UV-B Action Spectrum for UVR8-Mediated HY5 Transcript Accumulation in Arabidopsis
Abstract
Arabidopsis thalianaUV RESISTANCE LOCUS8 (UVR8) is a UV-B-specific signaling component that mediates low fluence, photomorphogenic responses to UV-B. It is required for UV-B-induced expression of the gene encoding the ELONGATED HYPOCOTYL5 (HY5) transcription factor. HY5 is a key effector of responses mediated by UVR8. In mature leaf tissue, HY5 transcript accumulation occurred rapidly in response to a brief UV-B treatment and no induction was observed in a uvr8 mutant over a broad range of UV wavelengths. In response to monochromatic light, maximal transcript accumulation occurred in wild-type plants at wavelengths 280–300 nm. HY5 transcript accumulation showed reciprocity between the fluence rate and duration of UV-B exposure, and on this basis conditions were chosen to generate an action spectrum for the UVR8 signaling pathway. Dose–response curves were produced for a range of UV wavelengths using 20 min exposure to UV and harvesting tissue 2 h after the start of illumination. Experiments using mutants defective in sinapate ester and flavonoid biosynthesis indicated that the presence of UV-absorbing compounds did not affect the construction of an action spectrum under the conditions employed. The action spectrum for the induction of HY5 by the UVR8 pathway showed a main peak at 280 nm with a smaller peak at 300 nm. The data are discussed in relation to the proposed mechanisms of UV-B photoreception.
Introduction
The UV-B (280–315 nm) component of sunlight has a variety of effects on plants, ranging from tissue and molecular damage to cellular stress, the accumulation of protective pigments, gene expression and the induction of photomorphogenesis (1–3). Although it is difficult to compare studies because of differences in the plant species, developmental stage and precise experimental and treatment conditions used, recent work has shown there are separate UV-B signaling pathways that may function optimally at different UV-B wavelengths (4–6) and/or fluence rates (7,8).
Some of the signaling components that play key roles in these pathways have been identified. These include the UV RESISTANCE LOCUS 8 (UVR8) protein (9,10), CONSTITUTIVE PHOTOMORPHOGENESIS 1 (COP1) (11) and the bZIP transcription factor ELONGATED HYPOCOTYL 5 (HY5) (6,9). The UVR8 protein is 35% identical to the REGULATOR OF CHROMATIN CONDENSATION 1 (RCC1) family of proteins (10) and accumulates in the nucleus in response to UV-B where it binds to chromatin in the region of the HY5 gene promoter, orchestrating the expression of UV-protective genes (9,12,13). HY5 along with the HY5 HOMOLOG (HYH) transcription factor was found to effect the UVR8-dependent UV-B signaling pathway in mature Arabidopsis thaliana leaf tissue (7,9). COP1, previously known to down-regulate the level of HY5 protein in dark-grown Arabidopsis seedlings (14), has recently been shown to be required for UV-B-stimulated HY5 gene activation in light-grown seedlings (11).
However, despite these recent advances in our understanding of the different signaling pathways no UV-B photoreceptor has been identified. Classically, comparison of action spectra for relevant responses with absorption or excitation spectra from candidate molecules has provided important insights into the identity of plant photoreceptors (15,16). However, the production of action spectra based on dose–response data, as distinct from relative-response spectra, is time-consuming and demanding (17). One particular difficulty in assessing the effectiveness of wavelengths below 315 nm on plant responses is that not only does the presence of screening pigments (such as flavonoids in epidermal cell vacuoles) have to be considered, but also the capacity of biomolecules (such as proteins and DNA) for UV absorption must be taken into account (1,18). In addition, it is possible that previously identified photoreceptors or a combination of redundantly acting receptors may be activated by UV-B absorption, although mutant studies suggest that the known photoreceptors do not mediate UV-B responses (6,7).
While several action spectra for UV-B stimulated responses in plants have been generated, the peak wavelengths and conclusions drawn have varied. For example, Eisinger et al. determined the UV action spectrum for stomatal opening in epidermal peels from broad bean leaves. They found that the response peaked at 280 nm and inferred from mixed wavelength treatments that ultraviolet light acts via a blue light photoreceptor (19). Takeda and Abe also found a peak at 280 nm in their action spectrum for anthocyanin formation in a carrot cell suspension culture (20). A previous study on anthocyanin synthesis in dark-grown Sorghum bicolor had determined an action spectrum containing several peaks. However only the largest of these peaks, which occurred at 290 nm, could not be nullified by far-red light (21). An even earlier action spectrum for anthocyanin formation in Spirodela oligorrhiza showed peaks only in the far-red region of the spectrum and at around 300 nm (22). More recently, Wellmann determined an action spectrum for flavonoid induction in parsley cell suspension cultures which also showed a single clear peak at 300 nm (23). Further studies from the same laboratory, performed in bean leaves, highlighted the possibility that short wavelength UV-B (<310 nm) might actually reduce flavonoid formation (24). Finally, Ioki et al. have shown for both cucumber and transgenic Arabidopsis seedlings that action spectra for activation of the CsPHR promoter peak at around 310 nm (25).
A variety of UV-B photoreceptors have been postulated that may account for some of these action spectra. These include biomolecules such as phenolics, DNA or proteins (26), provitamin D (27) or typical polypeptide photoreceptor molecules complete with a pterin or flavin chromophore (18,28).
Although it has long been suspected that plants could have distinct UV-B signaling pathways and that some of these might interfere with one another, previous investigations may have been hampered by uncertainty as to whether or not a particular response was attributable to a single pathway. In addition, responses that are significantly downstream of the photoreceptor (such as the production of secondary metabolites) may not accurately reflect the characteristics of the light absorption event. To the best of our knowledge, no photobiological characterization of a defined UV-B signaling pathway in plants has so far been completed.
In this study, we present a detailed study of the UVR8-dependent UV-B signaling pathway in mature Arabidopsis leaf tissue. We show that HY5 is expressed rapidly in response to UV-B but no expression occurs at any tested UV-B wavelength in the uvr8 mutant. We investigate which wavelengths of light induce most HY5 expression in wild-type plants and whether a reciprocal relationship between treatment duration and fluence rate exists for HY5 expression at 300 nm. We report that the UV-B action spectrum for HY5 expression in mature Arabidopsis leaf tissue shows a major peak at 280 nm with a minor peak at 300 nm and discuss the implications of these findings for the molecular identity of a UV-B photoreceptor. We also investigate whether screening pigments, including flavonoids and sinapic acid esters inhibit stimulation of the UVR8-dependent pathway under the treatment conditions used.
Materials and Methods
Plant material. Seeds of wild-type Arabidopsis thaliana ecotype Landsberg erecta (Ler) and those of Columbia-3 (Col-3), tt4 (Ler) and fah1-7 (Col) were obtained from the European Arabidopsis Stock Centre. The uvr8-2 mutant (also Ler) was generated in a screen carried out by Brown et al. and carries a premature stop codon which results in a total loss of UVR8-mediated, UV-B stimulated gene expression (9).
To examine the kinetics of HY5 and CHS gene expression, seeds were sown on compost, stratified at 4°C for several days, and then grown in low fluence rate white light (20–25 μmol m−2 s−1) at approximately 20°C for 21 days prior to broad-band UV-B treatment.
For all other experiments, Arabidopsis seeds were surface sterilized by a brief wash in 95% (v/v) ethanol, followed by a 5 min treatment with 50% (v/v) sodium hypochlorite (VWR) 0.05% (v/v) triton X-100 (Sigma) solution. Seeds were then rinsed three times using a sterile 0.05% (v/v) triton X-100 solution under a laminar flow hood and resuspended in 0.1% (w/v) sterile agarose for sowing onto 0.8% (w/v) agar plates. Growth media for seedlings consisted of 1 × Murashige and Skoog (MS) salts (Sigma), 1 × MS vitamins, 0.05% (w/v) Mes (pH 5.7). Seeds were stratified at 4°C for several days before transfer to a low fluence rate of white light (approximately 26 μmol m−2 s−1) at 20°C where they were grown for 17 days. Plates containing seedlings were then wrapped in aluminum foil for a further day (dark adaptation) before treatment with monochromatic light at the wavelengths described.
Light treatments. Broad-band UV-B illuminations were carried out in controlled environment rooms at approximately 20°C. UV-B was obtained from UVB-313 UV fluorescent tubes (Q-Panel) covered with cellulose acetate (West Design Products) as described previously (7).
For monochromatic light, the source was a 150 W xenon arc lamp (Thermo Oriel Instruments). Specific wavelengths were selected using a monochromator (model 77250; Thermo Oriel Instruments) which fed light directly into a black, plastic box used as a treatment chamber. Monochromatic light was directed against the far wall of the treatment chamber. Agar plates containing the dark-adapted seedlings had their lids removed and were affixed to the wall of the treatment chamber. A flap on the outside of the treatment chamber wall allowed access for a light measurement sensor and a cover was used to exclude extraneous light from the chamber during measurements and light treatments. The half bandwidth was 15 nm. After seedlings had been illuminated at the required fluence rate, wavelength and duration, lids were replaced and the agar plates covered once more with aluminum foil until 2 h had elapsed since the start of each illumination. Samples of leaf tissue were then harvested into liquid nitrogen and stored at −80°C until required. Seedlings of each genotype from one dark-adapted, untreated agar plate were harvested as a control.
White light (photosynthetically active radiation: 400–700 nm) was measured using a Skye RS232 meter equipped with a Quantum sensor (Skye Instruments). Fluence rates of broad-band UV-B (280–315 nm) were measured using a Skye RS232 meter equipped with a SKU 430 sensor. Monochromatic light was quantified using a Macam spectroradiometer (model SR9910; Macam Photometrics) set to collect wavelengths between 240 and 450 nm.
RNA isolation and transcript assays. Leaf tissue was ground with a mortar and pestle, and RNA extracted using the Purescript kit (Flowgen) with an additional chloroform extraction or the RNeasy plant mini kit (Qiagen). Following RNA extraction, a DNase treatment (DNA-free; Ambion) was used to eliminate contamination with genomic DNA. Complementary DNA was then synthesized using an oligo(dT) primer and avian myeloblastosis virus reverse transcriptase (Promega) at 48°C for 45 min.
For semiquantitative RT-PCR, reactions were carried out as described by Brown and Jenkins (7). Transcript levels in different RNA samples were compared using cycle numbers within the linear range of amplification.
For real-time PCR, cDNA was quantified using a Stratagene MX4000 real-time PCR machine (Stratagene) and Brilliant® SYBR® Green QPCR master mix (Stratagene) according to the manufacturer’s recommendations. Duplicate reactions were carried out in parallel for each biological sample. Each reaction contained 2 μL cDNA and 0.2 μm each primer in a total volume of 25 μL. PCR conditions were 10 min at 95°C, then 40 cycles of 30 s at 95°C, 1 min at 55°C (59°C for the kinetic work shown in Fig. 1) and 30 s at 72°C. To assay HY5 transcripts, primers 5′-GGCTGAAGAGGTTGTTGAGG-3′ and 5′-CAGCATTAGAACCACCACCA-3′ were used to amplify 222 bp of the HY5 cDNA sequence. For ACTIN2, primers 5′-CTCTCCCGCTATGTATGTCG-3′ and 5′-TCCATCTCCTGCTCGTAGTC-3′ were used to amplify 300 bp of the ACTIN2 cDNA sequence. Primers were checked for gene specificity using appropriate BLAST searches. Ct values were determined from SYBR-Green fluorescence using software provided by Stratagene. Amplification products were quantified using plasmids containing a corresponding fragment of HY5 or ACTIN2 cDNA as an external standard. For each sample, the value for the concentration of HY5 cDNA was normalized by dividing it by the concentration of ACTIN2 cDNA. The normalized level of expression in an untreated sample was then subtracted from that in each treated sample to generate values for the level of HY5 transcripts induced by each treatment.
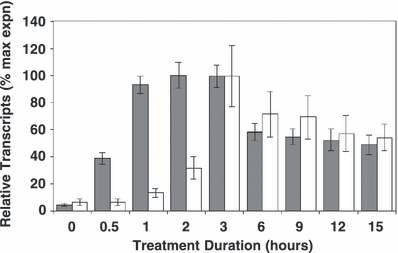
Kinetics of HY5 and CHS expression. Wild-type Ler plants were grown in 20–25 μmol m−2 s−1 white light and then exposed to 3 μmol m−2 s−1 UV-B. Leaf tissue was harvested and transcript levels quantified using real-time PCR. Expression was normalized using ACTIN2 transcript levels. Data shown (percentage of the maximal expression value of each gene) are means + SE from six independent experiments. Gray bars, HY5; white bars, CHS.
For analysis of the kinetics of HY5 and CHS expression (shown in Fig. 1), real-time PCR was carried out using the HY5 primers described above and for ACTIN2, primers 5′-ACTAAAACGCAAAACGAAAGCGGTT-3′ and 5′-CTAAGCTCTCAAGATCAAAGGCTTA-3′ were used to amplify 211 bp of the ACTIN2 cDNA sequence, while 5′-CTACTTCCGCATCACCAACA-3′ and 5′-TTAGGGACTTCGACCACCAC-3′ were used to amplify 195 bp of the CHS cDNA sequence. For each sample, the value for the concentration of HY5 and CHS transcripts was normalized by dividing it by the concentration of ACTIN2 transcripts.
Data analysis. The points for the action spectra were calculated from quantitative real-time PCR data taken from three independent experiments as follows. At each wavelength the level of HY5 transcript accumulation induced by each treatment (normalized expression in illuminated sample minus normalized expression in untreated sample) was plotted as a function of fluence rate. Hyperbolic curves were fitted to the data using the graphing software SigmaPlot (version 10.0, Systat). Hyperbolic behavior is consistent with a simple model of light-activated receptor stimulation as described by Eisinger et al. (19) and Hartman (29). Using the best-fit hyperbolic curves, the number of photons required to achieve two separate standard responses (0.02 and 0.04 units of HY5 expression) that occur on the approximately linear portion of each dose–response curve, was found using the “zoom” and “crosshairs” functions of SigmaPlot. The inverse of these fluence rate values was calculated and plotted against wavelength to produce the action spectra.
Results
HY5 transcript accumulation in mature Arabidopsis leaf tissue peaks after 2 h of continuous UV-B illumination and before CHS transcript accumulation reaches its maximal level
One of the protective genes induced by the UVR8-dependent UV-B signaling pathway is CHALCONE SYNTHASE (CHS). CHS is principally controlled at the level of transcription and catalyzes the first committed step of flavonoid biosynthesis. Previous UV action spectra have focussed on responses downstream of CHS gene expression, such as the biosynthesis of flavonoids or anthocyanin (21–23). We wanted to study a response controlled by the UVR8-dependent UV-B signaling pathway that was as close as possible to the photoreceptor in order to obtain the most accurate photobiological characterization. Since HY5 had previously been shown to be a key effector of this pathway and upstream of CHS gene expression we set out to determine the best time to assay leaf tissue for the highest level of HY5 transcript accumulation. This would ensure that our data and action spectrum would represent a high-resolution picture of the UVR8-dependent UV-B signaling pathway.
Wild-type Ler plants were grown for 3 weeks in a low fluence rate of white light that stimulates little or no UV-B-induced transcript accumulation in leaf tissue before being transferred to 3 μmol m−2 s−1 broad-band UV-B for various durations as shown in Fig. 1. As expected, HY5 was expressed rapidly in response to UV-B. Significant levels of HY5 transcripts could be measured after only 30 min of illumination and reached a peak after 2 h of continuous treatment. CHS transcripts accumulated more slowly and reached a peak approximately 1 h later.
We therefore conclude that (1) HY5 is expressed rapidly in response to UV-B and more rapidly than CHS transcripts; and (2) Arabidopsis leaf tissue harvested approximately 2 h after the start of a UV-B light treatment should have accumulated near maximal amounts of HY5 transcript.
HY5 is expressed in wild-type but not uvr8 mutant leaf tissue in response to monochromatic UV-B; maximal HY5 transcript accumulation occurs in response to 280–300 nm light
Our previous studies characterizing the UVR8-dependent UV-B signaling pathway had all been performed using a broad-band UV-B light source (7,9). We wanted to extend these studies by investigating the effect of a range of wavelengths of monochromatic UV light on HY5 transcript accumulation.
Wild-type Ler and uvr8 mutant plants were grown on agar plates for 17 days in a low fluence rate of white light- and dark-adapted for one further day. They were then transferred to 0.3 μmol m−2 s−1 monochromatic light of the wavelengths shown for 1 h (Fig. 2) and returned to dark conditions for a further 1 h before leaf tissue was harvested. The uvr8 mutant showed very little or no HY5 transcript accumulation at any of the tested wavelengths. By contrast, wild-type plants expressed HY5 clearly in response to wavelengths ranging from 260 to 310 nm. The highest levels of expression were found in response to 280–300 nm. A repeat experiment using 30 min illuminations at the higher fluence rate of 0.5 μmol m−2 s−1 produced a similar result and cDNA from both experiments was additionally quantified using real-time PCR. The data shown in Fig. 2 are representative of the findings. Note that the fluence rates used here are too low to induce HY5 expression in either wild-type Ler or uvr8 at any UV-A wavelength. Higher fluence rates of UV-A do however induce HY5 expression independently of the UV-B specific UVR8 pathway (9).
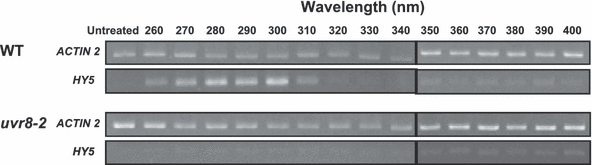
Effect of wavelength on HY5 transcript accumulation in dark-adapted wild-type and uvr8 leaf tissue. Plants grown in 26 μmol m−2 s−1 white light- and dark-adapted for 1 day were treated with 0.3 μmol m−2 s−1 monochromatic light of the wavelengths shown for 1 h and returned to darkness for a further 1 h. Leaf tissue was harvested and HY5 transcript levels measured by sqRT-PCR. ACTIN2 transcript levels are shown as a loading control.
We conclude that (1) UVR8 is required for stimulation of HY5 transcript accumulation in response to monochromatic UV-B and UV-C; and (2) the UVR8-dependent UV-B signaling pathway stimulates maximal HY5 transcript accumulation in response to 280–300 nm monochromatic light.
The UVR8-dependent UV-B signaling pathway that stimulates HY5 transcript accumulation shows a reciprocal relationship between treatment duration and fluence rate at 300 nm
If only photochemical reactions are involved in generating a particular response, then the strength of the response should be equal to the product of the number of photons and the treatment duration. An understanding of this reciprocal relationship between treatment duration and fluence rate is important in action spectroscopy because it can be used to assess whether particular treatment conditions are suitable for the construction of an action spectrum (17).
We wanted to construct an action spectrum for the UVR8-dependent UV-B signaling pathway using treatment durations and fluence rates that were not saturating. Since the preceding experiment (Fig. 2) had shown that stimulation of HY5 transcript accumulation in response to monochromatic UV-B was entirely dependent on UVR8 and since it also showed that a large amount of HY5 expression was generated in wild-type plants by illumination at 300 nm, we decided to examine the response to 300 nm light in more detail using a variety of fluence rates and treatment durations.
Wild-type Ler plants were grown on agar plates and dark-adapted as described above before treatment with 300 nm monochromatic light at one of four different fluence rates for the durations shown in Fig. 3. HY5 expression levels, quantified using real-time PCR, were proportional to both fluence rate and treatment duration over the range tested. This demonstrates the suitability of these treatments for the construction of dose–response curves that may be used to generate an action spectrum.
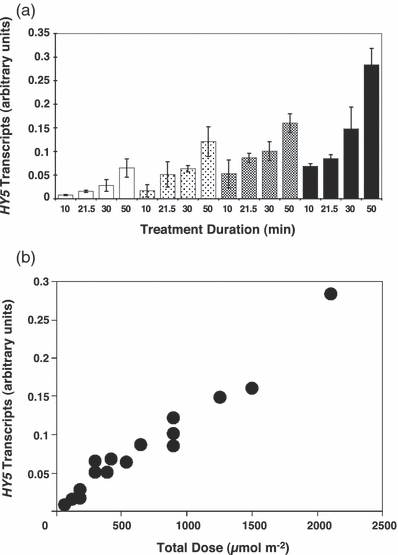
Reciprocal relationship between treatment duration and fluence rate in stimulating HY5 gene expression. (a) Wild-type Ler plants grown in 26 μmol m−2 s−1 white light- and dark-adapted for 1 day were treated with 300 nm monochromatic light at one of four different fluence rates for the durations shown and returned to dark conditions. Leaf tissue was harvested 2 h after the start of each treatment and HY5 transcript levels quantified using real-time PCR. HY5 expression was normalized using ACTIN2 transcript levels and the value from an untreated control subtracted. Mean expression levels from two independent experiments are shown. White bars, 0.1 μmol m−2 s−1; light gray bars, 0.3 μmol m−2 s−1; dark gray bars, 0.5 μmol m−2 s−1; black bars, 0.7 μmol m−2 s−1; Error bars are + 1 SD. (b) The data from (a) are replotted to illustrate the linear relationship between total dose (μmol m−2) and mean expression levels.
We conclude that (1) the UVR8-dependent UV-B stimulation of HY5 transcript accumulation shows reciprocity between treatment duration and fluence rate at 300 nm; (2) The response is not saturated by the range of treatment durations and fluence rates tested; and (3) These treatment durations and fluence rates are likely to be suitable for constructing dose–response curves and generating an action spectrum.
The action spectrum for the UVR8-dependent UV-B stimulation of HY5 transcript accumulation in mature Arabidopsis leaf tissue shows a major peak at 280 nm and a minor peak at 300 nm
For the action spectrum studies, wild-type Ler plants were grown on agar plates and dark-adapted as described above before treatment with monochromatic light at one of four different fluence rates at each wavelength for 20 min and then returned to dark conditions for a further 1 h 40 min before leaf tissue was harvested. HY5 expression levels were quantified using real-time PCR. The results of three independent experiments were combined.
Figure 4 shows the fluence response curves for HY5 expression at six wavelengths ranging from 260 to 310 nm. Hyperbolic curves show a good fit to the data generated, as Eisinger et al. (19) also discovered in their study of UV-induced stomatal opening in broad bean. Plots of response versus log fluence (not shown) showed linearity below saturation. These data indicate that UV-B stimulated HY5 transcript accumulation exhibits first-order kinetics as predicted for a single photochemical reaction.
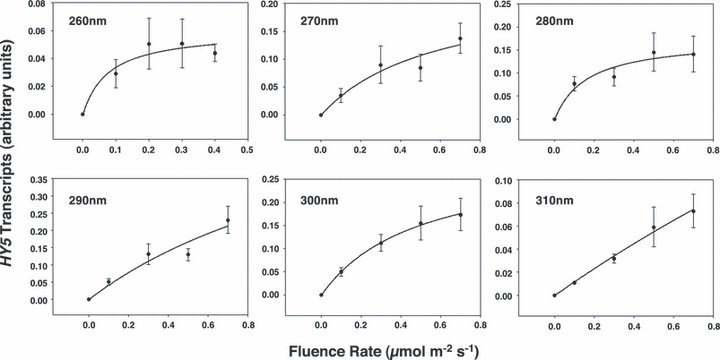
Effect of wavelength on HY5 transcript levels in dark-adapted Arabidopsis leaf tissue as a function of fluence rate. Hyperbolic curves were fitted to each data set. Error bars are ±1 SE (n = 3).
Using the data shown in Fig. 4, UV-B action spectra were generated by plotting the inverse of the number of photons required to produce two separate standard responses (0.02 and 0.04 units of HY5 transcripts respectively) found on the approximately linear portion of each dose–response curve. As shown in Fig. 5, the UV-B action spectrum for HY5 transcript accumulation in mature Arabidopsis leaf tissue shows a major peak at 280 nm and a minor peak at 300 nm.
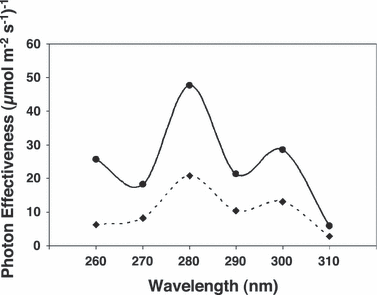
Short wavelength UV action spectrum for induction of HY5 gene expression in dark-adapted Arabidopsis leaf tissue. Photon effectiveness was calculated from the dose–response curves shown in Fig. 4 using two levels of standard response: 0.02 (solid line) and 0.04 (dotted line) units of HY5 transcripts, respectively.
Mutants deficient in the biosynthesis of flavonoids or sinapic acid esters do not show increased HY5 transcript accumulation in response to UV-B
For studies of UV-B responses in plants, it is important to consider the possibility that waxes, hairs and UV-absorbing compounds might affect the quantity of light reaching the photoreceptive site. When such screening effects are enhanced at selected wavelengths, due to the absorption characteristics of a particular screening pigment, then a corresponding effect might be expected on the shape of the UV-B effectiveness curve (17). Thus, in de-etiolated plant material nonphotoreceptor pigments may significantly alter action spectra as has been shown with the effect of chlorophyll, which absorbs blue light, on hypocotyl growth inhibition in both Sinapis alba (30) and Chenopodium rubrum (31,32). While chlorophyll should have little effect on our action spectrum since it absorbs UV-B wavelengths only poorly (33), the putative photoreceptor may be shaded by the vacuoles of epidermal cells containing UV-absorbing flavonoids and phenylpropanoids (34–36).
We wanted to investigate whether screening pigments might be reducing the level of HY5 transcripts accumulating under our experimental conditions and therefore decided to compare our response in mutants deficient in the production of flavonoids (the tt4 mutant) and sinapic acid esters (the fah1-7 mutant) with corresponding wild-type plants.
Wild-type Ler, wild-type Col-3, tt4 (Ler) and fah1-7 (Col) plants were grown on agar plates for 17 days in a low fluence rate of white light- and dark-adapted for one further day. They were then transferred to one of three fluence rates of monochromatic light at each of two wavelengths (290 and 300 nm) for 20 min and then returned to dark conditions for a further 1 h 40 min, before leaf tissue was harvested. HY5 expression levels were assayed using semiquantitative RT-PCR as shown in Fig. 6. It is clear from the results that mutants deficient in the biosynthesis of flavonoids or sinapic acid esters do not show increased HY5 transcript accumulation under our experimental conditions. We therefore conclude that flavonoids and sinapic acid esters do not impair stimulation of the UVR8-dependent pathway by UV-B under the treatment conditions used.
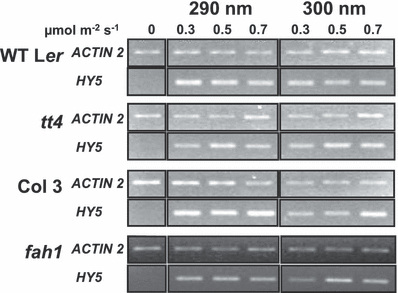
The tt4 and fah1 mutants do not show increased HY5 transcript accumulation. Wild-type and mutant plants grown in 26 μmol m−2 s−1 white light- and dark-adapted for 1 day were treated with monochromatic light at the wavelengths and fluence rates shown for 20 min and returned to darkness for 1 h 40 min. HY5 and control ACTIN2 transcripts were measured by sqRT-PCR.
Discussion
UVR8 is a UV-B specific signaling component with a key role in establishing UV protection in plants (9). Unlike a UVR8-independent UV-B signaling pathway that also stimulates gene expression in mature Arabidopsis leaf tissue, the UVR8-dependent pathway operates at low UV-B fluence rates (7). In this study we present a photobiological characterization of the UVR8-dependent UV-B signaling pathway based on expression of the HY5 gene, which is a very early response to UV-B illumination. The action spectrum for this response provides a starting point for understanding the mechanism of photomorphogenic UV-B photoreception in responses mediated by UVR8.
Constructing an action spectrum for the UVR8-dependent UV-B signaling pathway
In order to construct a reliable action spectrum, it was necessary to consider a variety of factors. The UV-B response selected for study was perhaps the most important of these. Previous work had shown that stimulation of CHS expression by UV-B was impaired in the hy5 mutant (7,9) indicating that HY5 acts upstream of CHS in the UV-B response pathway. In this study, we show, in agreement with the previous work, that HY5 transcript accumulation reaches a peak before CHS transcript accumulation (Fig. 1). Thus, HY5 transcript accumulation is more closely linked to UV-B photoreception than many responses that have previously been used to generate UV action spectra, such as flavonoid or anthocyanin biosynthesis which both require CHS.
Confirmation of reciprocity at 300 nm over the range of fluence rates used to generate the dose–response curves (Fig. 3) suggests that the UVR8-dependent pathway is controlled by photochemical reaction(s). Not only is HY5 transcript accumulation close to UV-B photoreception, and therefore likely to be a good reporter of the photobiological events, but it also occurs rapidly. It is clear from Fig. 1 that large quantities of HY5 transcripts have accumulated 2 h after illumination begins. It should be noted that a lengthy light treatment is not necessary for stimulating expression, but time is required for transcript accumulation to take place. In fact, Frohnmeyer et al. reported that millisecond UV-B treatments appear to be sufficient to activate a CHS gene promoter in transgenic parsley cell culture (37). Experiments prior to the present work also indicated that dark adapting wild-type plants grown on agar plates reduced the background level of HY5 gene expression in untreated leaf tissue. Thus, dark adaptation maximizes the difference between levels of transcript accumulation in untreated and illuminated tissue, increasing the resolution of gene induction measurements and leading to a better action spectrum.
Previous studies on UV-B responses in plants have produced a variety of action spectra
Action spectra have been produced for the biosynthesis of flavonoids and anthocyanin in several different species of plant and many show peaks of activity in the UV-B spectral region. The action spectrum for anthocyanin synthesis in maize coleoptiles which peaks between 290 and 300 nm (36) is typical of these. Action spectra determined for anthocyanin biosynthesis in Sorghum bicolour and Spirodela oligorrhiza showed UV-B peaks corresponding to 290 and 300 nm respectively (21,22) and an action spectrum for flavonoid synthesis in parsley cell cultures also peaked at 300 nm (23).
Our action spectrum for UV-B-stimulated HY5 transcript accumulation in Arabidopsis peaks at 280 nm and therefore differs from many previous action spectra for flavonoid and anthocyanin biosynthesis in plants. However, action spectra for anthocyanin formation in carrot cell suspension cultures (20), for stimulation of the PAL1 gene promoter in carrot protoplasts (38) and for UV-induced stomatal opening in broad bean (19) also peak at 280 nm.
Since no HY5 transcript accumulation was observed at any of the wavelengths tested in the uvr8 mutant (Fig. 2), UV-stimulated HY5 gene expression at low fluence rates is entirely dependent on UVR8 and so an activity spectrum for the UVR8 pathway may be unequivocally determined. Thus, we constructed dose–response curves from results generated in three separate experiments with wild-type plants and found that hyperbolas fitted the data better than straight line plots (Fig. 4). The fluence rates required to achieve standard responses occurring on the linear portion of each dose–response curve were calculated and the inverse of these fluence rates plotted as a function of wavelength to generate the action spectra (Fig. 5). It should be noted that the same action spectrum was obtained if the data were plotted as response versus log fluence. Since the first data point on each dose–response curve (the HY5 expression value at 0.1 μmol m−2 s−1) is critical in determining the initial slope of the curve, and since the expression value at this fluence rate is higher at 280 nm than at any other wavelength in each of the three independent experiments, we can be confident that the action spectrum for UV-B induced HY5 transcript accumulation in mature Arabidopsis leaf tissue does peak at 280 nm. Nevertheless, it should be noted that substantial HY5 transcript accumulation also occurs at 290 and 300 nm.
The plants used in the current study were grown in a low fluence rate of white light which should induce very little flavonoid biosynthesis and since the UV treatments applied were short, significant amounts of screening pigments should not be present. Nonetheless, HY5 transcript accumulation was examined in mutants deficient in flavonoid and sinapic acid ester biosynthesis and found not to be elevated compared to wild-type plants (Fig. 6). Therefore, it seems that flavonoids and sinapic acid esters do not impair UV-B induction via the UVR8-dependent pathway under the conditions used in this work and do not interfere with construction of the action spectrum.
Implications for UVR8-dependent UV-B photoreception
It is perhaps surprising that the action spectrum for the UVR8 pathway has a peak at around 280 nm because very little light below about 290 nm reaches the earth’s surface. It may be that this system has evolved to enable plants to act most effectively at the shortest, most damaging wavelengths they are ever likely to experience. It should be remembered that the levels of ambient UV-B at the time land plants appeared were probably higher than they are now. In nature, wavelengths between 290 and 310 nm are likely to initiate maximal activity of the UVR8 pathway. Although the peak of the action spectrum was at 280 nm, substantial levels of HY5 transcript accumulation occurred at 290 and 300 nm. Interestingly, activity spectra for UV-induced protective responses generated in ecological studies (e.g. Mazza et al.) also show that the shortest wavelengths of the solar spectrum are most effective (39).
In the past, action spectra for particular responses have been compared to absorption spectra of putative photoreceptors in an effort to identify the specific photoreceptor mediating a response (31). Since flavonoid and anthocyanin biosynthesis action spectra from various plants show peaks between 290 and 300 nm, it has been proposed that a UV-B photoreceptor may have a reduced flavin or pterin chromophore (18,40). Some experiments support this possibility (28,41,42). As mentioned above, several other action spectra for UV-B responses peak at 280 nm, similar to that presented here. We cannot exclude the involvement of a reduced flavin or pterin chromophore because the absorption spectrum of the chromophore in the molecular environment of the putative photoreceptor protein is unknown and can differ from the absorption spectrum in solution. Although UVR8 mediates HY5 expression in response to wavelengths below 280 nm (Fig. 2), the low level of expression generated at 260 nm indicates that DNA is unlikely to be the UVR8-dependent UV-B signaling pathway photoreceptor. This conclusion has been drawn in several other studies of low fluence UV-B responses, including for CHS expression (37), which is mediated by UVR8 (9). Furthermore, a lack of correlation between the number of cyclobutane pyrimidine dimers and levels of transcripts for defense genes has been demonstrated in Pisum sativum (43) and studies on DNA repair-deficient mutants do not show enhanced responses to UV-B in Arabidopsis (6,44,45).
An interesting possible mechanism for UV-B photoreception is the ligand-independent activation of plasma membrane receptor-like kinases. In mammalian cells reactive oxygen species (ROS) accumulation following UV-B exposure is reported to inactivate tyrosine phosphatase activity leading to activation of particular tyrosine receptors by autophosphorylation or phosphorylation by a separate kinase (46,47). The most effective wavelength is close to 280 nm, the absorption maximum of proteins. In plants, UV-B activates the systemin receptor, leading to activation of a wound signaling pathway (48), and an Arabidopsis mutant defective in the brassinosteroid receptor BRI1 is reported to have reduced UV-B induction of some genes (49). Although the mechanism of ligand-independent receptor activation by UV-B is unknown, it seems unlikely that sufficient ROS would be produced by low fluence UV-B to cause receptor activation in photomorphogenic responses and, moreover, ROS activation would not be specific to UV-B. A further possible mechanism of UV-B photoreception consistent with action at 280 nm is suggested by recent work in mammalian cells. The arylhydrocarbon receptor (AhR) is implicated in some UV-B responses and can be activated by the ligand 6-formylindolo[3,2-b]carbazole (FICZ), formed by UV-B irradiation of tryptophan in solution (50). It was demonstrated that FICZ is formed in cells following UV-B exposure and, moreover, depletion of cytosolic tryptophan impaired the UV-B stimulation of processes mediated by AhR. It is conceivable that a comparable mechanism could operate in plants but no evidence to support this notion has yet been presented.
An intriguing possibility is that UVR8 itself is the UV-B photoreceptor regulating HY5 expression. UVR8 is UV-B-specific and mutant screens have failed to identify any component that functions upstream of UVR8 (9,11). Interestingly, UVR8 has 14 highly conserved tryptophan residues, more than in most proteins of equivalent size (e.g. RCC1 has three), and these would permit effective absorption at 280 nm. It is possible that the protein environment could extend the absorption spectrum of one or more tryptophans to longer wavelengths but it is unclear whether this could account for the peak in the action spectrum at 300 nm; if not, an additional, unknown chromophore would be required. The possibility that UVR8 functions in UV-B photoreception therefore merits further investigation.
In summary, the action spectrum reported here for the rapid induction of HY5 transcript accumulation mediated by UVR8 suggests maximum photon effectiveness at around 280 nm with significant action at longer UV-B wavelengths. There are credible mechanisms for UV-B photoreception at these wavelengths based on research with plant and mammalian cells, but no studies have presented direct evidence of a plant photoreceptor that can mediate UV-B-specific responses. The present action spectrum for a defined UV-B-specific pathway may encourage further experimentation to identify the elusive mechanism of photomorphogenic UV-B photoreception.
Acknowledgements— This work was supported by a UK Biotechnology and Biological Sciences Research Council (BBSRC) grant to G.I.J. (B.A.B.) and a BBSRC PhD studentship (L.R.H.). We would like to thank the European Arabidopsis Stock Centre for providing seeds. Additionally, we would like to thank Dr. Joel Milner, Dr. Andrew Love and Janet Laird for their advice and guidance on technical aspects of the quantitative real-time PCR work and data analysis. We are grateful to Dr. Peter Dominy, Dr. John Christie, Kevin Zambaux and Adrian Hills for advice on generating the dose–response curves. We would also like to thank members of the Jenkins and Christie laboratories for helpful discussions.




