Ultraviolet Radiation-induced Transcription is Associated with Gene-specific Histone Acetylation
Abstract
UVR is an important environmental carcinogen and a powerful modulator of the cutaneous immune system. Exposure to UVR activates many signaling pathways leading to changes in the expression of several hundred genes. While the covalent modification of histones has been shown to play a central role in regulating gene expression, the impact of UVR on histone modifications and the contribution of histone acetyltransferases (HATs) and histone deacetylases (HDACs) to the UVR-induced transcriptional response have not been completely characterized. In this report, we have examined the impact of UVR on histone H3 K9/14 acetylation. The potential role of UVR-induced histone acetylation in the UVR transcriptional response was also explored using the HAT inhibitor curcumin and HDAC inhibitor trichostatin A (TSA). We found that UVR caused an increase in histone H3 acetylation within the promoter regions of ATF3, COX2, IL-8, MKP1 and MnSOD. In most of the regions examined, histone H3 acetylation peaked 24 h after UVR and then returned to baseline levels by 72 h. The induction of ATF3, COX2 and MKP1 was blocked in the presence of curcumin at doses that decrease in vivo histone H3 acetylation but not at lower doses that do not affect acetylation levels. We also provide evidence that for ATF3, a transcriptional superinduction occurs after repeat exposures to UVR that can be recapitulated when the second UVR exposure is replaced with TSA treatment. Thus, UVR can alter histone acetylation within human keratinocytes and these changes may contribute to the UVR-transcriptional response.
Introduction
As a complete carcinogen, UVR plays a major role in the development of skin cancer and has broad biological effects (1–6). Solar UVR is divided into UVA (320–400 nm), UVB (280–320 nm) and UVC (100–280 nm) based on wavelength. UVC irradiation does not reach the earth’s surface; however, humans are exposed to UVA and UVB irradiation from solar sources, as part of phototherapy for skin diseases, and from tanning beds. Both UVA and UVB irradiation can alter the expression of a large number of genes and both have been linked to mutagenesis (7,8). Despite the potentially dangerous adverse effects of UVR, it is a useful therapeutic for the treatment of many skin diseases, both inflammatory and neoplastic (9–15). The diverse biological responses to UVR are due in large part to alterations in gene expression induced by UVR. The transcriptional response to UVR is complex and involves a large number of genes (8,16–20). In addition, differences in the kinetics, magnitude and duration of these alterations underscore this complexity.
After UVR exposure, numerous signaling pathways are activated, including the mitogen-activated protein kinase (MAPK) pathway, epidermal growth factor receptor pathway, and those pathways activated in response to reactive oxygen species and direct DNA damage (21–25). How these multiple signaling pathways converge to ultimately regulate gene expression both acutely and under chronic conditions is not completely understood.
Despite the large number of factors and signaling cascades involved, a common end result of UVR-activated signaling is to alter gene expression. Many of the genes induced by UVR allow cells to respond to the damage caused by UVR. This includes DNA damage and damage to other cellular structures. While gene regulation is highly complex, a central theme for many transcriptional mechanisms involves altering chromatin structure. ATP-dependent chromatin remodeling complexes and enzymes that effect posttranslational modifications to nucleosomal core histones are the main activities governing chromatin structure as it relates to transcription (26,27).
While there is evidence that UVR can alter histone modifications, most of these studies focused on the role of these modifications in DNA repair (28–30) or on global changes in histone modifications that occur after UVR or modifications occurring in single cell eukaryotes (31,32). UVR exposure of yeast cells has been shown to increase acetylation of histone H3 and not H4 within the promoter of a repressed yeast locus (32,33). In addition, exposure of cells to UVR has been shown to lead to other histone modifications including H3 and H2AX phosphorylation, and H2A ubiquitylation (30,34,35). The role of UVR-induced histone modifications in the UVR-transcriptional response has been less well characterized.
To investigate the relationship between UVR-mediated gene induction and chromatin, a set of UVR-induced genes were selected for analysis. The RNA induction profiles and histone H3 K9/K14 acetylation profiles were determined for the set and compared over time courses extending to 72 h after irradiation. The results showed moderate to robust changes in histone acetylation that accompany gene induction. Additionally, depending on the induction profile, a transcriptional superinduction that correlated with peak promoter region nucleosome acetylation was observed, suggesting that histone modifications may contribute to adaptive transcriptional responses occurring in the setting of multiple UVR exposures.
Materials and methods
Cells and cell culture. HaCaT cells (36) from the Emory University Department of Dermatology Skin Diseases Research Center were generously provided by Dr. Norbert E. Fusenig. Cells were grown at 37°C and 5% CO2 in Dulbecco’s minimal essential medium supplemented with 10% fetal bovine serum (HyClone, Inc., Logan, UT), penicillin (50 U mL−1), streptomycin (50 μg mL−1) and l-glutamine (1 mm) (Life Technologies, Grand Island, NY).
Antibodies and treatment of cells. Immunoprecipitations were performed with anti-acetyl histone H3 (Lys 9/14) antibody (Upstate Biotechnology, Lake Placid, NY, catalog no. 06-599). UVB wavelength irradiation was performed using a UVR chamber containing four UVB FS20T12 bulbs (Daavlin; UVA/UVB Research Irradiation Unit, Bryan, OH) positioned 24 cm above the platform on which tissue culture dishes were placed. UVB wavelength irradiance was measured using a SEL240 UVB-detector connected to an IL1400A photometer (International Light, Inc., Peabody, MA). To eliminate wavelengths below 290 nm, a Kodacel cellulose acetate sheet (kindly provided by Dr. Craig Elmets, University of Alabama, Birmingham, AL) was placed between the light source and the tissue culture dishes. Prior to irradiation, cell culture dishes were washed twice in PBS and coated with PBS. Dish covers were removed and cells were irradiated with 0, 40 or 80 mJ cm−2 of UVB as indicated. Under the conditions used, 0, 40 and 80 mJ cm−2 resulted in >90%, 80% and 55% viability, respectively, at 24 h of culture following UVR as measured by Annexin V staining (data not shown). Curcumin (C7727; Sigma-Aldrich, St. Louis, MO) was dissolved in dimethylsulfoxide (DMSO) at 10 mg mL−1 and cells treated at a final concentration of 10 or 100 μm. Trichostatin A (TSA; T8552; Sigma-Aldrich) was dissolved in DMSO at 2 mg mL−1 and cells treated at a final concentration of 1.65 μm.
mRNA isolation and reverse transcription. RNA was isolated from cells using the RNeasy RNA isolation kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. cDNA synthesis by Superscript II reverse transcriptase (Invitrogen, Inc., Carlsbad, CA) was performed using 2 μg of total RNA in a total volume of 20 μL in PCR II buffer (Applied Biosystems, Foster City, CA) supplemented with MgCl2 to a final concentration of 5 mm.
Real-time PCR. Quantitative PCR analysis was performed as previously described with the iCycler and iCycler optical module (Bio-Rad Laboratories, Hercules, CA) (37–39). In all experiments, including chromatin immunoprecipitation (ChIP), incorporation of SYBR Green into double-stranded amplicons was measured. Products were evaluated using melt curve analysis and/or agarose gel electrophoresis to ensure a single product of the expected size. For analysis of genomic fragments in the ChIP assay, standard curves were performed on all primer pairs using HaCaT DNA that was previously quantified. Five-point standard curves using 500, 100, 20, 4 and 0.8 ng/reaction were performed for each experiment. Efficiencies between primer sets varied by <10%. All primer sequences were designed using Beacon Designer software (Premier Biosoft International, Palo Alto, CA) and are available upon request. The primers DRAW and DRAY used for the promoter of the major histocompatibility class (MHC) class II HLA-DRA gene have been reported previously (39).
Chromatin immunoprecipitation assays. ChIP assays were performed as described in Beresford and Boss (40) with the following modifications. HaCaT cells were plated at ∼15 × 106 cells per 15 cm2 dish 24 h before each experiment and one dish was used for each time point/condition. HaCaT cells were fixed with 1% formaldehyde for 10 min at room temperature. Cells were washed twice with 4°C PBS, PBS was poured off and discarded and cells scraped into 1.7 mL tubes in residual PBS. Cells were pelleted by centrifugation for 2 min at 2040 g using an Eppendorf 5415 C centrifuge at 4°C. Cells were resuspended in 1 mL of ice-cold lysis buffer (39) followed by a 5 min incubation on ice. Pellets were spun down for 5 min at 734 g and resuspended a second time in 1 mL of lysis buffer as above. Nuclei were pelleted as above and resuspended in 0.5 mL of ice-cold nuclear lysis buffer (39) and frozen at −80°C until they were used. Prior to use, chromatin was sonicated to an average DNA size of 600 bp and lysates were cleared by centrifugation at 15 994 g for 10 min at 4°C. For each ChIP, one-tenth of the total sonicated chromatin volume (500 μL) was used. Immunoprecipitations were performed overnight at 4°C with 5 μL of the indicated antibody. Chromatin–antibody complexes were immobilized to protein-A Sepharose beads (60 μL) and washed as described (40). The cross-links were reversed and DNA purified by phenol/chloroform extraction followed by ethanol precipitation. DNA was resuspended in 60 μL of water and 3 μL (1/20th of the total immunoprecipitated DNA) analyzed by real-time PCR for each 50 μL reaction.
Statistics. Statistical significance between treated and untreated samples was determined using the Student’s t-test.
Results
UVR induces changes in gene expression in HaCaT cells
To evaluate the impact of UVR on histone modifications and how such changes influence UVR-induced gene expression, a set of genes induced by UVR were selected to serve as a model for these studies. The genes selected were induced by UVR in human keratinocytes by previous microarray analysis (8) and included activating transcription factor 3 (ATF3), cyclo-oxygenase 2 (COX2), cystatin M, interleukin-8 (IL-8), MAPK phosphatase 1 (MKP1), manganese superoxide dismutase (MnSOD) and tumor necrosis factor-α (TNF-α). These genes were chosen because they represented those with robust induction from microarray studies and/or have been linked to inflammatory and malignant disease (8,16,41–43). To establish the kinetics of the response, a time course of the UVB-mediated induction of these genes was performed using the immortalized human keratinocyte cell line HaCaT (36). Three general patterns of induction were observed. ATF3, COX2 and TNF-α were induced transiently with a peak of steady-state RNA levels detected at 4 or 8 h following an 80 mJ cm−2 UVR treatment (Fig. 1). Cystatin M, MnSOD and IL-8 showed a delayed induction with the 24 h time point representing the highest level of induction (Fig. 1). MKP1 was induced early in response to 80 mJ cm−2 with continued expression and apparent plateau between 8 and 24 h. The transcriptional responses to the 80 mJ cm−2 dose of UVB were more robust than the 40 mJ cm−2 dose for ATF3, COX2, IL-8, MKP1 and TNF-α. These experiments confirmed that expression of these genes is increased in response to UVB in HaCaT cells and established an early time course and dose response for each gene.
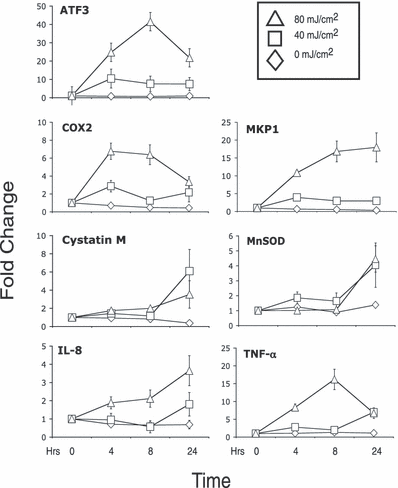
Induction of genes by UVB in HaCaT cells. HaCaT cells were exposed to 0, 40 or 80 mJ cm−2 of UVB wavelength UVR. RNA was isolated just prior to exposure (T = 0) and 4, 8 and 24 h after irradiation. RNA was analyzed by real-time RT-PCR and ΔCT calculated relative to GAPDH and fold change (ΔΔCT) calculated relative to time T = 0. Error bars represent ±SEM for three independent experiments.
One UVR exposure can impact the transcriptional response to subsequent exposures
Environmental and therapeutic exposures to UVR are typically multiple. In addition, the beneficial effects of therapeutic UVR typically occur in the setting of multiple suberythemogenic doses. It is therefore possible that some of the beneficial and harmful effects of UVR exposure are mediated by transcriptional changes occurring in the setting of multiple exposures. To examine this, cells were irradiated with UVR, rested for 24 h, re-exposed to another dose of UVR and RNA isolated 8 h following the second exposure (Fig. 2a). Using this approach, we found that ATF3 and COX2 exhibited a transcriptional superinduction in response to two UVR exposures whereas the other genes examined showed no superinduction (Fig. 2b). To determine the contributions of each exposure separately, mock irradiations were substituted for the first and/or second exposures. These combined treatments produced levels of induction that were comparable to or greater than that seen with a single dose of 80 mJ cm−2. This suggests that the events occurring after one UVB exposure are stable for at least 24 h and can impact the magnitude of subsequent transcriptional responses.
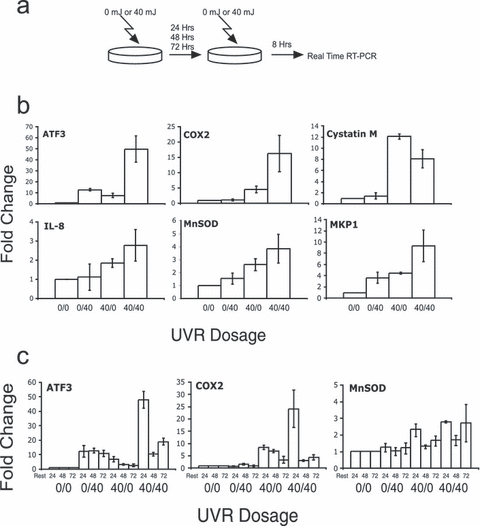
UVR transcriptional superinduction of ATF3 and COX2. (a) An overall schematic for the approach used in these experiments is shown. HaCaT cells were exposed to 0 or 40 mJ cm−2 on the first day and then rested for 24 h. On the second day cells were exposed to 0 or 40 mJ cm−2 and RNA was isolated 8 h later. RNA was analyzed by real-time RT-PCR and ΔCT calculated relative to GAPDH and fold change (ΔΔCT) calculated relative to cells irradiated with two 0 mJ cm−2 doses. Error bars represent ±SEM for three independent experiments. (b) mRNA levels of ATF3, COX2, Cystatin M, IL-8, MnSOD and MKP1 were analyzed using the above method. Doses are expressed as first day/second day in mJ cm−2 along the x-axis. mRNA levels were measured 8 h after the second exposure as indicated. Error bars represent ±SEM for three independent experiments. (c) HaCaT cells were exposed to 0 or 40 mJ cm−2 on the first day and then rested for 24, 48 or 72 h. On the second day of treatment, cells were exposed to 0 or 40 mJ cm−2 and RNA isolated 8 h later. RNA was analyzed by real-time RT-PCR and ΔCT calculated relative to GAPDH and fold change (ΔΔCT) calculated relative to cells irradiated with two 0 mJ cm−2 doses. Error bars represent ±SEM for three independent experiments. Doses are expressed as first day/second day in mJ cm−2 and rest periods indicated in hours along the x-axis. Error bars represent ±SEM for three independent experiments.
To further characterize the increased transcriptional responsiveness to UVR, the time between the two UVR exposures was increased. Thus, following a single UVR exposure, cells were rested for 24, 48 or 72 h, re-exposed to UVR and assayed for gene expression after 8 h (Fig. 2a). ATF3 and COX2 were examined as these genes were transiently expressed and displayed a superinduction in response to dual UVR exposures. As above, a superinduction was observed when the UVR doses were 24 h apart (Fig. 2c). Extension of the time between doses to 48 or 72 h resulted in responses that were similar to the primary dose, suggesting that the mechanism(s) responsible for the superinduction lasts for 24 but not 48 h. The MnSOD gene showed minimal changes in response to 40 mJ cm−2 and no evidence of UVR superinduction. None of the other genes tested showed evidence of UVR superinduction (data not shown).
UVR sensitizes cells to TSA treatment
The covalent modifications of histones contribute to several processes including transcription (26), transcriptional memory (44) and DNA repair (45). Because histone modifications may be stable and can affect the binding and/or assembly of transcriptional complexes, we hypothesized that changes in histone modifications induced by one UVR exposure may be able to alter the transcriptional response to subsequent UVR exposures. To determine whether histone acetylation contributes to UVR-mediated gene induction, the histone deacetylase (HDAC) inhibitor TSA was used in combination with UVR exposure. HaCaT cells were exposed to two doses of UVR or a combination of UVR and TSA (Fig. 3a). These experiments revealed that 24 h after 40 mJ cm−2 of UVR, TSA could induce the expression of ATF3 at 8 h to levels approximating the second UVR exposure (Fig. 3b bar 40/40 vs 40/T in the first panel). In contrast, the effect of TSA on COX2 and MnSOD expression was less robust. The above suggests that for some genes such as ATF3, histone acetylation plays an important role in the transcriptional response to UVR. We have also found that of the genes examined, ATF3 is strongly induced by TSA alone at 24 h (data not shown). Others have reported the induction of ATF3 by TSA in lung cancer cell lines (46).
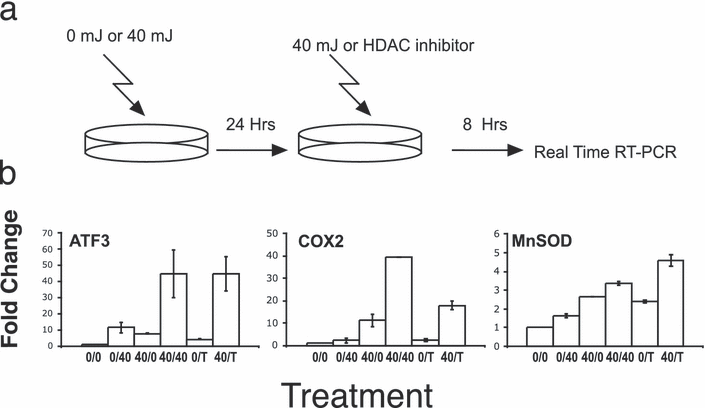
UVR can sensitize cells to TSA transcriptional induction. (a) A schematic of the experiment is shown. HaCaT cells were treated with 0 or 40 mJ cm−2 and rested for 24 h. Cells were then treated with 40 mJ cm−2 or TSA and RNA isolated 8 h after this second treatment and changes in the expression of the indicated genes were performed using real-time RT-PCR. (b) Trichostatin A (T) was used at 1.65 μm in combination with 40 mJ cm−2 UVR (indicated as 40) and changes in expression of the indicated genes were analyzed as above. Values are expressed as fold change relative to 0/0 values after normalization to GAPDH. Error bars represent ±SEM for three independent experiments.
UVB induction is inhibited by curcumin
Curcumin (diferuloylmethane), derived from Curcuma longa Linn, has a wide range of biological effects and has been shown to be an inhibitor of the histone acetyltransferase (HAT) activity of p300 and CBP, two coactivators associated with the induction of many genes (47–49). In addition, curcumin has been shown to inhibit angiogenesis (49) and suppress signaling pathways (50) at concentrations lower than those required to effectively inhibit HAT activity (47). The antioxidant activity of curcumin is also seen at lower concentrations of curcumin (51,52). The impact of curcumin on gene expression also varies with concentration in a gene-specific manner (53,54). To examine the effect of low and high concentrations of curcumin on the UVR induction of the genes under investigation, cells were treated with 10 or 100 μm concentrations of curcumin following irradiation. Curcumin has been shown to induce apoptosis in HaCaT cells (55). Under our conditions, incubation in 100 μm curcumin for 8 h led to Annexin V staining in ∼95% of cells, suggesting that at this time point the cells had initiated apoptosis. However, there were no changes in cell size, cell adhesion or light scattering (data not shown). Whereas 100 μm curcumin blocks or attenuates the induction of ATF3, COX2 and MKP1, 10 μm has little or no impact on the induction of these genes by UVR (Fig. 4a). The above suggests that 100 μm curcumin inhibits processes not affected at 10 μm or inhibits the same processes more effectively. To examine the effect of curcumin at additional time points following UVR exposure, cells were exposed to UVR and treated with 100 μm curcumin. RNA was isolated from cells at 4, 8 and 24 h following irradiation. Curcumin blocked the induction of all the genes analyzed (Fig. 4b). The inhibition of both rapid (ATF3, COX2) and delayed (MnSOD) types of UVB induction suggests that curcumin targets key components of the UVR transcriptional response. While this observation may be due to actions of curcumin not solely involving the inhibition of p300/CBP, these results suggest that chromatin modifications by the p300/CBP enzymes may be central to UVR-induced gene expression.
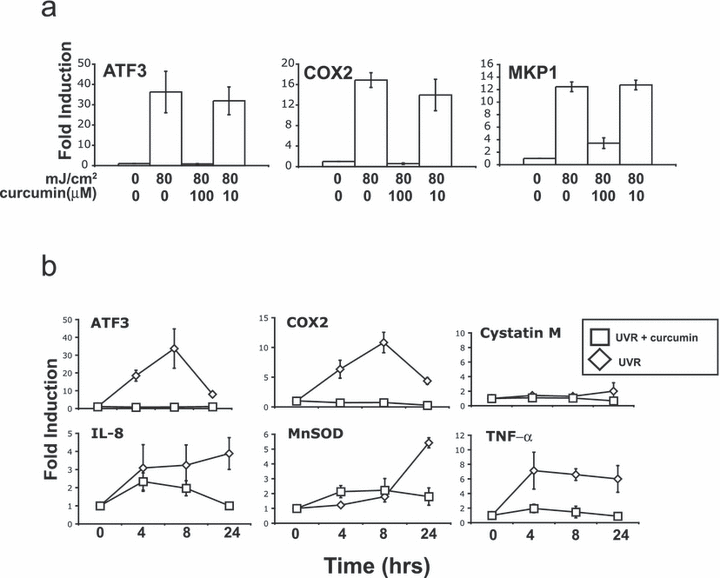
Effect of curcumin on the induction of UVR-regulated genes. (a) HaCaT cells were exposed to 80 mJ cm−2 of UVB and after irradiation incubated with media containing curcumin (100 or 10 μm). RNA was isolated just prior to treatment (0 mJ cm−2) and 8 h after UVR (80 mJ cm−2) exposure. RNA was analyzed by real-time RT-PCR and ΔCT calculated relative to GAPDH and fold change (ΔΔCT) calculated relative to time T = 0. Error bars represent ±SEM for three independent experiments. (b) HaCaT cells were exposed to 80 mJ cm−2 of UVB and after irradiation incubated with media containing curcumin (100 μm) or media alone. RNA was isolated just prior to treatment (T = 0) and 4, 8 and 24 h after UVR exposure. RNA was analyzed by real-time RT-PCR and ΔCT calculated relative to GAPDH and fold change (ΔΔCT) calculated relative to time T = 0. Error bars represent ±SEM for three independent experiments.
UVR induction of histone acetylation
To correlate changes in histone acetylation with mRNA levels, we performed an extended time course of steady-state mRNA levels following a single exposure to 40 mJ cm−2 of UVB. Following 40 mJ cm−2, mRNA levels of ATF3, COX2 and MKP1 peaked at 8 h (Fig. 5a). MnSOD mRNA levels peaked at 24 h. In contrast, IL-8 levels began to increase at 72 h in response to 40 mJ cm−2. The above suggests that the UVR induction of gene expression may involve alterations in histone acetylation. To directly evaluate the changes in histone acetylation occurring after UVR exposure within specific genomic regions, ChIP experiments were undertaken. The loci examined were within 750 bp of the start site of transcription. In addition, because ATF3 expression was the most sensitive to TSA following UVR exposure, a region further upstream harboring numerous putative transcription factor-binding sites was also included for analysis (56). These experiments focused on histone H3 K9/K14 acetylation because these marks have been shown to be associated with gene activation and have been well characterized (39,40,57,58). To define the kinetics and stability of the UVR-induced changes in histone acetylation, chromatin was isolated at 8, 24, 48 and 72 h post 0 or 40 mJ cm−2 UVR exposure. HaCaT cells grew to confluence by 72 h after 40 mJ cm−2. These experiments revealed that at the promoter regions tested for ATF3, COX2, IL-8, MKP1 and MnSOD, 40 mJ cm−2 of UVB induces an increase in histone acetylation that peaks 24 h after the exposure (Fig. 5b). Thus, 40 mJ cm−2 of UVR can increase acetylation at the promoter regions of UVR-induced genes and this increase returns to baseline by 72 h postexposure. Intriguingly, the region of the ATF3 gene further upstream from the transcriptional start site displayed a prolonged increase in histone H3 acetylation compared with the other regions analyzed. In contrast, the promoter region of the MHC class II gene, HLA-DRA, showed very low levels of acetylation and no increases after UVR exposure. Thus, histone acetylation is increased after 40 mJ cm−2 of UVB irradiation with most of the regions analyzed showing a peak of acetylation 24 h after exposure.

The effect of a single 40 mJ cm−2 treatment on mRNA levels and histone H3K9/14 acetylation. (a) HaCaT cells were exposed to 0 or 40 mJ cm−2 of UVB wavelength UVR. RNA was isolated just prior to exposure (T = 0) and 8, 24, 48 and 72 h after irradiation. RNA was analyzed by real-time RT-PCR and ΔCT calculated relative to GAPDH and fold change (ΔΔCT) calculated relative to time T = 0. Error bars represent ±SEM for three independent experiments. (b) HaCaT cells were exposed to 0 or 40 mJ cm−2 of UVB wavelength UVR and chromatin isolated at 8, 24, 48 and 72 h after irradiation. ChIP was performed using antibodies against acetylated histone H3K9/14. After immunoprecipitation of chromatin, DNA was purified and analyzed by quantitative real-time PCR. The genes analyzed are indicated in each panel along with the position of the amplicons relative to the transcriptional start site of each gene. The percentage of input chromatin is shown along the y-axis and represents 1/20th of the total DNA isolated by each ChIP. Time (h) is indicated along the x-axis. Error bars represent ±SEM for three independent experiments. The differences in acetylation levels between 0 and 40 mJ cm−2 were statistically significant (P < 0.05) for all regions except COX2 (P = 0.057) and HLA-DRA (P = 0.146). (c) HaCaT cells were subject to the same protocol as described in the legend for Fig. 2a except that chromatin was isolated 8 h after the second treatment. ChIP was performed using antibodies against acetylated histone H3K9/14. After immunoprecipitation, DNA was purified and analyzed by quantitative real-time PCR. The percentage of input chromatin is shown along the y-axis and represents 1/20th of the total DNA isolated by each ChIP. Doses of UVB in mJ cm−2 are expressed as first day/second day. The amplicon location relative to the start of ATF3 transcription is shown in the panel.
To examine the effect of multiple doses of UVR on histone acetylation, we repeated our double exposure experiments (Fig. 2a) and analyzed histone H3K9/14 acetylation levels 8 h after the second exposure. As seen previously in our ChIP time course experiment, levels of histone H3K9/14 acetylation were increased 8 h following 40 mJ cm−2 (Fig. 5c, bar 0/0 vs 0/40). In addition, this increase in H3K9/14 acetylation was stable 32 h after 40 mJ cm−2 (Fig. 5c, bar 40/0). The two doses of UVR failed to result in additional increases in histone H3K9/14 acetylation levels (Fig. 5c, bar 40/40) suggesting that in this experimental system no additional histone H3K9/14 acetylation occurs with the second exposure. This analysis confirms the increase in acetylation in the ATF3 promoter region 8 h after UVR and suggests that the second dose of UVR may induce ATF3 through mechanisms other than additional H3K9/14 acetylation.
Inhibition of histone acetylation by curcumin
The ability of curcumin to inhibit p300/CBP activity in vitro is concentration dependent with near-complete inhibition at 80 μm (47). Because we only observed an inhibitory effect of curcumin at 100 μm and not at 10 μm, we used ChIP analysis to evaluate the effect of these two concentrations of curcumin on histone acetylation. All loci demonstrated a significant (P < 0.05) loss of histone H3K9/14 acetylation in response to 100 μm (and not 10 μm) curcumin (Fig. 6a). Thus, concentrations of curcumin required to block UVR-induced transcription are associated with a decrease in histone acetylation levels at the loci examined.
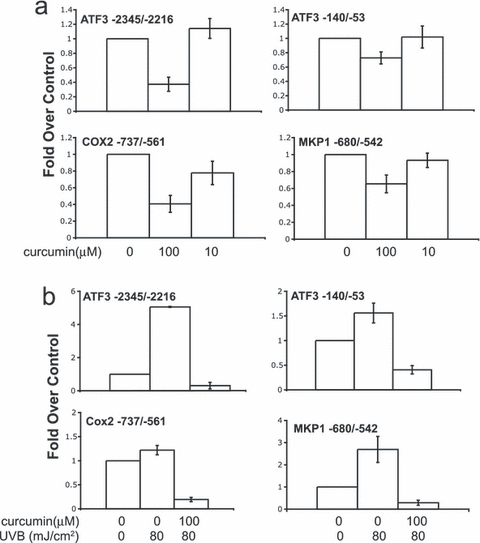
Curcumin decreases histone H3K9/14 acetylation and prevents UVR-induced histone acetylation. (a) Acetylation of histone H3K9/K14 was analyzed using ChIP. HaCaT cells were left untreated (0 μm), treated with 10 or 100 μm curcumin and chromatin isolated after 8 h. After immunoprecipitation of chromatin, DNA was purified and analyzed by quantitative real-time PCR using a standard curve of HaCaT DNA. For each independent experiment, the calculated percentage input for each ChIP was divided by the percentage input of the untreated (0 μm) control sample and these values are indicated along the y-axis. The average of two or three independent experiments ±SEM is shown. The effect of 100 μm curcumin vs the untreated sample was statistically significant (P < 0.05) in all cases whereas none of those with 10 μm curcumin were statistically significant. The concentrations of curcumin used are indicated along the x-axis. (b) HaCaT cells were mock irradiated (0 mJ cm−2) or irradiated with 80 mJ cm−2 of UVB. Immediately following radiation, cells were left untreated (0 μm) or treated with 100 μm of curcumin. Chromatin was isolated 8 h following irradiation and subject to ChIP. For each condition, the calculated percentage input was divided by that of the mock-irradiated control sample. These values are indicated along the y-axis as fold over control. The average of two independent experiments is shown ±SEM. All increases in acetylation seen after UVR were statistically significant (P < 0.05) except those for COX2. The effect of curcumin on acetylation levels vs the untreated sample was statistically significant (P < 0.05) in all cases. Concentrations of curcumin and UVB irradiation are indicated along the x-axis.
To examine the impact of curcumin on UVR-induced histone acetylation, cells were exposed to mock irradiation, UVB or UVB plus curcumin. Eight hours following irradiation, histone acetylation was analyzed by ChIP. As seen previously, we observed varying increases in histone H3 acetylation 8 h after UVR exposure (Fig. 6b). Some regions, such as ATF3 (−2345/−2216), displayed a large increase in acetylation 8 h following UVR, whereas the increases in acetylation at other loci were less robust. In addition to blocking the UVR-induced increases in acetylation, curcumin decreased acetylation levels to below those of mock-irradiated samples. Thus, curcumin can block UVR-induced increases in acetylation and promote deacetylation at the loci examined. These experiments demonstrate that HDAC activity is present in these regions and when HAT activity is inhibited, the balance between HAT and HDAC activity is shifted to favor deacetylation.
Discussion
The results presented suggest a model where exposure to UVR activates signaling pathways that impact histone acetylation and gene expression (Fig. 7). These pathways likely include those initiated by extranuclear events and those triggered by direct DNA damage within the nucleus. Given the importance of histone acetylation in the regulation of gene expression and the large number of genes whose expression is altered by UVR, it is likely that at least some of the pathways activated by UVR will affect HAT and/or HDAC function. Here, we show that the induction of gene expression by UVR is blocked by concentrations of curcumin that decrease histone H3K9/14 acetylation but not at lower doses that do not have this effect on histone acetylation. In addition, a superinduction of ATF3 by TSA (at 8 h) only occurs if preceded by UVR exposure. Treatment by TSA in this system induced ATF3 to levels nearly identical to that seen with a second UVR exposure, suggesting that these two treatments may share a common mechanism. Using ChIP analysis, we show that UVB wavelength UVR can induce an increase in histone H3 K9/K14 acetylation within the promoter regions of several UVR-regulated genes. This increase was not seen at the promoter of the MHC class II HLA-DRA gene, which is not induced by UVR. The increase in acetylation for some loci, such as those within the promoter region of ATF3 (−140/−53 and −2345/−2216), is present at 8 h post-UVR. For most regions analyzed, the increase in acetylation peaks 24 h post-UVR. This peak of acetylation levels corresponds to the presence of a transcriptional superinduction for ATF3 and COX2. These findings suggest that UVR can impact histone acetylation and that for some UVR-induced genes, changes in histone acetylation may contribute to the UVR transcriptional response.
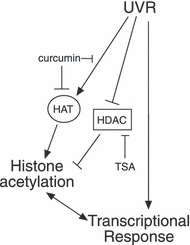
Model of the role of UVR-induced histone acetylation. UVR activates multiple signaling pathways that either increase HAT activity or decrease HDAC activity at regulatory regions of UVR-induced genes. This leads to increases in histone acetylation and contributes to the transcription of some UVR-induced genes. HDAC inhibitors such as TSA can recapitulate some of this effect by promoting acetylation of histones and other proteins. Curcumin blocks UVR-transcriptional induction by inhibiting UVR-induced signaling and/or HAT activity. For some genes, the increase in acetylation following UVR may be secondary to increased transcription and may not be required for the UVR-transcriptional response.
Histone acetylation contributes to transcriptional activation via several mechanisms. Acetylation can impact interactions of histones with DNA, chromatin compaction, and transcription factor and co-factor binding via bromodomains. Our data suggest that UVR-induced histone acetylation contributes to the UVR transcriptional response. However, the role of this increased acetylation is likely gene dependent. For some genes, such as ATF3, that are expressed at low levels prior to irradiation, the increase in acetylation may be required to relieve the repressive effect of chromatin thereby allowing efficient transcription to occur. In contrast, for other genes (especially those that are well expressed prior to UVR), the increase in acetylation reported here may be a result of increased transcription rather than vice versa.
A delay in peak acetylation (in relation to the peak of steady-state mRNA levels) was seen for most of the regions analyzed. In addition, most of the regions examined displayed sustained increases in acetylation despite steady-state mRNA returning to near-baseline levels. Thus, in our experimental system, transcription can be repressed despite the presence of increased histone acetylation. While this is in contrast to the established role of histone acetylation in transcriptional activation, there are several possible explanations for this observation. The acetylation sites may help recruit repressive complexes through bromodomain:acetyllysine interactions (59). In addition, UVR may induce transcriptional repressors (such as ATF3) whose binding to DNA is either unaffected or enhanced by the increased acetylation. Consistent with this, using ChIP, we have detected ATF3 bound to its own promoter 24 h after UVR despite increased levels of histone acetylation (data not shown). The repair of UVR-induced DNA damage involves modifications to histones that include acetylation (28,32,60). Our data suggest that for the loci examined, UVR can induce an increase in histone acetylation that contributes to the UVR transcriptional response. How does UVR exposure increase acetylation levels? Several possibilities exist. As histone acetylation levels are dynamic, UVR may increase the binding of HATs in these regions and thus tip the balance in favor of additional acetylation. Conversely, UVR may promote a loss of HDAC recruitment in these regions with the same result. It is also likely that the changes in acetylation induced by UVR represent a gene-dependent response and will vary based on the specific regulatory region being analyzed. The UVR-dependent induction of ATF3 by TSA suggests that following UVR, a loss of HDAC activity is sufficient to induce ATF3 expression. This suggests a central role for histone acetylation in the control of ATF3 expression. The data with COX2 suggest that factors other than acetylation are required for the superinduction.
Changes in the transcriptional response mediated through chromatin can optimize how cells respond to their environment. This is true in yeast and mammals. In yeast, SWI/SNF is required for transcriptional memory at the GAL gene cluster (61) and in mice CBP plays an important role in the regulation of the fosB gene in response to cocaine (62). We provide evidence for a type of transcriptional adaptation occurring in keratinocytes in response to UVB. Both ATF3 and COX2 exhibited a UVR superinduction that was present 24 h after the initial UVR exposure. This effect was manifested as an increased sensitivity to the second of two UVR exposures. Levels of mRNA (with two 40 mJ cm−2 doses) were more than additive and similar to those seen with an 80 mJ cm−2 dose. This type of response to UVB may help cells cope with multiple UVR exposures. For example, ATF3 is known to prevent p53 degradation (63), thus its induction by UVR likely impacts p53-mediated cell fate decisions (41). Such an effect might be protective against UVR-induced skin cancer. Our data with ATF3 suggest that acetylation plays an important role in the superinduction of ATF3. However, other components of the stress response, such as transcription factors, other types of covalent modifications and signaling pathways, are also likely involved.
There are multiple reports describing a role for UVR-induced histone modifications (or histone replacement) in the repair of UVR-induced DNA damage. Specifically, these include histone H3 S10 phosphorylation (34), histone H2A ubiquitylation (35), histone H3 and H4 ubiquitylation (64), histone H3 acetylation (32), histone H2AX phosphorylation (30) and the incorporation of histone H3.1 (65). In addition, p53-dependent histone acetylation and chromatin relaxation in response to UVR has been reported (66,67). Most of these reports suggest that these events are important in the repair of UVR-induced DNA damage by nucleotide excision repair. It is also likely that UVR-induced chromatin modifications can occur in the absence of DNA damage. UVR can induce dramatic clinical responses at suberythemogenic doses that likely involve changes in gene expression that do not require DNA damage. In addition, peaks of acetylation, at least for the genes studied, appear to be localized to the promoter regions of UVR-induced genes and did not occur at the MHC class II HLA-DRA promoter. Moreover, the extent of UVR-induced acetylation varied between promoter regions and between the two loci examined upstream of the ATF3 transcriptional start site. These differences are more likely due to the impact of UVR on the specific HAT and/or HDAC complexes present in these regions rather than the amount of DNA damage caused by UVR within these regions. Finally, the large-scale p53-dependent changes in chromatin that occur after UVR (66) likely require chromatin modifications in regions not directly damaged by UVR.
We provide evidence that UVR exposure results in temporary increases in histone H3 K9/14 acetylation within the promoter regions of UVR-regulated genes. It is likely that other histone modifications are affected by UVR. Defining the types and locations of histone modifications induced by UVR will provide a more complete picture of the events regulating transcriptional changes induced by UVR. In addition, this knowledge will aid in the rational application of compounds targeting histone-modifying enzymes to treat diseases caused or exacerbated by UVR exposure.
Acknowledgements— We appreciate comments from members of the Boss laboratory, Jack L. Arbiser, William G. Kelly, Robert A. Swerlick and Paul A. Wade. This work was supported by a research grant from the American Skin Association (B.P.P.) and a Physician-Scientist Career Development Award from the Dermatology Foundation (B.P.P.).




