Do scattered white spots in the duodenum mark a specific gastrointestinal pathology?
Abstract
OBJECTIVE: To discover whether scattered white spots (SWS) in the duodenum are related to a specific kind of disease. We also scrutinized other upper endoscopic findings which might be associated with SWS.
METHODS: Among the patients who were admitted for upper gastrointestinal system endoscopy, those having the endoscopic appearance of SWS in duodenum were enrolled in this study. In total 107 patients [70 women, 37 men, mean age: 51.6 ± 16.88 years (range: 17–82 years)] were included. At least three duodenal biopsies were taken from each patient and histopathological examinations were done by experienced pathologists.
RESULTS: The histopathological examination revealed that 39 (36.4%) patients had intestinal lymphangiectasia (IL), 15 (14%) patients had giardiasis (G) and 30 (28.1%) patients had chronic non-specific duodenitis (CD). Two patients with IL were also found to have G. Twenty patients had both IL and CD. One had both G and CD. G was the least common etiology for SWS in the duodenum. The most common reasons for SWS in the duodenum in this study group were IL and CD, in order of decreasing frequency. There was no significant relationship with the other upper endoscopic findings in all these patients.
CONCLUSION: Histopathological examinations should be provided for each patient with SWS in the duodenum to assess the etiology.
INTRODUCTION
Scattered white spots (SWS) in the duodenum are a relatively uncommon endoscopic finding in upper gastrointestinal endoscopy.1–5 They are usually seen diffusely in the proximal duodenum and cannot be washed or rubbed away (Fig. 1). Tiny SWS can also be seen together with white villi, especially in patients with intestinal lymphangiectasia (IL).3–5 These are thought to indicate stasis of lymphatic flow. However, these spots may also be seen in individuals without symptoms or in patients with disorders including giardiasis.1,6
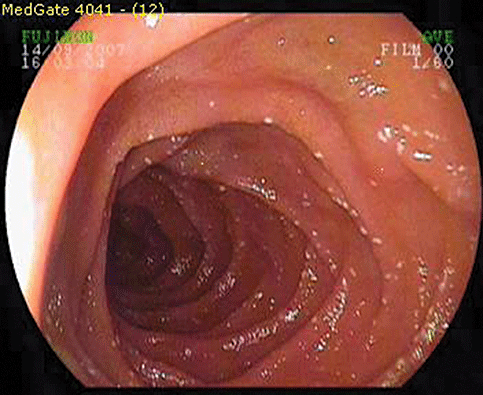
Endoscopic appearance of scattered white spots in duodenum.
To our knowledge there has been no study investigating the spectrum of pathologies in a group of patients with this endoscopic appearance. Moreover, there are no data in the literature of the other endoscopic findings which may be associated with or relevant SWS in the proximal duodenum. Hence, we aimed to investigate the specific histopathological diagnosis in patients with SWS diffusely scattered throughout the duodenum and document the other upper gastrointestinal endoscopic findings in those patients.
MATERIALS AND METHODS
All patients undergoing an upper gastrointestinal endoscopy (UGE) at Ankara Education and Research Hospital during the period January 2006 to September 2007 were included in this prospective study. We surveyed 9008 consecutive adult patients (≥16 years old) who were referred for endoscopy from several departments and outpatient clinics for upper gastrointestinal system symptoms. Patients requiring an urgent endoscopy for upper gastrointestinal bleeding and incomplete or duplicate procedures were excluded. Data collection was performed in the endoscopy reports. Overall 107 patients were diagnosed as having SWS in the duodenum.
Food intake was withheld at least 15 hours before the endoscopic examination. Written informed consent was obtained from all patients before the endoscopic procedures. The UGE was performed by four endoscopists using a videogastroscope with forward viewing (Fujinon EVE S400, Saitama City, Japan). All examinations were performed with the patient in the left lateral decubitus position. Premedication consisted mostly of topical anesthesia only, and in combination with midazolam 2.5–5 mg and/or meperidine 25–50 mg i.v. when needed. Special attention was paid to the duodenum. We also documented the endoscopic findings of upper gastrointestinal system including esophagitis, Barrett's esophagus, hiatal hernia, open cardia, gastritis, bulbitis and ulcer in all patients.
At least three duodenal biopsies for histological investigation were taken from the second and or third portion of the duodenum containing diffusely scattered, tiny white spots. The biopsy samples were put in a 10% formalin solution and then embedded in paraffin. The histopathological examinations were done by two experienced pathologists. HE stained sections from three duodenal biopsies from each patient were evaluated for pathogens, e.g., giardia lamblia and cryptosporidium species, lamina propria inflammation, intraepithelial lymphocytes and villous architecture. In addition, a periodic acid-Schiff (PAS) stain was performed to rule out Whipple's disease. Biopsies obtained during lower endoscopy were examined in HE and trichrome-(van Gieson elastin) stained slides. All slides were independently examined by two pathologists who were blind to the clinical and endoscopic data. Activity, inflammation, atrophy, architecture of villi, erosions and ulcerations and infiltration of neutrophils, lymphocyte, eosinophils and plasma cell were evaluated by the pathologists. Chronic non-specific duodenitis (CD) was described as mild (inflammation with edema and infiltration of leukocytes and increased number of plasma cells in the lamina propria) and overt duodenitis (additionally architectural distortion with shortening and blunting of intestinal villi).
Data analysis was performed using SPSS for Windows, version 11.5 (SPSS, Chicago, IL). Nominal data were expressed as the number and percentage of the patients. Categorical comparison was done by using Pearson χ2 and continuity corrected χ2 or Fisher's exact tests where applicable. A P-value less than 0.05 was considered statistically significant.
RESULTS
Of 107 patients, 37 (34.5%) patients were men and 70 (65.5%) were women. The mean age was 51.6 ± 16.8 years (range; 17–82 years).
The histological examination revealed the following pathologies in decreasing order: intestinal lymphangiectasia (IL), chronic non-specific duodenitis (CD) and giardiasis (G). There were also patients with an overlap of those disorders (Tables 1 and 2).
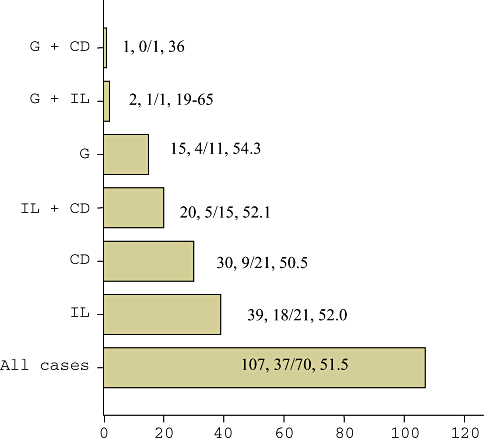
| Frequency | Percentage (%) | |
|---|---|---|
| IL | 39 | 36.4 |
| G | 15 | 14.0 |
| CD | 30 | 28.1 |
| IL+G | 2 | 1.9 |
| IL+CD | 20 | 18.7 |
| G+CD | 1 | 0.9 |
| Total | 107 | 100 |
- CD, chronic non-specific duodenitis; G, giardiasis; IL, intestinal lymphangiectasia.
A duodenal histology revealed reduced villous heights in four patients (3.73%), two of whom had CD, one had IL and the rest had both CD and IL simultaneously. Three patients who had lymphoid follicle at histology had giardiasis. We did not find any patient with a histology consistent with Celiac disease (no patient with increased intraepithelial lymphocytes, total villous atrophy, etc.). There were also no patients with PAS-positive macrophages in duodenal biopsy samples.
Other UGE findings of esophagus, stomach and bulbus of the duodenum were similar in the three groups. The whole upper endoscopic findings are summarized in Table 3.
| Giardiasis N (%) | P | IL N (%) | P | Chronic duodenitis N (%) | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| − | + | − | + | − | + | ||||
| Barrett's esophagus | 0 | 1 | 1 | 0 | 0 | 1 | |||
| (0) | (5.6) | 0.168 | (2.2) | (0) | 0.430 | (0) | (2.0) | 0.477 | |
| LES insufficiency | 8 | 4 | 5 | 7 | 8 | 4 | |||
| (9.0) | (22.2) | 0.116 | (10.9) | (11.5) | 1.000 | (14.3) | (7.8) | 0.454 | |
| Hiatus hernia | 5 | 0 | 3 | 2 | 2 | 3 | |||
| (5.6) | (0) | 0.587 | (6.5) | (3.3) | 0.650 | (3.6) | (5.9) | 0.668 | |
| Esophagitis | 17 | 6 | 15 | 8 | 10 | 13 | |||
| (19.1) | (33.3) | 0.211 | (32.6) | (13.1) | 0.028 | (17.9) | (25.5) | 0.337 | |
| Gastric ulcer | 8 | 1 | 3 | 6 | 5 | 4 | |||
| (9.0) | (5.6) | 1.000 | (6.5) | (9.8) | 0.729 | (8.9) | (7.8) | 1.000 | |
| Gastritis (total) | 87 | 18 | 45 | 60 | 55 | 50 | |||
| (97.8) | (100) | 1.000 | (97.8) | (98.4) | 1.000 | (98.2) | (98.0) | 1.000 | |
| Duodenal ulcer | 4 | 1 | 4 | 1 | 2 | 3 | |||
| (4.5) | (5.6) | 1.000 | (8.7) | (1.6) | 0.162 | (3.6) | (5.9) | 0.668 | |
| Duodenitis | 14 | 6 | 9 | 11 | 15 | 5 | 0.045 | ||
| (15.7) | (33.3) | 0.100 | (19.6) | (18) | 1.000 | (26.8) | (9.8) | ||
| Superficially erosive gastritis | 20 | 4 | 12 | 12 | 11 | 13 | |||
| (22.5) | (22.2) | 1.000 | (26.1) | (19.7) | 0.431 | (19.6) | (25.5) | 0.469 | |
| Erythematous exudative gastritis | 62 | 11 | 30 | 43 | 38 | 35 | |||
| (69.7) | (61.1) | 0.477 | (65.2) | (70.5) | 0.562 | (67.9) | (68.6) | 0.932 | |
| Polypoid gastritis with erosions | 2 | 3 | 0.033 | 4 | 1 | 4 | 1 | ||
| (2.2) | (16.7) | (8.7) | (1.6) | 0.162 | (7.1) | (2.0) | 0.366 | ||
| Atrophic gastritis | 6 | 1 | 2 | 5 | 4 | 3 | |||
| (6.7) | (5.6) | 1.000 | (4.3) | (8.2) | 0.696 | (7.1) | (5.9) | 1.000 | |
- LES, lower esophageal sphincter; IL, intestinal lymphangiectasia.
DISCUSSION
The present study showed that diffuse SWS in the duodenum have heterogenous etiologies. IL is the most common cause in these endoscopic finding. IL is characterized by generalized dilatation of lymphatic channels.7 Three endoscopic characteristics, SWS, white villi and chyle-like substances covering mucosa have been described.8,9 The histology revealed markedly dilated lymphatics (Fig. 2). These tiny white spots might be indicative for stagnant lymphatic flow in the intestine.4 White villi were also present in each patient and these whitish villi are due to chylomicrons in the stromal area and lipoprotein-like substances in the columnar epithelium. These findings unequivocally revealed a fat transport defect from the epithelial cells to the lymphatics.4
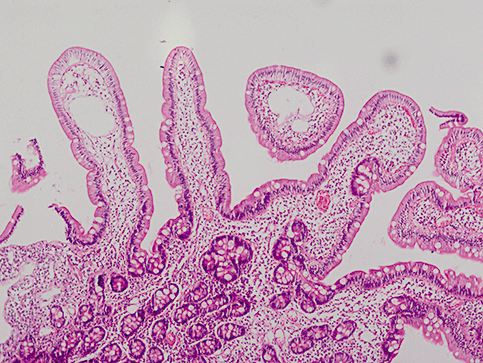
Microscopic appearance of intestinal lymphangiectasia in a patient with duodenal scattered white spots (10 × 10 HE).
Giardiasis is an endemic parasitic infection in developing countries. The infestation rate of giardiasis is about 3–5% in Turkey.10 It has been shown to occur in young people with dyspeptic complaints and/or diarrhea.11 One of the endoscopic findings of giardiasis is scattered tiny white spots (Fig. 3).1 There may be reddened, raised non-eroded mucosal patches in the duodenum11 that we did not detect in our series. This infection has also been thought to be related etiologically to IL.12 It was hypothesized that giardiasis, when present for long periods of time, may cause secondary IL.12 In our series only two patients with giardiasis had histological signs of IL. The other patients had no microscopic signs of a fat transport defect in the intestinal epithelium. Thus we cannot make a firm conclusion on this subject.
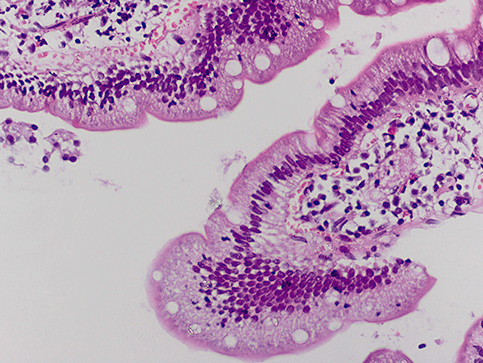
Histology showing giardiasis in a patient with duodenal scattered white spots (4 × 10 HE).
In our study CD was the second most common etiology for SWS in the duodenum (Fig. 4). Interestingly, there were few patients with endoscopic signs of reddened, raised or non-raised patchy mucosa. All of patients had dyspeptic symptoms with or without abdominal pain. However, we do not have data about Helicobacter pylori infection in these patients. The mechanism for forming tiny white spots is not clear in these patients. Possibly, chronic inflammation induced changes in the epithelial lymphatic flow (not a blockage but a slow lymphatic drainage in the mucosa). However, this is a speculative theory since there was no overt sign of a fat transport defect on microscopy of intestinal epithelium in these patients.
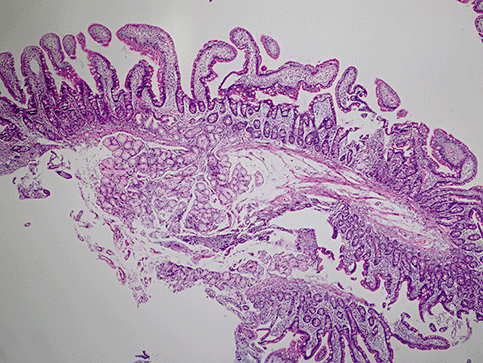
Chronic non-specific duodenitis was diagnosed histologically in a patient with duodenal scattered white spots (4 × 10 HE).
In conclusion, SWS in the duodenum is not a characteristic sign of any disease. Although IL is the most common cause, a similar endoscopic appearance may be detected in CD and giardiasis. Moreover, a combination of any of these three etiologies may also be present. Hence, a biopsy should be taken to differentiate them from each other whenever SWS is detected on a routine endoscopic examination of the duodenum.




