39 (17.49) | 19.01 | 16.07–21.94 | 87.31 (12.78) | 8.96 | 7.26–10.66 | 45.79 | .0132 | |||
| AML/BEN (5558) | 155.75 (16.29) | 19.01 | 16.07–21.95 | 86.98 (12.84) | 9.24 | 7.54–10.94 | 50.59 | .9277 | |||
| Combined LDC | 156.72 (16.99) | 17.88 | 17.43–18.34 | 83.40 (12.62) | 7.55 | 7.30–7.80 | 40.77 | <.01 | 0.70 | <.01 | |
| CKD | AML/OM (293) | 156.07 (18.76) | 20.19 | 16.95–23.42 | 82.24 (14.72) | 10.00 | 8.12–11.87 | 25.60 | – | ||
| AML/VAL (607) | 154.92 (18.4) | 18.10 | 15.05–21.14 | 81.11 (13.10) | 9.07 | 7.30–10.83 | 24.38 | .6924 | |||
| AML/BEN (1104) | 152.24 (18.36) | 18.79 | 15.80–21.77 | 80.53 (12.99) | 9.07 | 7.34–10.80 | 28.08 | .3979 | |||
| Combined LDC | 153.91 (18.10) | 14.99 | 14.33–15.66 | 80.02 (12.66) | 5.77 | 5.39–6.16 | 23.45 | .03 | 0.67 | .03 | |
- Abbreviations: CI, confidence interval; SD, standard deviation. See text for all other abbreviations.
For the subgroup of patients who were identified as obese, the mean regression-adjusted difference in SBP/DBP change was significantly greater in the AML/OM cohort vs the AML/VAL (1.49/0.68 mm Hg, P<.01), AML/BEN (2.01/1.10 mm Hg, P<.01), and combined LDC AML/ARB (1.57/0.50 mm Hg, P<.01 and P=.02, respectively) cohort. Among obese patients, the likelihood of JNC 7 goal attainment was significantly lower with AML/VAL (OR=0.87, P=.03), AML/BEN (OR=0.78, P=.04), and the combined LDC cohort (OR=0.79, P<.01) relative to patients taking AML/OM.
For the subgroup of patients who were identified as overweight, the mean regression-adjusted difference in SBP/DBP change was significantly greater in the AML/OM cohort compared with the combined LDC AML/ARB cohort (2.05/0.89 mm Hg, P<.01). Relative to the AML/OM cohort, patients in the LDC AML/ARB cohort were significantly less likely to attain JNC 7 BP goal (OR=0.70, P<.01).
Among patients with CKD, the mean regression-adjusted difference in SBP change was significantly greater in the AML/OM cohort compared with the AML/VAL (2.41, P=.02) and combined LDC AML/ARB cohorts (2.42 mm Hg, P=.01). Relative to the AML/OM cohort, patients in the LDC AML/ARB cohort were significantly less likely to attain JNC 7 BP goal (OR=0.67, P=.03).
Table IV presents the treatment comparisons of JNC 7 goal attainment rates for patients who were identified as being not at goal and switched from monotherapy and LDC therapy to FDC therapy. Among monotherapy switchers, 1538 switched to AML/OM, 2174 to AML/VAL, and 4317 to AML/BEN. Monotherapy switchers to AML/BEN had a lower likelihood (OR=0.86, P=0.03) of getting to JNC 7 goal relative to AML/OM. The likelihood of JNC 7 goal attainment for switchers from LDC was not significantly different between AML/OM and AML/VAL or AML/OM and AML/BEN.
| Patients Who Switched Treatments | |||
|---|---|---|---|
| AML/OM | AML/VAL | AML/BEN | |
| Switchers from monotherapy (OM, VAL, AML) or FDC (OM/HCTZ, VAL/HCTZ) not at goal at switcha | N=1538 | N=2174 | N=4317 |
| JNC 7 goal attainment (post-switch) | |||
| Yes, % | 49.15 | 46.73 | 48.23 |
| No, % | 50.85 | 53.27 | 51.77 |
| Odds ratio (95% confidence interval) of attaining goal relative to AML/OM | – | 0.94 (0.81–1.08) P=.37 | 0.86 (0.74–0.99) P=.03 |
| Switchers from LDC AML/ARBb | n=324 | n=551 | n=560 |
| JNC 7 goal attainment (post-switch) | |||
| Yes, % | 41.05 | 36.84 | 37.32 |
| No, % | 58.95 | 63.16 | 62.68 |
| Odds ratio (95% confidence interval) of attaining goal relative to AML/OM | – | 0.85 (0.62–1.16) P=.30 | 0.85 (0.60–1.20) P=.34 |
- See text for abbreviations. Covariates for logistic regression models: treatment group propensity score, hydrochlorothiazide use, baseline blood pressure, sex, age, race, body mass index (overweight, obese, undetermined), comorbidities (IHD, nephritis, CVD, DM, CKD, CHF), baseline hypertensive medicine count, baseline dosage indicator (low, high). aPatients switching from monotherapy to combination therapy. bPatients switching from LDC therapy to combination therapy.
Discussion
The primary objective of this study was to analyze the comparative effectiveness of CCB (amlodipine) and ARB FDCs and LDCs in achieving BP reduction and JNC 7 goal attainment using EMR data from real-world clinical practice; while the EMR-derived data are retrospective in nature with uneven baseline parameters, they reflect real-world therapeutic practices in a community-based setting. All combinations of ARBs and amlodipine studied were effective in lowering BP and these reductions were sustained in the study. Crude goal attainment rates ranged from 35.0% for the AML/LOS LDC cohort to 45.7% for the AML/OM cohort. Patients prescribed AML/OM attained significantly greater reductions in SBP compared with patients prescribed AML/BEN, AML/VAL, and LDC therapy with AML/ARBs, as well as significantly greater reductions in DBP compared with patients prescribed AML/VAL and LDC AML/ARB therapy. Compared with AML/OM, patients prescribed AML/VAL and LDC AML/ARB therapy were significantly less likely to attain JNC 7 BP goal.
These findings may have clinical relevance to hypertensive patients and their providers. Incremental reductions in SBP of between 2.0 mm Hg and 15 mm Hg have been reported to produce significant reductions in cardiovascular outcomes including myocardial infarction, cardiovascular mortality, and stroke.20 Risk of incident atrial fibrillation also increases with increasing SBP. Patients with SBP >140 mm Hg account for >82% of the increase in incident atrial fibrillation.20 In addition to various individual criteria, superior outcomes in terms of JNC 7 goal attainment may be considered as a parameter in guiding treatment decisions. The results in this study suggest that, at comparable doses and baseline BP levels, treatment with FDC therapy vs LDC therapy is associated with increased likelihood for patients to attain JNC 7 goal. It was observed that in general, goal attainment rates and reductions in SBP and DBP were greater in the FDC therapy cohorts (AML/OM, AML/VAL, AML/VAL) compared with the combined LDC cohort.
Several studies have demonstrated that the benefits of FDCs in a single tablet are associated with greater adherence, superior BP outcomes, and lower health care costs compared wtih the corresponding free-drug components given separately. A recent meta-analysis compared health care costs, adherence, and persistence between groups of patients taking antihypertensives as single-pill combinations (SPCs) with free-equivalent components.21 Combined total annual hypertension-related and all-cause health care costs were nearly $1350 lower for SPC than for free-equivalent component groups, and both adherence and persistence were greater for patients prescribed SPCs.
Another meta-analysis found that, compared with free-drug combinations, FDCs of antihypertensive agents were associated with a significant improvement in compliance and with nonsignificant beneficial trends in BP and adverse effects.22
Bangalore and colleagues23 compared patient compliance in 4 studies that involved FDC vs free-drug components of the same antihypertensive regimen given separately. FDC (pooled relative risk, 0.76; 95% confidence interval, 0.71–0.81; P<.0001) decreased the risk of medication noncompliance by 24% compared with LDC regimens (N=5750). Furthermore, improved adherence has been associated with a lower risk of cardiovascular events. In a study of patients newly treated with antihypertensive agents between 1999 and 2002 and followed-up for a 3-year period, it was found that patients with low adherence were more likely to have coronary disease, cerebrovascular events, and CHF within the 3-year follow-up period.24
Subgroup Analyses
This study confirms previous findings that goal attainment among African Americans, patients with diabetes and CKD, and obese patients is more difficult than for the general population.18 The results in the subgroups mirror the differences seen in the overall sample; treatment with AML/OM was associated with higher rates of JNC 7 goal attainment among African Americans and obese and overweight patients, relative to AML/VAL and the combined LDC cohort. Statistically significant differences were not observed between treatment cohorts in diabetic and CKD subgroups.
The efficacy of the AML/OM combination has been well established in difficult-to-treat subpopulations through prospective clinical trials, including African Americans, diabetic hypertensive patients, and obese patients.25–27
Data from previous randomized clinical trials comparing these ARBs suggest that OM and IRB offer the greatest reduction in BP, followed by VAL and LOS.16,17 In a previous, large (N=73,012) real-world retrospective analysis that utilized EMR data representative of ambulatory patients over 13 months, Ram and colleagues found that adjusting for covariates, overall BP reductions with OM, and OM/HCTZ were 1.88/0.86 mm Hg, 1.21/0.52 mm Hg, and 0.89/0.51 mm Hg greater than for LOS and LOS/HCTZ, VAL and VAL/HCTZ, and IRB and IRB/HCTZ, respectively.18 Additionally, mean differences were higher for monotherapy: 2.43/1.16 mm Hg, 2.18/0.93 mm Hg, and 1.44/0.91 mm Hg, respectively (all P values <.0001). In another analysis using medical charts (N=1293) and claims data, Miller and associates concluded that treatment with OM was associated with the highest proportion of patients achieving JNC 7 goal relative to VAL, LOS, and IRB.19 Additionally, an analysis that utilized real-world, retrospective claims data from a large, national managed care plan showed that during an average follow-up of 2.5 years among patients with newly initiated ARB therapy (N=65,579), treatment with OM was associated with lower risk of cardiac events and lower healthcare resource utilization vs VAL, LOS, and IRB.28
Effect of Switching the Treatment
Patients who were not at BP goal on monotherapy with AML, OM, or OM/HCTZ, and VAL or VAL/HCTZ, and switched to FDC therapy, were significantly more likely to attain recommended BP goals when switched to AML/OM than to AML/BEN. For switchers from LDC therapy to FDC therapy, statistically significant differences were not observed between the three FDCs therapies. These observations are in line with the findings of the prospective, open-label, titrate-to-goal Blood Pressure Control in All Subgroups With Hypertension (BP-CRUSH) study, in which 999 patients with hypertension uncontrolled on monotherapy were switched to fixed-dose AML/OM and uptitrated in terms of dose and/or addition of HCTZ as triple therapy.29 The cumulative percentage of patients achieving seated systolic BP <140 mm Hg (<130 mm Hg for patients with diabetes) by week 12 was 75.8% and by week 20 the cumulative BP threshold of <140/90 mm Hg was achieved by 90.3% of patients.
Study Limitations
Since our study employed a retrospective observational design, patient assignment to treatment group was dependent on clinical assessment by the individual’s physician. This was a study limitation due to differences in the indications for the different ARBs. It was also possible that a patient received an antihypertensive prescription from another physician that was not recorded in the EMR database. We employed propensity scoring to minimize the selection bias inherent in retrospective database studies. However, it is possible that some variables were not available for analysis and inclusion in the propensity score calculation, and it is difficult to know whether this may have impacted our study’s results.
It is possible that certain comorbidities such as CKD or diabetes may have been under-reported in the database; furthermore, ICD-9-Clinical Modification codes were solely used to identify these conditions, and better sensitivity may have resulted from the use of laboratory data (CKD) and/or antidiabetic medication use (diabetes). Additionally, information regarding race was not always present, and other socioeconomic and clinical patient characteristics, such as insurance type, income, and duration of hypertension, were not included in the analysis. In addition, since our study was retrospective in nature, BP measurements were not standardized and we cannot rule out measurement error in our study. However, as reported in an earlier study using the EMR database,18 it is unlikely that these omissions or that BP measurement errors systematically biased the findings in one treatment cohort over another. Another limitation of these data is the inability to extract meaningful safety information.
Details regarding prescription refills and medication compliance and persistence were not available, but given their similar tolerability profiles, compliance and persistence between different ARBs would be expected to be similar. Despite these limitations, this study has considerable implications in terms of the real-world data clinical practice trends.
Conclusions
Comparative effectiveness research, as carried out in this study, provides a practical and cost-effective way to complement randomized clinical trial data on treatment of hypertension. In this way, the effect of FDC and LDC therapy on BP reduction and goal attainment has been compared in the real-life clinical setting and provides an insight into treatment effectiveness in traditionally hard-to-treat populations, including African Americans, patients with diabetes, overweight and obese patients, and patients with CKD. A CCB/ARB combination regimen has been found to be a rational choice based on the complementary mechanisms of action of these two classes. The finding that goal attainment rates and reductions in SBP and DBP were, in general, numerically greater in the FDC treatment groups compared with the LDC groups indicates the potential benefits of improved adherence using a FDC therapy such as AML and OM. Given the potential long-term health benefits of even modest improvements in BP control, consideration of FDC therapy with the greatest real-world effectiveness could play an important role in hypertension management, particularly in hard-to-treat patient subgroups. Among the two ARB-based fixed-dose options studied, AML/OM demonstrated better goal attainment in the subgroups of African Americans and obese and overweight patients relative to AML/VAL. Finally, this study’s findings highlight the value of EMR databases in the evaluation of treatment-to-guideline recommendations in primary care settings.
Acknowledgments and disclosures: CER & Outcomes writing support was provided by Poornima Whomsley, PhD. This analytical research project was supported by funds from Daiichi Sankyo USA. All authors declared no competing interests.




