Time-resolved Luminescence and Singlet Oxygen Formation After Illumination of the Hypericin–Low-density Lipoprotein Complex
Abstract
Time-resolved fluorescence and phosphorescence study of hypericin (Hyp) in complex with low-density lipoproteins (LDL) as well as the evolution of singlet oxygen formation and annihilation after illumination of Hyp/LDL complexes at room temperature are presented in this work. The observed shortening of the fluorescence lifetime of Hyp at high Hyp/LDL molar ratios (>25:1) proves the self-quenching of the excited singlet state of monomeric Hyp at these concentration ratios. The very short lifetime (∼0.5 ns) of Hyp fluorescence at very high Hyp/LDL ratios (>150:1) suggests that at high local Hyp concentration inside LDL molecules fast and ultrafast nonradiative decay processes from excited singlet state of Hyp become more important. Contrary to the lifetime of the singlet excited state, the lifetime (its shorter component) of Hyp phosphorescence is not dependent on Hyp/LDL ratio in the studied concentration range. The amount of singlet oxygen produced as well as the integral intensity of Hyp phosphorescence after illumination of Hyp/LDL complexes resemble the dependence of the concentration of molecules of Hyp in monomeric state on Hyp/LDL until a concentration ratio of 60:1. This fact confirms that only monomeric Hyp is able to produce the excited triplet state of Hyp, which in aerobic conditions leads to singlet oxygen production. The value of singlet oxygen lifetime (∼8 μs) after its formation from the excited triplet state of Hyp in LDL proves that molecules of singlet oxygen remain for a certain period of time inside LDL particles and are not immediately released to the aqueous surrounding. That Hyp exists in the complex with LDL in the monodeprotonated state is also demonstrated.
Introduction
Hypericin (Hyp), 7,14-dione-1,3,4,6,8,13-hexahydroxy 10,11-dimethyl-phenanthrol [1,10,9,8-opqra] perylene (Fig. 1), is a naturally photosensitizing pigment occurring in plants of the genus Hypericum and some insect species. It can be prepared synthetically as well. Plants of the genus Hypericum have been used for a long time in traditional folk medicine, for example in the treatment of depression and wound healing. The recent interest in Hyp is provoked by the discovery that under light illumination Hyp possesses antiproliferative and cytotoxic effects (necrosis as well as apoptosis) on many tumor cell lines and virucidal activity against several types of enveloped viruses. These properties together with minimal dark toxicity, tumor selectivity and high clearance rate from the host body make Hyp a very promising agent for photodynamic therapy (PDT) of cancer and virus diseases (for reviews, see Refs. [1–4]).
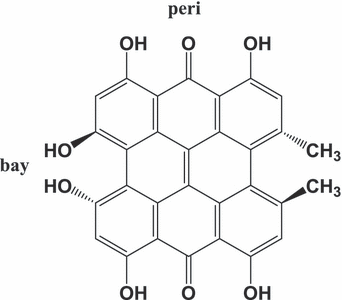
Molecular structure of hypericin.
Several mechanisms have been proposed to clarify its biological activities, including oxygen-dependent Type I and Type II photodynamic reactions resulting in the formation of the superoxide anion radical (5–8) and singlet oxygen (6–10), formation of Hyp radicals (7–12) and excited state proton transfer from Hyp to the surroundings leading to a pH drop (13,14).
Singlet oxygen is supposed to play a major role in the photoactivity of Hyp under aerobic conditions (15–17). Its generation via excitation energy transfer from the triplet state of Hyp to the molecules of oxygen in the ground state was theoretically predicted (18) as well as experimentally confirmed in several organic solvents and lipid environments (7–9,19–22). The quantum yield of singlet oxygen formation varied between 0.3 and 0.4 in organic solutions, liposomal structures and mitochondria (20–22). However, there exist several other processes which can depopulate the triplet excited state of Hyp: phosphorescence, radiation-less deactivation, electron transfer to ground-state oxygen, triplet-triplet annihilation or quenching by the ground-state Hyp (5,7,9,11,18,23). The relative contribution of the individual processes depends on various factors, e.g. the type of solvent, the concentration of Hyp and O2, the pH of the solution or the presence of electron acceptors or donors.
It is well known that serum proteins are predominantly responsible for the transportation of photosensitizers throughout the body (24–28). Upon administration into the bloodstream Hyp associates with serum proteins, mainly with low-density lipoproteins (LDL) and to a lesser extent with human serum albumin and high-density lipoproteins (29).
Up to now, only a few articles concerning the interaction of Hyp with plasma lipoproteins have appeared (30–34), although the study of complexes of lipoproteins with hydrophobic and amphiphilic photosensitizers and/or other drugs is not unusual (23–27). The importance of the study of drug–lipoprotein complexes is confirmed by the fact that the US Food and Drug Administration encouraged the inclusion of lipoprotein–drug interaction studies as a part of any investigational new drug application that contains a hydrophobic compound (35).
Low-density lipoproteins could play a key role in the targeted delivery of hydrophobic and/or amphiphilic photosensitizers to tumor cells in PDT (24–28,36–42) due to the enhanced expression of specific LDL receptors (regulated by the cholesterol needs of the cell, usually higher in fast growing cells, like tumor cells and tumor endothelial cells) in many types of transformed cells when compared with nontransformed cells (41,42).
Our previous studies in this field showed that high Hyp/LDL ratios (>30:1) lead to a significant decrease in the quantum yield of Hyp fluorescence (32). We have suggested that this decrease is caused by the formation of Hyp aggregates inside of LDL molecules as well as by self-quenching of Hyp fluorescence. It was shown that photoactivated Hyp oxidizes LDL in a light dose and excitation wavelength-dependent manner. We have also demonstrated an important role of the LDL receptor pathway for Hyp delivery to U-87 MG cells in the presence of LDL (33). The substantial increase in Hyp uptake was revealed when the number of LDL receptors on the cell surface was elevated. Moreover, colocalization experiments showed the lysosomal localization of Hyp following uptake and that the concentration of Hyp in these organelles was enhanced in the cells with elevated number of LDL receptors when the incubation medium contained LDL. The recent publication of Laggner et al. (43) paradoxically shows Hyp as a potent antioxidant in LDL and as an inhibitor of LDL atherogenic modification.
This work presents data about the dependence of the kinetics of Hyp luminescence (fluorescence as well as phosphorescence) and singlet oxygen formation and annihilation after illumination of the Hyp/LDL complex on the Hyp/LDL ratio. Moreover, integral Hyp phosphorescence and singlet oxygen luminescence are evaluated as a function of Hyp concentration. The obtained results contribute to the more detailed view of the physicochemical properties of the complexes of photosensitizers (in this case Hyp) with LDL and elucidate some aspects of singlet oxygen formation and annihilation following illumination of photosensitizers embedded in the lipid environment.
Materials and methods
Chemicals. Hypericin and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich, LDL (purity >95% of total lipoprotein content by electrophoresis) from Calbiochem (Darmstadt, Germany).
Preparation of Hyp-LDL solutions. Stock solutions of Hyp (0.1 and 2 mm) were prepared by dissolving solid Hyp in 100% DMSO and were kept in the dark at 4°C. A stock solution of LDL at 10 μm concentration was prepared in 150 mm NaCl aqueous solution at pH = 7.4 with 0.01% EDTA. Solutions for experiments were obtained by dilution of stock solutions of LDL and Hyp with phosphate buffer (PBS; pH = 7.4). The final quantity of DMSO in all experiments was less than 1.5%. Such prepared solutions were then stored in the dark at 4°C for 12 h before measurements.
Fluorescence lifetime measurements. For time-resolved fluorescence studies we utilized a custom-built, diode-pumped picosecond pulsed YAG laser. The laser provided ∼30 ps pulses with pulse energy of up to 0.1 mJ at 1064 nm; its repetition frequency was set to 10 Hz to match the detector sampling rate (44). The laser pulses were first converted to 532 nm by the second harmonic generator. The sample was excited through the side window of a 1 × 1 cm cell cuvette and the emission was monitored at right angles to the direction of excitation. The image of the horizontal line, formed by the fluorescence following the laser beam path within the cell, was spatially filtered by a 100 μm wide slit and imaged onto the photocathode of the streak camera K008 (BIFO) (45), using photographical lens. The emission of the sample was filtered by long-pass filter before entering the streak camera system to detect only photons within the ∼600–800 nm range. In the streak operation mode, the deflection plates in the tube sweep a photoelectron image over the micro-channel plate, creating a linear time base perpendicular to the spatial axis. The photoelectron pattern is then intensified by the micro-channel plate and converted back to optical signal using the phosphor screen. The readout of the signal was realized using a CCD videocamera, coupled to a computer. The signal derived from several tens of laser pulses was averaged to decrease noise and to get the maximal possible intensity range. The fluorescence decay curves were obtained by summing the streak image over all pixels with the same time coordinate. In subsequent multiexponential decay analysis in Origin 7.0 (OriginLab) we used only the decaying part of the data, thus reducing the convolution effects. Half-width of the instrument response of the whole system, comprising picosecond laser excitation and the streak camera with fast gating electronics and CCD readout, was estimated at 0.098 ± 0.003 ns.
The experiments were performed in two complementary ways. First, the concentration of LDL was kept constant (c = 10−7 m) and the concentrations of Hyp were varied between 2 × 10−7 and 1.75 × 10−5 m to obtain molar ratio Hyp/LDL from 2:1 to 175:1. Second, the concentration of Hyp remained constant (5 × 10−7 m) and the concentrations of LDL were varied between 2.5 × 10−9 and 2.5 × 10−7 m to obtain molar ratios Hyp/LDL from 2:1 to 200:1.
The fluorescence decay of Hyp was fitted as a monoexponential function until the Hyp/LDL ratio was 50:1. Beyond this ratio the experimental data were necessary to be fitted as a biexponential function. All measurements were realized at room temperature and were repeated for every Hyp/LDL ratio at least three times.
Phosphorescence measurements. For the phosphorescence experiments the concentration of LDL was kept constant (c = 10−7 and 2 × 10−7 m) and the concentrations of Hyp were varied between 2 × 10−7 and 4 × 10−5 m. Both time- and spectral-resolved phosphorescence of Hyp and singlet oxygen luminescence were measured simultaneously using an infrared (IR)-sensitive emission spectrometer. A detailed description of the experimental setup and procedures is presented in our previous publication (46). The samples were excited through the optically polished bottom of the fluorescence cell by laser pulses at 597 nm with energy of ≈30 μJ. The IR emission was detected by an IR-sensitive photomultiplier together with a photoncounter/multiscaler with 5 ns per channel.
The phosphorescence decay curve of Hyp has a complicated course, which did not allow us to fit experimental data as a monoexponential function. The observed experimental data can be reliably fitted as a biexponential decay; however, we realize that Hyp triplet state deactivation is a more complex process and that biexponential function is only a very rough approximation of the real situation.
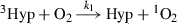 and annihilation
and annihilation 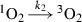 were fitted by the equation
were fitted by the equation
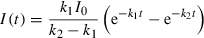 (1)
(1)All measurements were realized at room temperature and were repeated for every Hyp/LDL ratio minimally three times. Time-resolved phosphorescence spectra were treated by Microcal Origin, version 7.5 (Microcal Software, Inc., Northampton, MA).
Results
Absorption, fluorescence and phosphorescence spectra of Hyp in complex with LDL
Figure 2 presents normalized absorption, fluorescence and phosphorescence spectra of Hyp in complex with LDL at a Hyp/LDL concentration ratio of 20:1. It was previously shown that molecules of Hyp exist in monomeric state at these concentration conditions (32). The positions of maxima of absorption (597 nm) and fluorescence spectra (599 nm) of Hyp are in good agreement with the previously determined values in lipid environment (DMPC liposomes [22,47], EPC liposomes [48]). On the other hand, the Hyp phosphorescence maximum (around 830 nm) is redshifted with respect to the already determined ones: 754 nm (49), 730 nm (50) and 759 nm (51). However, these works presented the phosphorescence spectra of Hyp recorded at low temperatures (1.2 and 77 K) and in organic solvents. The theoretical calculation of the lowest triplet excitation of Hyp predicts this transition at 765 nm for neutral and at 833 nm for monodeprotonated Hyp (18). Therefore, our results suggest that Hyp molecules in the complex with LDL at pH = 7.4 exist in a monodeprotonated state. This conclusion is in good accordance with the results of other works concerning the protonation behavior of Hyp in various media (2,52,53). It is generally accepted that the first deprotonation occurs in the bay-region of Hyp (2,52). It should be noted that the observed fluorescence and absorption spectra of Hyp in Hyp/LDL complexes are fully consistent with the previous published spectra for the monodeprotonated state of Hyp (53,54).
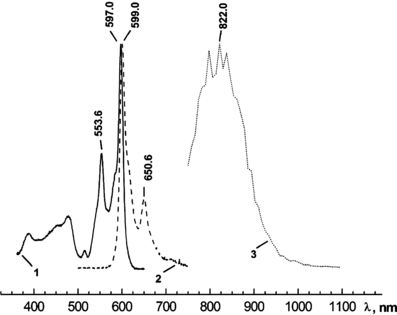
Absorption (1), fluorescence (λexc = 488 nm) (2) and phosphorescence (λexc = 597 nm) (3) spectra of Hyp in complex with LDL (molar ratio Hyp/LDL = 20:1) at room temperature. The concentration of LDL was 2 × 10−7 m and concentration of Hyp was 4 × 10−6 m.
The positions of the maxima of the absorption and luminescence spectra of Hyp in the presence of LDL are not significantly influenced by the changing Hyp/LDL ratio. The position of Hyp phosphorescence maximum cannot be exactly determined because of the low resolution of the monochromator used for measurements of phosphorescence spectra (∼8 nm). The shapes of the phosphorescence spectra of Hyp are almost identical for various Hyp/LDL ratios under given resolution of the experimental setup; however, slight shifts in Hyp phosphorescence maxima for different Hyp/LDL values cannot be excluded.
The absorption spectra of Hyp as a function of Hyp/LDL ratio were measured in our previous work (32). These spectra confirmed the formation of Hyp aggregates at high Hyp/LDL ratios (>30:1). Moreover, using the ratio of absorbance of two main absorption bands of Hyp in the visible region (A554/A597), we have determined the dependence of the concentration of monomeric Hyp on Hyp/LDL. The course of this function is utilized in this work for comparison with the dependence of the integral Hyp and singlet oxygen phosphorescence intensities on Hyp/LDL ratio (see Fig. 7).
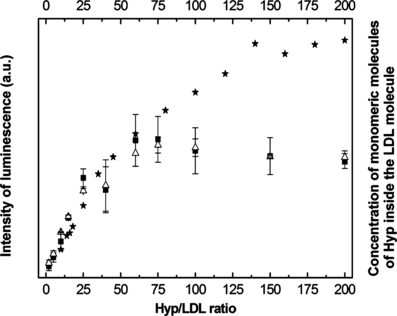
Integral intensity of Hyp phosphorescence () and singlet oxygen luminescence (△) after illumination of the Hyp/LDL complex (excitation line, 597 nm) and concentration of monomeric Hyp ( ) as a function of the Hyp/LDL ratio. The concentration of LDL was constant at 2 × 10−7 m.
) as a function of the Hyp/LDL ratio. The concentration of LDL was constant at 2 × 10−7 m.
Fluorescence kinetics of Hyp embedded in LDL
The dependence of Hyp fluorescence lifetime on the Hyp/LDL ratio is presented in Fig. 3. Fluorescence kinetics of Hyp in complex with LDL follow monoexponential decays for low Hyp/LDL ratios. The value of the lifetime is around 7.3 ns and it remains constant up to a Hyp/LDL ratio of 15:1. This lifetime is quite longer than the lifetime of Hyp fluorescence decay in organic solvents (up to 6 ns) (55) but is in very good accordance with the published data for the fluorescence lifetimes of Hyp in lipid media (48). With an increase in the Hyp/LDL ratio above 15:1, a substantial shortening of this lifetime occurred (Fig. 3, Table 1A). This result clearly confirms the expected occurrence of the self-quenching of Hyp fluorescence accompanying the formation of aggregates of Hyp under elevated local Hyp concentration inside LDL. Moreover, starting at ca Hyp/LDL = 50:1, Hyp fluorescence kinetics exhibit more complicated behavior with two fluorescence lifetimes detected. As has been previously shown (48,56), the multiple decay components may indicate that various fractions of Hyp molecules are differently self-quenched in different parts of the LDL particle. The appearance of two populations with different fluorescence lifetimes at Hyp/LDL ratio of 60:1 (the first value for which the decay kinetics is necessary to be fitted as biexponential function) with almost identical contributions (Table 1A) indicates that at lower Hyp/LDL ratios the lifetimes of both components are very similar and so fluorescence decays can be fitted as monoexponential functions. Nevertheless, the fraction of population with shorter lifetime increases with increasing Hyp concentration to the extent that it creates almost all fluorescence signals at Hyp/LDL ratios exceeding 150:1. The very short lifetime (∼0.5–0.6 ns) of Hyp fluorescence at these Hyp/LDL ratios suggests that in accordance with the previous studies of Hyp photophysics in phospholipid membranes (57), ultrafast and fast nonradiative decay processes from the excited singlet state of Hyp become more important at very high Hyp local concentrations. Subnanosecond resolution experiments have to be employed for deeper elucidation of Hyp decay at its very high local concentration. We can only speculate that the existence of two populations with different fluorescence lifetimes is a consequence of two regions inside LDL particles (phospholipid+cholesterol surface shell and region close to the hydrophobic core) (58), where Hyp can be located and that with the increasing Hyp/LDL ratio the fluorescence from monomeric Hyp embedded in the phospholipid shell is quenched due to the formation of aggregates and only fluorescence of Hyp located closer to the hydrophobic core of LDL is observed with decreasing lifetime at increasing Hyp/LDL ratios. With respect to the work of Losi (57) it can be concluded that the most probable region for the internalization of Hyp into LDL is the polar zone close to the phospholipid–water interface but with the increasing Hyp/LDL ratio the population of Hyp molecules localized closer to the hydrophobic core is increased.
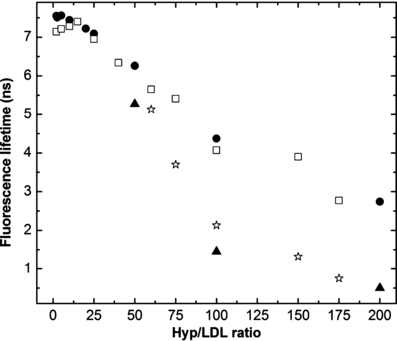
The dependence of Hyp fluorescence lifetime on Hyp/LDL ratio at constant LDL concentration (10−7 m) (□—longer component,  —shorter component) and at constant Hyp concentration (5 × 10−7 m) (•—longer component, —shorter component). The excitation wavelength was 532 nm.
—shorter component) and at constant Hyp concentration (5 × 10−7 m) (•—longer component, —shorter component). The excitation wavelength was 532 nm.
| Hyp:LDL | τ 1 (ns) | a 1 (%) | τ 2 (ns) | a 2 (%) |
|---|---|---|---|---|
| A: Constant concentration of LDL (10−7 m) | ||||
| 2:1 | 7.14±0.11 | 100 | ||
| 5:1 | 7.21±0.11 | 100 | ||
| 10:1 | 7.28±0.09 | 100 | ||
| 15:1 | 7.40±0.05 | 100 | ||
| 25:1 | 6.95±0.12 | 100 | ||
| 40:1 | 6.34±0.02 | 100 | ||
| 60:1 | 5.65±0.42 | 46.1±32.3 | 5.13±0.09 | 53.9±32.3 |
| 75:1 | 5.41±0.09 | 34.1±3.2 | 3.70±0.14 | 65.9±3.2 |
| 100:1 | 4.07±0.15 | 33.5±3.4 | 2.13±0.05 | 66.5±3.4 |
| 150:1 | 3.90±0.45 | 8.7±1.7 | 1.32±0.21 | 91.3±1.7 |
| 175:1 | 2.77±1.62 | 10.3±5.7 | 0.75±0.31 | 89.7±5.7 |
| B: Constant concentration of Hyp (5 × 10−7 m) | ||||
| 2:1 | 7.55±0.04 | 100 | ||
| 2.5:1 | 7.51±0.03 | 100 | ||
| 5:1 | 7.56±0.05 | 100 | ||
| 10:1 | 7.44±0.10 | 100 | ||
| 20:1 | 7.22±0.03 | 100 | ||
| 25:1 | 7.09±0.07 | 100 | ||
| 50:1 | 6.26±0.63 | 35.6±0.5 | 5.27±0.23 | 64.4±0.5 |
| 100:1 | 4.37±0.15 | 23.3±1.6 | 1.45±0.08 | 76.7±1.6 |
| 200:1 | 2.74±0.55 | 6.8±2.9 | 0.50±0.16 | 93.2±2.9 |
- Hyp = hypericin; LDL = low-density lipoprotein.
In addition, we have realized complementary experiments with a constant concentration of Hyp and changing concentrations of LDL to obtain various Hyp/LDL ratios. The observed values of Hyp fluorescence lifetimes at different Hyp-LDL ratios (Fig. 3, Table 1B) are almost identical with those obtained in the situation with a constant concentration of LDL. It means that the concentration ratio of Hyp/LDL is more important than the total concentration of Hyp and LDL for the determination of the photophysical properties of Hyp in the presence of LDL.
The results confirm and complement our previous work where it was found that the fluorescence intensity of Hyp linearly increases up to Hyp/LDL ratio of 30:1 and above this molar ratio the quenching of Hyp fluorescence was observed (32). The fluorescence intensity decrease was not linear and at a high Hyp/LDL ratio (>200:1) the fluorescence almost disappeared. On the basis of the steady-state as well as the time-resolved fluorescence measurements it can be concluded that both the formation of aggregates of Hyp inside LDL and the self-quenching of Hyp fluorescence contribute to the observed decay of Hyp fluorescence quantum yield at high Hyp/LDL ratios.
Phosphorescence kinetics of Hyp and formation and annihilation of singlet oxygen after illumination of the Hyp/LDL complex
Figure 4 presents time- and spectral-resolved phosphorescence of Hyp and singlet oxygen after illumination of the Hyp/LDL complex at room temperature (spectra for Hyp/LDL = 25:1 are shown as an example). Hyp embedded in LDL particles exhibits phosphorescence with the position of its maximum around 830 nm. As already mentioned, this value indicates that Hyp incorporated into LDL molecules exists in the monodeprotonated state. Similarly as in the fluorescence measurements, no Hyp phosphorescence was observed in the aqueous solution. This result confirms the assumption about the formation of nonluminescent aggregates of Hyp in aqueous solutions.
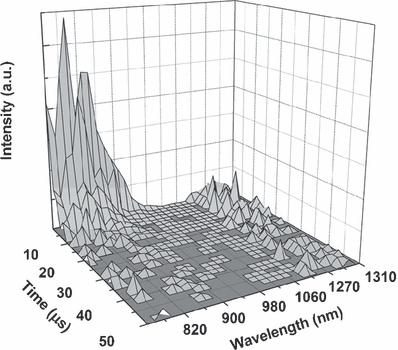
Time- and spectral-resolved phosphorescence of Hyp and singlet oxygen luminescence after illumination of the Hyp/LDL complex at room temperature. Excitation line is 597 nm, concentration of LDL is 2 × 10−7 m and Hyp concentration is 5 × 10−6 m.
The maximum for singlet oxygen luminescence detected at 1278 nm has the expected value and is in accordance with previously published data (59). It is not possible to fit the decay curve for Hyp phosphorescence as a monoexponential function (Fig. 5A). Experimentally observed Hyp phosphorescence decay can be reliably fitted by using a two-exponential decay curve. The value of the shorter component is about 2.1 μs and is independent of the Hyp concentration in the studied range of Hyp/LDL ratios. The longer component of Hyp phosphorescence decreases with increasing ratio between concentrations of Hyp and LDL, from 9 μs at low Hyp/LDL ratios to around 6 μs at Hyp/LDL = 200:1.
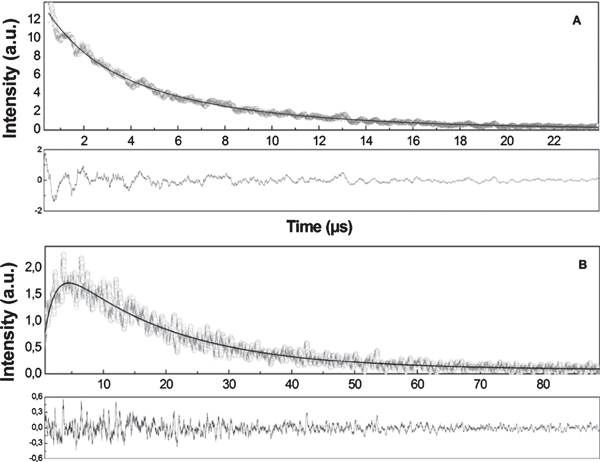
(A) Kinetics of Hyp phosphorescence and (B) evolution of the signal of singlet oxygen luminescence (1278 nm) after illumination of the Hyp/LDL complex. Excitation line is 597 nm, concentration of LDL is 2 × 10−7 m and Hyp concentration is 5 × 10−6 m.
The kinetics of singlet oxygen formation and depopulation is depicted in Fig. 5B. The experimental data were fitted by Eq. (1) (see Materials and Methods). The rise time of singlet oxygen production is about 2 μs which is very close to the shorter component of Hyp phosphorescence decay. This value only slightly decreases with the increase in the Hyp/LDL ratio (Fig. 6). The almost perfect coincidence of Hyp phosphorescence lifetime and singlet oxygen rise time (Fig. 6) together with the independence of Hyp phosphorescence shorter lifetime on Hyp concentration suggest that under the given experimental condition, the main process of the depopulation of Hyp triplet state is the interaction between triplet Hyp and molecular oxygen in the ground-state, which results in the formation of singlet oxygen.
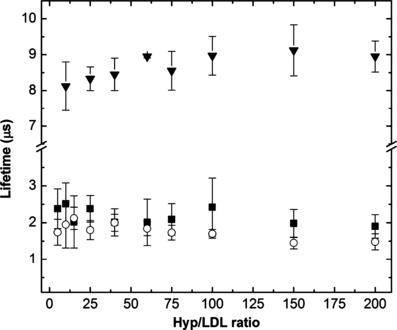
Dependence of Hyp phosphorescence lifetime (), the rise time of singlet oxygen formation (○) and singlet oxygen lifetime () on the Hyp/LDL ratio after illumination of the Hyp/LDL complex (excitation line, 597 nm). The concentration of LDL was constant at 2 × 10−7 m.
The independence of the shorter component of Hyp phosphorescence lifetime on Hyp/LDL concentration ratio excludes all depopulation processes whose rate constants depend on Hyp concentration, such as autoionization of triplet excited Hyp by other Hyp molecules in ground or triplet excited states or triplet–triplet annihilation. With respect to the above-mentioned fact, only the following processes, in addition to singlet oxygen formation, could contribute to the depopulation of the excited triplet state of Hyp in complex with LDL: (1) the formation of superoxide anion radical by direct electron transfer from triplet state Hyp to oxygen with the formation of superoxide anion radical; (2) radiation-less deactivation; and (3) the electron and/or proton transfer between Hyp and surrounding molecules.
The possibility of the formation of superoxide anion radical by direct electron transfer from triplet state Hyp to oxygen was studied in several works (5,47) and the conclusion of these works was that this process can occur only with a very low probability. Radiation-less deactivation has also a very low probability because the phosphorescence lifetime measured under the anaerobic condition in organic solution is about a millisecond (49,51). A possibility remains that Hyp in its triplet state can interact with some molecules which are part of LDL leading to the formation of Hyp radicals (anion as well as cation radicals). Hyp anion radical can consequently interact with oxygen and superoxide anion radical could be formed. This process was observed, suggesting that it may contribute to the Hyp activity in lipid environment (9,23). Our data do not allow us to unambiguously decide whether this process can substantially contribute to the depopulation of the excited triplet state of Hyp in complex with LDL.
For explaining the decay kinetics of singlet oxygen, the approach proposed by Ehrenberg et al. (22) was utilized. They studied the decay of singlet oxygen (formed by the illumination of Hyp immersed in liposomes) that resides in two phases and diffuses from the lipid phase to the aqueous phase. It was proposed that the decay kinetics in such a situation are not a linear combination of the two characteristic decay times in the two phases, but are convoluted with the diffusion rate of singlet oxygen from the lipid phase to the aqueous surrounding, generating a quasimonoexponential decay curve. Using this approach, we have determined the lifetime of singlet oxygen after its formation from the triplet excited state of Hyp embedded in LDL. Only a slight increase in this lifetime with an increase in the Hyp/LDL ratio (from 8.2 μs at Hyp/LDL = 10:1 to 9.2 μs at Hyp/LDL = 175:1) was detected (Fig. 6). A possible explanation for this observation is that at high Hyp/LDL ratios more Hyp molecules are immersed close to the hydrophobic core of LDL from which the distance to the aqueous surrounding is longer than that from the phospholipid shell of LDL, where the molecules of LDL are dominantly located at lower Hyp/LDL ratios. The intrinsic lifetime of singlet oxygen in a lipid environment, depending on the type of lipid, was found in the range of 13–35 μs (22) and about 4 μs in pure water (60). The observed values of the singlet oxygen lifetime between these numbers (ca 8–9 μs) demonstrate that singlet oxygen molecules, formed by the illumination of Hyp/LDL complex, spend part of their life inside LDL particles.
Integral phosphorescence intensity of Hyp and quantity of singlet oxygen production
Figure 7 presents dependence of total Hyp and singlet oxygen phosphorescence intensities on the Hyp/LDL ratio after illumination of this complex together with the concentration of monomeric Hyp as a Hyp/LDL function, which was determined in our previous work (32). The character of these dependencies clearly suggests that the amount of produced singlet oxygen and intensity of Hyp phosphorescence correlate very well with the quantity of monomeric molecules of Hyp inside the LDL particles for Hyp/LDL < 100:1. The increase of Hyp/LDL ratio above this value leads to a difference between the studied dependencies. Meanwhile, the concentration of monomeric Hyp slightly rises with increasing Hyp/LDL, the integral intensities of Hyp and singlet oxygen phosphorescence slightly decrease. This is probably caused by the following reason: The high local concentration of Hyp in LDL at Hyp/LDL >60:1 can lead to the not very profound, but detectable deactivation of Hyp triplet state by processes dependent on Hyp concentration (the electron and/or proton transfer between Hyp and surrounding molecules, triplet–triplet and/or triplet–ground state annihilation). The consequence of this fact is the observed slight decrease in the integral intensity of Hyp and singlet oxygen phosphorescence. However, the existence of significant Hyp phosphorescence and singlet oxygen production, even at these high Hyp-LDL ratios, supports our suggestion about the existence of monomeric Hyp inside LDL also for very high Hyp/LDL ratios.
Conclusion
The study of time-resolved fluorescence and phosphorescence of Hyp and singlet oxygen formation after illumination of Hyp/LDL complex showed that self-quenching of the excited singlet state of Hyp plays an important role at very high Hyp/LDL ratios. On the contrary, the kinetics of the depopulation of the triplet state of Hyp are not significantly dependent on Hyp concentration. It was demonstrated that singlet oxygen production and integral intensity of Hyp phosphorescence follow the course of the dependence of the concentration of monomeric Hyp on Hyp/LDL ratio until the value of 60:1. Further increasing Hyp/LDL leads to a slight decrease in Hyp and singlet oxygen phosphorescence intensities, which is caused by the high local concentration of Hyp inside LDL. The high local concentration of Hyp enables the deactivation processes of the Hyp triplet state, which are dependent on Hyp concentration (e.g. triplet–triplet annihilation) to become significant. However, we have concluded that the primary process of depopulation of Hyp triplet state inside LDL is its interaction with ground-state molecular oxygen with consequent production of singlet oxygen. The value of singlet oxygen lifetime after its formation in LDL suggests that molecules of this reactive species are not immediately diffused out of the LDL particle but spend a part of their life inside this macromolecule. Moreover, phosphorescence spectra reveal that molecules of Hyp embedded in LDL particles exist in monodeprotonated state. All these results complement our previous study of the optical spectroscopic properties of the Hyp/LDL complex (32) and can provide valuable insight into the kinetics of the photophysics and photochemistry of Hyp embedded in the lipid environment.
Acknowledgments
Acknowledgements— This work was supported by the Slovak Research and Development Agency under the contract APVV-0449-07, by the Scientific Grant Agency of the Ministry of Education of Slovakia under the grant VEGA 2278 and by the Czech Ministry of Education research project MSM 0021620835. We thank Martin Klein for critical reading of the manuscript.




