Diagnostic value of a group of biochemical markers of liver fibrosis in patients with non-alcoholic steatohepatitis
Abstract
OBJECTIVE: The objectives of this study were to investigate the use of non-invasive biochemical markers to evaluate the severity of liver fibrosis in patients with non-alcoholic steatohepatitis (NASH).
METHODS: This was a cross-sectional study of patients with histopathologically confirmed NASH between January 2005 and December 2006. The patients’ characteristics were recorded and the body mass index was calculated for each patient. All patients underwent ultrasound-guided liver biopsy and a fibrosis assessment was performed using the Brunt criteria. The non-invasive laboratory markers measured were insulin resistance, tumor necrosis factor (TNF-α), type IV collagen and hyaluronic acid (HA).
RESULTS: Thirty patients were recruited, of whom 18 (60%) were men. Their mean age was 45 ± 13.9 (18–71) years. About 83% of patients had fibrosis stage 1–2. In bivariate analysis, age, TNF-α and type IV collagen concentrations showed a weak but significant correlation with the fibrosis stage. When the patients were grouped into mild fibrosis (stages 1–2) and advanced fibrosis (stages 3–4), the mean concentrations of HA and type IV collagen were significantly higher in those with advanced fibrosis than those with mild fibrosis (180.8 ± 49.63 vs 543.6 ± 360.45 ng/mL; for HA; P = 0.026 and 125.3 ± 32.11 vs 288.0 ± 171.22 ng/mL for type IV collagen; P = 0.010).
CONCLUSION: Our study showed that the degree of liver fibrosis was significantly correlated with age, TNF-α and type IV collagen concentrations. The level of HA and type IV collagen could differentiate between mild (F1–2) and advanced fibrosis (F3–4).
INTRODUCTION
Non-alcoholic steatohepatitis (NASH) is a metabolic liver disorder in which insulin resistance and hepatic steatosis are complicated by chronic necroinflammatory change. This condition has the potential to become progressive liver fibrosis. This definition implies that the diagnosis of NASH should be based on histopathological evaluation from a liver biopsy specimen. Currently, liver biopsy is still considered the gold standard for fibrosis assessment. However, due to its invasiveness, many investigators have began to develop non-invasive tests to evaluate the presence and severity of liver fibrosis.
For instance, a panel consisting 13 non-invasive parameters has been proposed recently to predict NASH in patients with non-alcoholic fatty liver disease (NAFLD), i.e., age, sex, height, weight and serum levels of triglycerides, cholesterol, α2-macroglobulin, apolipoprotein A1, haptoglobin, gamma-glutamyl transpeptidase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin. These panels, called the NASH test, were nearly 80% able to predict the presence of NASH (the area under the receiver operating characteristic curve [AUC] 0.78 with a 95% confidence interval [CI] 0.71–0.83).1 Furthermore, another test called the FibroTest, including total bilirubin, GGT, α2-macroglobulin, apolipoprotein A1 and haptoglobulin has also been designed as a non-invasive quantitative assessment of liver fibrosis.2 However, these tests have not included markers of liver extracellular matrix components.
Immunological mechanisms have an important role in the pathogenesis of NASH. Studies both in human and animal models have shown the role of pro-inflammatory cytokines in causing hepatic injury.3 The pathogenesis involved increased tumor necrosis factor-α (TNF-α) which was found in obese patients and contributed to the development of insulin resistance. The production of TNF-α is believed to be the first event in liver injury, triggering the production of other cytokines that recruit inflammatory cells, destroy hepatocytes and initiate a healing response, including fibrogenesis.4 Other potential non-invasive markers are components of the extracellular matrix, such as hyaluronic acid (HA) and collagen. Serum HA has been shown to reflect the severity of liver inflammation, fibrosis and fibrogenesis in alcoholic liver disease as well as being a marker of fibrosis. The cut-off value of 85–100 µg/L has been associated with 51–87% sensitivity and 74–93% specificity for detection of severe fibrosis.5–7 Serum type III procollagen, type IV collagen and the 7S domain of type IV collagen have been showed to be highly correlated with the collagen amount in the liver in patients with chronic viral hepatitis.8
In addition, liver steatosis has been associated with several metabolic parameters such as body mass index (BMI), waist circumference, glucose intolerance and insulin resistance.9,10 Few data from Asia have been generated to assess liver fibrosis using non-invasive markers. This study aimed to investigate the use of several biochemical markers as non-invasive predictors of liver fibrosis in patients with NASH, including their levels of HA and type IV collagen. Several markers were randomly selected to address this issue, i.e., the patient's age, BMI, homeostasis model assessment of insulin resistance (HOMA-IR), TNF-α, HA and type IV collagen.
MATERIALS AND METHODS
Study design and patient recruitment
This was a cross-sectional study of patients with histopathologically confirmed NASH between January 2005 and December 2006. The patients were consecutively enrolled. Inclusion criteria were that they were hepatitis B antigen negative, anti-hepatitis C virus negative, anti-nuclear antibody negative and with no history of alcohol abuse and drug use. Their BMI was calculated as body weight in kilograms divided by body height in square meters (kg/m2).
Fibrosis staging
All the patients underwent an ultrasound-guided liver biopsy using a 16-gauge Menghini needle (Hepafix, B. Braun Melsungen AG, Germany) under local anesthesia. Specimens were graded and staging according to Brunt criteria.11 The stage of fibrosis was measured on a five-point scale (F0 = normal connective tissue, F1 = foci of perivenular and/or perisinusoidal fibrosis in zone 3, F2 = perivenular or pericellular fibrosis affecting zones 3 and 2, F3 = septal or bridging fibrosis and F4 = cirrhosis). The fibrosis stage was independently examined by a senior pathologist who was blinded to the laboratory results.
Laboratory markers
Venous blood samples were drawn from the patients after they had fasted over night for measurement of the serum ALT, glucose, insulin, type IV collagen and HA. TNF-α was measured with the enzyme-linked immunosorbent assay technique (Quantikine ELISA; R&D Systems, Minneapolis, MN, USA) which were done in a private laboratory (PRODIA Laboratory, Jakarta). The HA was measured by a latex agglutination immunoassay and type IV collagen was measured by a radioimmunology assay. Insulin resistance was measured with HOMA-IR using the following formula: HOMA-IR = fasting insulin (µU/mL) × plasma glucose (mg/dL)/405. This model was developed by Matthews12 and later modified.13
Statistical analyses
Characteristics of the study subjects were presented descriptively. Correlations between non-invasive markers and fibrosis stages were tested using Spearmen's correlation test. Mean differences of HA and type IV collagen between mild and advanced fibrosis were tested using Mann–Whitney's U-test. A P-value less than 0.05 was considered statistically significant. Analyses were performed by using the software Statistical Package for Social Studies (SPSS) version 12.0 for Windows PC (SPSS, Chicago, IL, USA).
RESULTS
There were 30 patients recruited in this study, predominantly men (60%). The patients’ mean age was 45 ± 13.9 years, ranging from 18 to 71 years. More than 80% of the patients had fibrosis stage 1 or 2. Most patients were overweight with a BMI of more than 25 kg/m2. There were 18 (60%) patients with HOMA-IR >3.02. The patients also showed high levels of serum HA (238.8 ± 191.64 ng/mL) and type IV collagen (152.4 ± 93.25 ng/mL) (Table 1). Age, TNF-α concentration and type IV collagen concentration showed a weak but significant correlation with the patients’ fibrosis stage. In contrast BMI, insulin resistance and HA showed no correlation with the fibrosis stage (Table 2). When patients were grouped into two fibrosis categories, the mean concentrations of HA and type IV collagen were significantly higher in patients with advanced fibrosis (Table 3, 1, 2).
| Characteristic | Mean ± SD | N | % |
|---|---|---|---|
| Sex | |||
| Male | 18 | 60 | |
| Female | 12 | 40 | |
| Age, years | 45 ± 13.9 | ||
| Age group (years) | |||
| <30 | 4 | 13 | |
| 31–40 | 5 | 16.7 | |
| 41–50 | 9 | 30.0 | |
| 51–60 | 7 | 23.3 | |
| 61–70 | 3 | 10.0 | |
| >70 | 1 | 3.3 | |
| Body mass index (kg/m2) | 27.2 ± 5.35 | ||
| Alanine aminotransferase (IU/mL) | 79.8 ± 61.93 | ||
| Insulin (µU/L) | 18.0 ± 24.04 | ||
| HOMA-IR | 5.1 ± 5.92 | ||
| TNF-α (pg/mL) | 3.6 ± 1.70 | ||
| Hyaluronic acid (ng/L) | 238.8 ± 191.64 | ||
| Type IV collagen (ng/L) | 152.4 ± 93.25 | ||
| Fibrosis stage | |||
| F1 | 5 | 16.7 | |
| F2 | 20 | 66.7 | |
| F3 | 2 | 6.7 | |
| F4 | 3 | 10 | |
- HOMA-IR, homeostasis model assessment of insulin resistance; TNF-α, tumor necrosis factor alpha.
| Markers | Spearman correlation (r) | P-value* |
|---|---|---|
| Age (years) | 0.347 | 0.014 |
| Body mass index (kg/m2) | 0.218 | 0.247 |
| HOMA-IR | 0.075 | 0.693 |
| TNF-alpha (pg/mL) | 0.375 | 0.041 |
| Hyaluronic acid (ng/mL) | 0.218 | 0.294 |
| Type IV collagen (ng/mL) | 0.347 | 0.004 |
- *P < 0.05. HOMA-IR, homeostasis model assessment of insulin resistance; TNF-α, tumor necrosis factor-α.
| Parameter | F1–F2 | F3–F4 | P-value* |
|---|---|---|---|
| Age (years) | 43 ± 13.0 | 58 ± 12.3 | 0.048 |
| Body mass index | 28.0 ± 5.56 | 23.6 ± 1.51 | 0.028 |
| HOMA-IR | 5.3 ± 6.46 | 3.7 ± 1.28 | 0.781 |
| TNF-α (pg/mL) | 3.4 ± 1.50 | 5.0 ± 2.16 | 0.042 |
| Hyaluronic acid (ng/mL) | 180.75 ± 49.64 | 543.60 ± 360.45 | 0.026 |
| Type IV collagen (ng/mL) | 125.34 ± 32.11 | 287.96 ± 171.22 | 0.010 |
- * P < 0.05 Mann–Whitney U test.
- HOMA-IR, homeostasis model assessment of insulin resistance; TNF-α, tumor necrosis factor alpha.
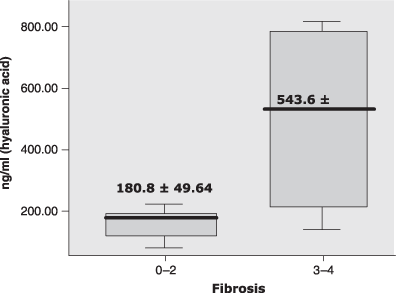
Mean difference of hyaluronic acid level between mild and advanced fibrosis. P, 0.026, Mann–Whitney U test.
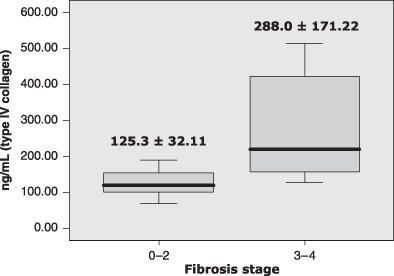
Mean differences of type IV collagen level between mild and advanced fibrosis. P, 0.016, Mann–Whitney U test.
DISCUSSION
The assessment of metabolic parameters and extracellular matrix components in patients with NASH is a relatively new approach in Indonesia and has not been established in routine clinical practice. This research is a preliminary study to evaluate the potential of several non-invasive markers to assess the severity of liver fibrosis in patients with histopathologically confirmed NASH.
The characteristics of our patients were almost similar to a study in Brazil. In that study, 30 patients were enrolled, 18 (60%) among whom were men. The patients’ mean age was 44.9 years. All patients had BMI more than 25 kg/m2 with a mean of 31 kg/m2.14 A study in Japan, which included 70 patients with NASH, found that 54 (77.1%) among them were women and had a slightly higher mean age (53.8 years) and BMI (29.6 kg/m2).15
Our results showed that age, TNF-α and type IV collagen showed a significant positive correlation with the fibrosis stage. There have not been many publications relating increasing age to a higher stage of fibrosis. One study found a non-significant difference of age between patients with mild fibrosis (49.5 years) and advanced fibrosis (55.6 years), although it showed that patients with advanced fibrosis tended to be older.16
TNF-α is a pro-inflammatory cytokine which has emerged as a key player in liver disease. It mediates the early stage of fatty liver disease as well as the transition to a more advanced stage of liver disease.17 Serum HA and TNF-α levels were significantly higher in patients with NASH and cirrhosis than in healthy controls. The level of serum HA in patients with NASH was 187.3 ± 139.21 ng/mL in stage 2–3 fibrosis and 143.5 ± 93.14 ng/mL in stage 0–1.18
Components of the extracellular matrix have been studied previously in other chronic liver diseases before investigators applied them in the study of NAFLD and NASH. In patients with chronic viral hepatitis, serum HA, procollagen type III and collagen type IV were used to define liver fibrosis at any stage, including cirrhosis. Among them, serum HA showed the highest accuracy. In particular, the cut-off value of HA for cirrhosis screening was 210 µg/L with a 96.2% sensitivity and 85.3% specificity, whereas the cut-off value of collagen type IV was 90 µg/L with a 75.8% sensitivity and 47.8% specificity.19
An early study of patients with NAFLD has found that the serum levels of HA were significantly different among fibrosis stages, i.e., 40 ± 66.3, 32 ± 25.7, 67 ± 56.2, 125 ± 152.9, and 246 ± 218.7 ng/mL, respectively, for stages 0–4 (P < 0.0001, anova with logarithmic transformation). When the comparison was done between patients with mild fibrosis (stages 0–2) and advanced fibrosis (stages 3–4) their serum HA also showed a significant difference (39 ± 46.7 ng/mL vs 203 ± 202.7 ng/mL, respectively; P < 0.0001, t-test with logarithmic transformation).20
A study in Japan used type IV collagen 7S domain and HA to predict the presence of NASH. At the cut-off value of 5.0 ng/dL, type IV collagen 7S domain had an AUC 0.828 (95%CI 0,754–0.902), 70% sensitivity, 81% specificity, and 86% positive predictive value (PPV) whereas serum HA level at 43 ng/dL had an AUC 0.797 (95%CI 0.716–0.879), 65.7% sensitivity, 90.5% specificity, and 92% PPV.15 A slightly lower cut-off has been proposed to predict advanced fibrosis (stage 3) in NASH, i.e., the levels of type IV collagen 7S domain at 4.25 ng/dL (AUC 0.767, 88.9% sensitivity, 59.7% specificity, 42.4% PPV and HA at 32.5 ng/dL (AUC 0.754, 77.8% sensitivity, 65.8% specificity, 42.7% PPV).16
One study assessed serum HA, leptin and laminin in patients with NASH. The results showed that NASH patients with fibrosis had significantly higher HA and laminin than NASH patients without fibrosis. The mean serum HA level was 332.7 ± 223.28 ng/mL in NASH patients with fibrosis and 55.1 ± 57.93 ng/mL in NASH patients without fibrosis (P < 0.001, Mann–Whitney test).21
Our data did not show any significant relation between HOMA-IR and the fibrosis stage. HOMA-IR is a marker for insulin resistance or metabolic syndrome, which is a risk factor for NAFLD. HOMA-IR may not be elevated in the early stage of NASH. Therefore, the association with liver fibrosis may not readily be seen in patients with NASH. In contrast, type IV collagen and HA are markers for fibrosis, which is a pathological component of NASH. However, a study did show significant difference of HOMA-IR between simple steatosis and early NASH. Furthermore, the investigators showed that the sensitivity in the detection of early stage NASH was increased to 94% when type IV collagen 7S, adiponectin and HOMA-IR were used as markers.22 Another study showed that HOMA-IR was significantly correlated with the degree of hepatic steatosis, but not with the fibrosis stage. The significance was seen in multiple regression analysis adjusting for age, sex and BMI. It was concluded that HOMA-IR was associated with insulin resistance in NAFLD. However, the participants in this study were mostly diabetic patients, who might had higher HOMA-IR than the normal population.23
Our preliminary results were consistent with those of many of the reported studies mentioned above. However, further prospective studies with larger samples are needed before applying them in clinical practice.
Conclusion
In conclusion, our study showed that the degree of liver fibrosis was significantly correlated with age, TNF-α and type IV collagen concentrations. The level of HA and type IV collagen could differentiate between mild fibrosis (stages 1–2) and advanced fibrosis (stages 3–4). Further prospective studies with larger samples are needed.




