Characteristics of pancreatic diabetes in patients with autoimmune pancreatitis
Conflict of interest: the authors have no competing interests to declare.
Abstract
OBJECTIVE: Although patients with autoimmune pancreatitis (AIP) tend to have concurrent diverse disorders, very few studies have focused on diabetes mellitus (DM) coexisting with AIP.
METHODS: In total 102 AIP patients with DM were divided into three groups. Those with DM before the onset of AIP were labeled group A (n = 35), those who developed DM and AIP simultaneously were labeled group B (n = 58) and those who developed DM after steroid therapy for AIP were labeled group C (n = 9). The characteristics of DM among the three groups were evaluated.
RESULTS: No significant differences were noted in the age of DM onset among the three groups. However, the mean duration of DM was significantly longer in group A (8.7 years) than in groups B and C. AIP developed 6.8 years after DM onset in group A, whereas it developed 1.8 years after steroid therapy in group C. Group A had the highest rate (25.7%) of family members with a history of AIP. Levels of serum albumin, total cholesterol and triglyceride were significantly lower in group A. No correlations were found between glycated hemoglobin and benzoyl-tyrosyl para-aminobenzoic acid. Hypoglycemia was observed in 20% of patients under insulin therapy. Most of them were habitual drinkers and received no pancreatic enzymes. Group A showed a high prevalence of retinopathy, nephropathy and macrovascular disorders than group B.
CONCLUSION: Aspects of AIP-associated pancreatic diabetes were clarified. AIP-associated DM must be controlled by a full assessment of the pancreatic endocrine and exocrine function.
INTRODUCTION
Autoimmune pancreatitis (AIP) is a distinctive type of pancreatitis, and it is thought that the pathogenesis of AIP involves autoimmune mechanisms.1–6 Recently AIP has been regarded as a systemic disease because it is often accompanied by various extrapancreatic lesions including cholangitis, sialadenitis, retroperitoneal fibrosis, hilar lymphadenopathy and chronic thyroiditis.7–10 Furthermore, AIP is often associated with pancreatic exocrine and endocrine dysfunction. Pancreatic exocrine dysfunction is reported in 83%–88% of AIP cases and diabetes mellitus (DM) in 42%–78%.10–14 However, very few reports have been published on AIP-associated pancreatic diabetes and its pathophysiology, complications and treatment have not been investigated at all. We recently examined the epidemiology of pancreatic diabetes in Japan in 2005 using a nationwide stratified random sampling method.15 In this report, no analysis of AIP-associated pancreatic diabetes was included. Accordingly, in the current study we selected AIP patients from the pancreatic diabetes patients surveyed and conducted a detailed analysis of the pathophysiology, complications and treatment of AIP-associated pancreatic diabetes.
MAERIALS AND METHODS
We conducted a nationwide survey on patients with pancreatic diabetes who were treated in 2005 in Japan.15 The stratified random sampling method was used to select hospital departments for survey. The departments of internal medicine, metabolism, diabetes, endocrinology, and surgery in each hospital were selected. The sampling rate was 5%, 10%, 20%, 40%, 80% and 100% for the general hospitals with less than 100 beds, 100–199 beds, 200–299 beds, 300–399 beds, 400–499 beds and 500 or more beds, and the university hospitals were selected in the same proportion. We added other relevant departments where many patients with pancreatic diabetes were expected to be treated in order to increase the efficiency of this survey.
The study consisted of two surveys, each using a different questionnaire. In the first survey, a simple questionnaire was used to ascertain the number of patients with pancreatic diabetes who were treated in 2005. Using the abovementioned sampling rate, this questionnaire was mailed directly to the heads of 3459 randomly selected departments. Next, the second questionnaire was forwarded to those departments from which patients with pancreatic diabetes were reported on the first questionnaire. The second questionnaire asked for detailed clinical information on individual patients regarding the etiology, symptoms and procedures for the diagnosis of pancreatic diseases as well as diabetic complications, treatment and prognosis. No patient with type 1 diabetes was included in the present survey.
In the present study we focused on patients with pancreatic diabetes associated with AIP. In all 102 AIP cases (84 men and 18 women; mean age 68.9 ± 10.7 years) were collected for the second survey. All the patients fulfilled the clinical diagnostic criteria for AIP of the Japan Pancreas Society in 2002. The 102 AIP patients with DM were divided into three groups at the time when DM was diagnosed: the patients who had DM before onset of AIP were put in group A (n = 35), the patients who developed DM and AIP simultaneously were put in group B (n = 58), and the patients who developed DM after the initiation of steroid therapy for AIP were put in group C (n = 9). Details regarding their age at AIP onset, their age at DM onset, the duration of DM, the family history of DM, nutritional status, condition of DM control, pancreatic exocrine function, treatment of DM and diabetic complications were examined. Their nutritional status was examined in terms of their body mass index (BMI), the levels of serum in total protein (TP), albumin (ALB), total cholesterol (TC) and triglyceride (TG). DM control levels were examined by dividing the patients into three groups according to their glycated hemoglobin (HbA1c) level: ≤6.5, 6.6–7.5 and ≥7.6%. Levels ≥7.6% were defined as poor DM control. Pancreatic insufficiency was investigated by dividing the patients into three groups according to the results of N-benzoyl-L-tylosyl-p-aminobenzoic acid (BT-PABA) test: <50%, 50%–70% and >70%. Patients with levels ≤70% were defined as having pancreatic exocrine dysfunction, among them <50% were defined as severe pancreatic exocrine dysfunction, BT-PABA test levels > 70% were defined as normal value.
As the present survey was conducted to study general pancreatic diabetes serum levels of immunoglobulin G4, the extent of pancreatic involvement (e.g. diffuse or segmental) and the steroid dose were unfortunately not addressed in the present survey. Furthermore, 30 out of 35 patients (85.7%) in group A, 51 out of 58 patients (87.9%) in group B, and all nine patients (100%) in group C were treated with steroids.
Statistical analysis
Statistical analysis was performed using StatView 5.0 (SAS Institute Inc., Cary, NC, USA). The data are presented as mean ± standard deviation (SD). An anova followed by Tukey's test was used for statistical analysis. A P-value of less than 0.05 was considered significant for all analyses.
RESULTS
Characteristics of pancreatic diabetes in patients with AIP (Table 1)
| Year | Group A (n = 35) | Group B (n = 58) | Group C (n = 9) |
|---|---|---|---|
| Age | 71.7 ± 9.5 | 62.1 ± 10.2* | 65.9 ± 8.7 |
| Age at onset of AIP | 68.5 ± 9.8 | 58.3 ± 11.1* | 61.0 ± 9.4 |
| Age at onset of diabetes | 62.7 ± 11.0 | 58.6 ± 10.6 | 62.8 ± 8.7 |
| Morbidity period of diabetes (years) | 8.7 ± 4.2 | 3.5 ± 2.8* | 3.1 ± 1.8* |
- * P < 0.05 with respect to group A. Data are presented as mean ± standard deviation (SD).
The mean age of patients in groups A, B and C was 71.7 ± 9.5, 62.1 ± 10.2 and 65.9 ± 8.7 years, respectively. The mean age of group B patients was significantly lower than that of group A patients (P < 0.001). The age of AIP onset in group B was significantly lower than that in group A (P < 0.001). No significant differences were observed in the mean age of patients in DM onset among the three groups. However, the duration of DM in group A patients (8.7 ± 4.2 years) was significantly longer than in groups B (3.5 ± 2.8 years) (P < 0.001) and C (3.1 ± 1.8 years) (P < 0.001). Further, the interval between DM onset and AIP onset was 6.8 ± 5.1 years in group A. In group C, the interval between DM onset and steroid therapy following AIP onset was 1.8 ± 1.6 years. A family history of DM was found in 21.6% of all patients. The rate of a DM family history in groups A, B and C was 25.7%, 19.0% and 11.1%, respectively. Group A had the highest rate among the three groups.
Assessment of nutritional status in patients with AIP-associated pancreatic diabetes (Table 2)
| Group A (n = 35) | Group B (n = 58) | Group C (n = 9) | |
|---|---|---|---|
| Body mass index (kg/m2) | 22.1 ± 2.9 | 22.1 ± 3.1 | 21.2 ± 1.1 |
| Total protein (g/dL) | 7.1 ± 0.73 | 7.0 ± 0.63 | 6.9 ± 0.60 |
| Albumin (g/dL) | 3.8 ± 0.55* | 4.0 ± 0.38 | 4.0 ± 0.50 |
| Total cholesterol (mg/dL) | 179.4 ± 40.7* | 202.1 ± 46.8 | 213.1 ± 70.5 |
| Triglyceride (mg/dL) | 119.9 ± 70.6* | 138.0 ± 73.8 | 136.4 ± 34.9 |
- * P < 0.05 with respect to group B. Data are presented as mean ± standard deviation (SD).
No significant differences were observed in the BMI among three groups. However, the levels of ALB, TC, and TG were significantly lower in group A than in group B: P < 0.05, P < 0.05, and P < 0.05, respectively.
Relationship between diabetic control and pancreatic exocrine function (Table 3)
| Group A % (n = 35) | Group B % (n = 58) | Group C % (n = 9) | |
|---|---|---|---|
| HbA1c (%) | |||
| ≤6.5 | 37.1 | 43.6 | 33.3 |
| 6.6–7.5 | 28.6 | 30.9 | 22.2 |
| ≥7.6 | 34.6 | 25.5 | 44.5 |
| Group A % (n = 18) | Group B % (n = 27) | Group C % (n = 5) | |
|---|---|---|---|
| BT-PABA (%) | |||
| <50 | 27.8 | 18.5 | 20.0 |
| 50–70 | 55.5 | 44.5 | 60.0 |
| >70 | 16.7 | 37.0 | 20.0 |
- BT-PABA, N-benzoyl-L-tylosyl-p-aminobenzoic acid; HbA1c, glycated hemoglobin.
Patients with poor DM control (HbA1c ≥ 7.6%) constituted 34.6% of group A, 25.5% of group B and 44.5% of group C, respectively. Meanwhile, pancreatic exocrine dysfunction (BT-PABA ≤ 70%) was noted in 74.0% of all patients, and observed in 83.3% of group A, 63.0% of group B, and 80.0% of group C, respectively. Next, we examined the relationship between DM control (HbA1c values) and pancreatic exocrine function (BT-PABA values) among the three groups, but no significant difference was observed (P = 0.486).
Treatment of AIP-associated pancreatic diabetes
Among treatment regimens for all patients with AIP-associated pancreatic diabetes, insulin therapy (49.0%) was the most frequent, followed by diet therapy (28.4%), oral hypoglycemic agent (21.6%) and unknown (1.0%) (Fig. 1). Insulin was used in 57.1% of group A patients, which was slightly higher than that of groups B and C (Table 4). On the other hand, the mean insulin dosage was the highest in group A (27.0 ± 9.6 units), and it was significantly higher than that of group B (18.1 ± 8.6 units) in particular (P < 0.05) but it was not significantly different from that of group C (23.7 ± 19.5 units). Among 50 patients under insulin therapy, 10 experienced hypoglycemia at least once a month (four patients in group A and six patients in group B). The profile of these 10 patients is shown in Table 5. Nine of the 10 patients were alcohol drinkers, and five were continual drinkers at the time of the survey. Despite the diagnosis of pancreatic exocrine disorder in all patients, eight patients were not treated with pancreatic enzymes.
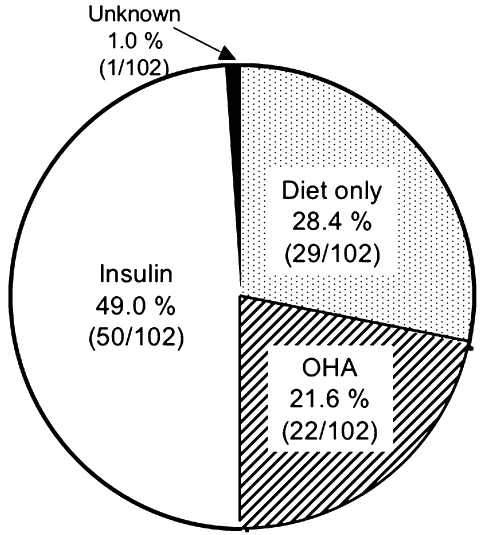
Treatment for pancreatic diabetes in patients with autoimmune pancreatitis. OHA: oral hypoglycemic agent.
| Group A (n = 35) | Group B (n = 58) | Group C (n = 9) | |
|---|---|---|---|
| Diet only | 22.9 | 32.8 | 22.2 |
| Oral hypoglycemic agent | 20.0 | 20.7 | 33.3 |
| Insulin | 57.1 | 44.8 | 44.5 |
| Unknown | 0 | 1.7 | 0 |
| Case | History of drinking alcohol | Continued drinking alcohol | Treatment with enzymes | Total dose of insulin (units) |
|---|---|---|---|---|
| Group A | ||||
| 1 | Yes | No | No | 25 |
| 2 | Yes | Yes | No | 30 |
| 3 | Yes | Yes | No | 28 |
| 4 | Yes | Yes† | Yes | 40 |
| Group B | ||||
| 1 | Yes | No | No | 28 |
| 2 | No | No | Yes | 20 |
| 3 | Yes | No | No | 22 |
| 4 | Yes | No | No | 12 |
| 5 | Yes | Yes | No | 26 |
| 6 | Yes | Yes† | No | 31 |
- † Heavy drinkers.
Complications of AIP-associated pancreatic diabetes
DM complications in AIP patients included retinopathy (6.9%), neuropathy (11.8%), nephropathy (7.7%), cerebrovascular disorders (3.9%) and cardiac diseases (8.8%) (Fig. 2). Group A showed a high prevalence of both microvascular disorders (retinopathy and nephropathy) and macrovascular disorders (cerebrovascular and cardiac diseases) than group B (Table 6).
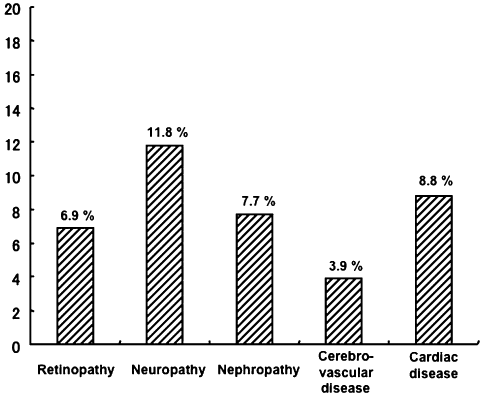
Frequency of diabetes-related complications in patients with autoimmune pancreatitis.
| Group A % (n = 35) | Group B % (n = 58) | Group C % (n = 9) | |
|---|---|---|---|
| Retinopathy | 8.6 | 6.9 | 0 |
| Neuropathy | 11.4 | 12.1 | 11.1 |
| Nephropathy | 11.4 | 5.2 | 11.1 |
| Cerebrovascular disease | 8.6 | 1.7 | 0 |
| Cardiac disease | 20.0 | 3.4 | 0 |
DISCUSSION
There have been very few epidemiological studies on DM coexisting with AIP and thus opportunities to discuss the characteristics of AIP-associated pancreatic diabetes are limited. Previously, Nishimori et al. conducted a field survey on AIP in 2002 in Japan.11 The annual number of AIP in Japan was estimated at approximately 900 (95% CI 670–1100) or 0.71 per 100 000 individuals in the Japanese population. In this survey it is reported that patients with AIP were associated with DM in the rate of 66.5%. Among those, 33.3% had DM before the onset of AIP and 51.6% started developing DM concurrently with the onset of pancreatitis. Furthermore, 14% developed DM after steroid treatment.11 In the present study, 34.3% of patients (35/102, group A) already had diabetes before AIP onset; 56.9% of patients (58/102, group B) developed diabetes concurrently with AIP and 8.8% of patients (9/102, group C) developed diabetes after steroid administration was started because of the therapy for the onset of AIP. These findings nearly concur with the data reported by Nishimori et al.11 AIP patients in group B are considered as having truly AIP-induced pancreatic diabetes. On the other hand, AIP patients in group A are thought to have developed type 2 diabetes before the onset of AIP; therefore, the frequency of a family history of diabetes may have been higher compared with groups B and C. Further, compared with groups B and C, patients with poor nutritional condition and those under insulin therapy were more frequently found in group A because of the longer duration of their diabetes. Furthermore, the incidence of diabetic complications was probably higher in group A because of the longer duration of DM. It is known that the duration and type of DM control are substantial factors for the development of diabetic complications in patients with pancreatic diabetes, as in the case of patients with types 1 and 2 diabetes.15,16,17 Therefore, it is considered important to assess complications and monitor the clinical treatment of pancreatic diabetes over time.18–20 Meanwhile, the mechanism of the concurrent onset of DM with AIP in group B was considered to be impaired blood flow to islet cells10,21 and damaged function of the islets of Langerhans because of fibrosis and inflammation,10,22 although further studies are needed.23
A certain consensus has been reached concerning the initial treatment of AIP, especially remission induction therapy with oral steroids, and this therapy has broadly been followed.10,23,24 However, no consensus has been obtained regarding the advisability of maintenance therapy after remission induction therapy, including applicable cases, maintenance dosage of steroids and duration of maintenance therapy.6 AIP tends to occur in relatively elderly people, especially people in their sixties.10,11 Many of these elderly patients concurrently have lifestyle-related diseases such as diabetes, hyperlipidemia and hypertension as well as issues such as osteoporosis that are often induced by steroids. Therefore, long-term steroid therapy requires risk-benefit considerations.10,11,23 Our study also revealed that the average age of AIP onset was as high as 68.9 years. Moreover, nine of the 102 patients (8.8%) developed diabetes an average of 1.8 years after the initiation of steroid treatment for AIP (group C). Therefore, even if diabetes does not exist at the time of AIP onset, the possibility of DM onset should always be kept in mind in patients who continuously receive steroid treatment over long periods of time. Further, consensus will have to be reached quickly concerning maintenance therapy after remission induction, especially duration of treatment and criteria for discontinuation.
Although little is known about the long-term prognosis of AIP, steroid therapy has brought about improvement of pancreatic endocrine and exocrine functions in some AIP patients.2 Interestingly, it has been reported that pancreatic exocrine and endocrine functions improved after treatment with steroids in 38%14 to 50%10 and in 25%14 to 45%10 of patients with AIP, respectively. Pancreatic endocrine and exocrine functions are possibly improved by steroid therapy through an increase of enzyme secretion because of regression of infiltrative inflammatory cells and fibroblasts, the improvement of pancreatic juice flow caused by narrowing of pancreatic duct,21 and the possible regeneration of the islets of Langerhans following the suppression of local cytokine production in the pancreas.22 Unfortunately, we did not conduct a follow-up survey in this study. Therefore we could not investigate the changes of glucose after the follow-up period. In the future, a follow-up investigation will be required. A recent report suggested that steroid therapy reduces inflammation and restores both pancreatic enzyme and hydrogen carbonate secretion in patients with AIP by regenerating acinar cells and correcting cystic fibrosis transmembrane conductance regulator localization in pancreatic duct cells.25 On the other hand, DM control worsens after steroid therapy in 75% of AIP patients with pre-existing type 2 diabetes.10 As a result, the pancreatic endocrine and exocrine function is not necessarily improved in all AIP patients.
The present study showed that as many as 49.0% of patients with AIP-associated pancreatic diabetes were treated with insulin. Hypoglycemia was experienced by 20% of patients under insulin therapy (10/50) at least once a month. Interestingly, nine of these 10 patients were alcohol consumers, and four were continually drinking alcohol. Further, despite the diagnosis of pancreatic exocrine dysfunction, pancreatic enzymes were not administered in eight patients. As a basic treatment for pancreatic diabetes, pancreatic enzymes should be administered at the dosage appropriate for each patient's level of pancreatic exocrine dysfunction and then the insulin dosage should be determined upon consideration of blood glucose control that may temporarily be worsened by the administration of pancreatic enzymes.19 Thus, a patient's nutritional condition can be well maintained and hypoglycemia can be prevented.19 Further, according to a long-term follow-up survey on chronic pancreatitis patients in Japan, diabetes developed in 28.9% of these patients during the 8-year period, and the greatest risk factor for diabetes onset was continual drinking.26 Another report stated that patients with true pancreatic diabetes and alcohol-induced chronic pancreatitis frequently experienced hypoglycemia leading to death if they continually drank alcohol and used insulin.15 Therefore, patients with AIP-associated pancreatic diabetes also should receive appropriate lifestyle guidance focusing on drinking cessation.
In conclusion, the present study yielded a new finding on pancreatic diabetes with AIP. A full assessment of the pancreatic endocrine and exocrine function is important in managing AIP-associated pancreatic diabetes. Furthermore, our study indicated that lifestyle guidance, including drinking cessation, is important for AIP patients.
ACKNOWLEDGMENTS
This study was supported by the Research Committee of Intractable Pancreatic Diseases (principal investigator: Tooru Shimosegawa) provided by the Ministry of Health, Labour, and Welfare of Japan. The authors are most grateful to the doctors who responded to the questionnaires.




