Photosensitized Oxidation of Hypoxanthine and Xanthine by Aluminum Phthalocyanine Tetrasulfonate. Role of the Alkylating Quinone 2,5-Dichloro-diaziridinyl-1,4-benzoquinone
Abstract
Photoirradiation of nitrogen-saturated aqueous solutions containing aluminum phthalocyanine tetrasulfonate (AlPcS4) at 675 nm in the presence of 2,5-dichloro-diaziridinyl-1,4-benzoquinone (AZDClQ) and hypoxanthine (HX) produces the oxidized HX derivatives, xanthine (X) and uric acid (UA). Concentrations of the AZDClQ semiquinone, X and UA increase at the expense of HX with an increase in irradiation time. Almost negligible decomposition of HX, as well as very low amounts of X, are detected if photolysis occurs under identical conditions but in the absence of AZDClQ. Addition of calf-thymus DNA produces quinone-DNA covalent adducts after photolysis of anaerobic samples containing quinone, DNA and AlPcS4, in the presence or absence of HX and at pH 5.5. However, larger amounts of quinone-DNA adducts are detected if HX is present. The results presented here could have applications in the photodynamic treatment of hypoxic tissues such as solid tumors, under conditions of high HX concentration, where Type-I pathways could be more important than singlet oxygen generation.
Introduction
Photodynamic therapy (PDT) is a cancer treatment that uses a combination of red laser light, a photosensitizing agent, and molecular oxygen to produce the therapeutic effect (1,2). Porphyrins, phthalocyanines, chlorins and other dyes are currently being used in photodynamic treatment of tumors due to their large absorption coefficients in the 500–800 nm range. In the presence of dioxygen, these photosensitize the production of singlet oxygen and superoxide (1,2). Singlet oxygen production, the so-called Type-II pathway, is claimed to be the most important process that kills tumor cells (1,2). However, Type-I pathways, those involving photoreduction or photooxidation of substrates, have also been proposed as photocytotoxic events in PDT, especially in hypoxic environments (3).
Solid tumors are often hypoxic (4). Therefore, direct killing of the hypoxic cells by singlet oxygen is very limited (2,5). However, as red–light-absorbing dyes also photoreduce oxygen (6–8), these should also photoreduce molecules having nearly equal or more positive redox potentials than oxygen in anoxic/hypoxic cells. If the reducible substrate is a DNA-alkylating quinone, which is activated by reduction, DNA alkylation should be expected, with the consequent cell death, if repair of the DNA damage is insufficient.
Diaziridinylbenzoquinones are alkylating agents by means of their aziridine groups (9,10). These compounds have significant activity against many tumor models and have been in clinical use for several years (10). They contain a quinone moiety that can be reduced and an aziridinyl group that can form covalent bonds with a variety of cellular components, including DNA. All aziridinyl compounds can be activated to alkylate DNA by protonation of the aziridine groups followed by nucleophilic attack. Indeed, it was demonstrated several years ago that simple aziridinylbenzoquinones in aqueous solution can cross-link DNA in the absence of reduction and this process is pH dependent (11). The quinones that can undergo bioreduction have an added advantage in many cases: although the pK for the aziridine moiety is around 3–4, the pK increases to around 5–6 when the quinone is reduced, and hence the aziridine should more readily react with DNA bases (12). Both the two-electron reduced species, hydroquinone (QH2) (13) and the semiquinone (Q•−) of aziridinyl quinones (14), have been proposed as the DNA-alkylating species. Bioreductively activated drugs such as these are of potential use against solid tumors containing hypoxic regions (15). In a previous study, we found that PDT-type dyes under anaerobic conditions photosensitize the reduction of alkylating quinones with the subsequent binding of the reduced quinones to calf-thymus DNA (16). Evidence suggested that, in the presence of DNA, reducing equivalents were transferred from DNA to the alkylating quinone.
Instead of DNA photoxidation by PDT dyes, sacrificial electron donors could be used to improve the photosensitized reduction of alkylating quinones. Sacrificial electron donors are compounds that donate electrons to excited-state dyes. Some of the biomedically relevant electron donors are mannitol, glucose, sucrose, maltose and amino acids such as tryptophan, tyrosine, histidine, methionine, serine (17) and cysteine (18). Other potential sacrificial electron donors are hypoxanthine (HX) and xanthine (X). HX and X are substrates of xanthine oxidase (XO). These purines are enzymatically oxidized to the final product, uric acid (UA), by XO, if a suitable reducible substrate, such as oxygen, is present (19). Promising antitumor activity has been obtained in mice after administration of a polythyleneglycol-bound XO (but not free XO) followed by HX injection (20). Also, purine catabolites, including HX, accumulate in solid tumors that are exposed to hyperthermia (21). Thus, photolysis of PDT dyes in the presence of both diaziridinylquinone and HX, or in combination with hyperthermia, could enhance quinone reduction and macromolecule alkylation and thus improve PDT of solid tumors.
In this work, we conduct a proof-of-principle test of a mechanism whereby PDT could damage biomolecular targets under hypoxic conditions. Specifically, we demonstrate that aluminum phthalocyanine tetrasulfonate (AlPcS4), under nitrogen-saturated conditions (to simulate solid tumor regions where oxygen participation is limited) photooxidizes HX to produce X and X to produce UA in the presence of AZDClQ but not in its absence. In addition, the presence of HX increases the photosensitized formation of aziridinylquinone-DNA covalent adducts under these conditions, thus suggesting a Type-I process that could be exploited in the PDT of solid tumors.
Materials and Methods
Materials. The dye, AlPcS4, was obtained from Frontier Scientific and used as received. AZDClQ, HX, X and UA were purchased from Sigma-Aldrich Chemicals and used as received. The quinone 2,5-dichloro-diaziridinyl-1,4-benzoquinone was either purchased from Sigma-Aldrich Chemicals or synthesized and characterized, starting from the tetrachloro quinone, as reported elsewhere (22). Calf-thymus DNA and Sephadex G-25 (superfine, DNA grade), were purchased from Sigma-Aldrich Chemicals. All other chemicals were of the highest purity commercially available and were used without further purification. Aqueous DNA stock solutions were freshly prepared in water each day and their concentration determined from the absorbance at 260 nm and using a molar absorption coefficient of 6.6 × 103 m−1 cm−1. Deionized and Chelex-treated water was used in the preparation of all stock and sample solutions. All reagent stock solutions were deaerated by flushing with water-saturated nitrogen prior to mixing with other reagents. Oxygen was then excluded from samples by keeping a positive pressure of N2 gas in the sample.
Sample photoirradiation. Nitrogen-saturated samples (1.00 mL) containing 875 μm quinone in the presence or absence of 1 mg mL−1 calf-thymus DNA, in the presence or absence of 2 mm HX and a dye concentration to produce an absorbance of 1, at the dye maximum wavelength of 675 nm (6 μm), in 20 mm phosphate buffer at either pH 5.5 or 7.4, were photolyzed in quartz cuvettes of 1.00 cm light path under continuous stirring. The pH value of 5.5 was selected to simulate hypoxic tumor pH conditions (23). Wanting to keep the same constituents at the two pH values and recognizing that phosphate would not be an efficient buffer at pH 5.5, the pH value was measured before and after irradiation and found to remain constant within ±0.1 pH units. In addition, to avoid interference with the reactions, phosphate was always used. Photoirradiation was allowed to occur for periods of time ranging from 0 to 20 min at 675 ± 10 nm with a radiant power of 8 mW. Samples were protected from external light during and after preparation. Irradiations were performed using a 1000-W xenon arc lamp coupled to a Schoeffel grating monochromator.
HX and X photooxidation. Samples were prepared and photolyzed as indicated above followed by HPLC analysis of HX and the products X and UA. HPLC analyses were performed using two Alltech Platinum EPS C-18 (4.6 × 250 mm) columns and gradient elution going from 100% ammonium acetate at pH 7.4 to 1:1 ammonium acetate (pH 7.4):methanol in 15 min followed by 100% methanol. In order to exclude oxygen from the eluant mixture, solvents were purged with argon 30 min before injection and during the chromatographic analysis. The flow rate of elution was 1 mL min−1. The column was washed daily with methanol for 1 h and then with water for 40 min and stored in methanol:water (1:4, v/v). The column was re-equilibrated to initial conditions for 60 min before injection. A Shimadzu 10A analytical HPLC system, equipped with a Shimadzu SPD-10 multiwavelength absorbance detector, was used at a wavelength of 267 nm. Standard curves were prepared with HX, X and UA solutions and used to obtain the concentrations of those species in irradiated samples.
Samples of HX, X and UA of the irradiated solutions were also collected from the HPLC analysis peaks and submitted to electrospray ionization mass spectrometry (ESI-MS). These were performed on a Micromass Quattro Micro API triple quadrupole mass spectrometer with an ESI source (Micromass, Inc., Manchester, UK). Samples were injected into the MS by direct infusion through capillary tubing at a flow rate of 4 μL min−1. The full scan ESI conditions for the monitored compounds were as follows: negative ionization mode, capillary voltage (3.07 kV), cone voltage (20.00 V), extractor voltage (2 V), source temperature (90°C), desolvation temperature (300°C) and desolvation gas flow of 400 L h−1.
Photosensitized semiquinone formation. Samples with the same composition as that described above were photolyzed, at pH 7.4, for increasing periods of time followed by transference of these to deaerated EPR quartz flat cells. EPR spectra were then recorded on a Bruker ER-200D spectrometer at 100 kHz magnetic field modulation. Semiquinone (Q•−) concentrations were obtained by comparing the semiquinone overmodulated EPR double-integrated spectral area with that corresponding to a 3-carbamoyl-2,2,5,5-tetramethyl-3-pyrrolin-1-oxide spin standard.
Quinone covalent binding to DNA. The procedure used in this determination was adopted from that described by Lusthof et al. (24) and used previously by us (16,25). Solutions of DNA, dyes and quinone were prepared, some were photolyzed and the samples were then left standing for 20 h at room temperature under an N2 atmosphere and shielded from light by aluminum foil. The nonphotolyzed samples served as matched controls for the photolysis. Then, quinone-bound and -free DNA were separated from unbound quinone on a Sephadex spun exclusion column (26). The DNA fractions were analyzed by absorption spectroscopy. The presence of a quinone absorption band at wavelengths above 300 nm was taken as evidence of DNA-bound quinone. For example, the DNA-2,5-bis(1-aziridinyl)-1,4-benzoquinone adduct has an absorption maximum wavelength at 343 nm (24). For each photolyzed sample, Sephadex spun exclusion chromatography was continued until no, or a small constant value of, quinone absorption was detected in the matched but nonphotolyzed control solution. Samples and controls were then extracted with an equal volume of chloroform and then with an equal volume of diethyl ether, followed by DNA precipitation and redissolution in water, as described elsewhere (27). Samples and controls treated in this manner were then submitted to absorption spectroscopic analysis. Sample pH values were measured before and after photolysis and before Sephadex extraction and were found to be constant. The molar ratio of covalently bound quinone to DNA bases was estimated from the quinone maximum absorbance in the 340–360 nm region, assuming the same molar absorption coefficient as that of the parent quinone, and DNA absorbance at 260 nm.
Results and Discussion
HX and X photo-oxidation
When a nitrogen-saturated sample containing 2 mm HX, 875 μm AZDClQ and AlPcS4 (A = 1 at 675 nm) in 20 mm phosphate buffer at pH 7.4 was photolyzed, the HX concentration decreased while the X and UA concentrations increased with irradiation time (Fig. 1). In addition to the retention time, ESI-MS analyses of the collected HPLC peaks show molecular masses, using negative ionization mode, of [M-H]135.2, 151.4 and 167.3 corresponding to HX, X and UA, respectively.
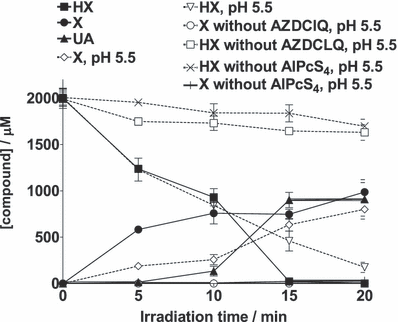
Dependence of HX, X and AU concentrations on photolysis time and pH. The N2-saturated sample contained 875 μm AZDClQ, 2.0 mm HX, AlPcS4 (A = 1 at 675 nm) in 20 mm phosphate buffer at pH 7.4 or pH 5.5 (only pH 5.5 is indicated). Data are presented as the mean ± SEM of three samples for each condition.
HX and X have been reported to be oxidized anaerobically to yield X and UA, in the case of HX oxidation, and UA in the case of X oxidation. For example, the anaerobic nonphotosensitized oxidation of HX or X by XO, which is followed by UA detection, has been reported previously (28,29). The photosensitized oxidation of X to UA by peroxydisulphate has also been reported previously (30). Addition of a water molecule to the X cation radical to form UA was proposed in that work and used in the present work to propose a potential mechanism for X and UA production (see below). However, the photosensitized oxidation of X by Rose Bengal under aerobic conditions produces other products instead of UA (30). The photosensitized aerobic oxidations of HX, X and UA have also been reported by others, although the products of those reactions were not characterized (31,32).
As tumor environments are characterized by acidic pH values (23), we also determined the yields of HX oxidation and X formation at pH 5.5 (Fig. 1). The chromatographic peak corresponding to UA was not quantified in this case, due to severe overlap of this peak with others of unidentified products of side reactions. As shown in Fig. 1, the yield of X, as well as the extent of oxidation of HX, do not change, within experimental error, upon going from pH 7.4 to 5.5.
Furthermore, previous reports have shown that dyes and visible light are able to photooxidize DNA under anoxia, especially through guanine oxidation (33,34). However, addition of DNA to samples undergoing photolysis under anaerobic conditions at pH 5.5 containing AlPcS4 in the presence of AZDClQ did not change the extent of HX oxidation within experimental error (Fig. 2). The latter result could imply that the rate of photo-oxidation of HX by AlPcS4 is much greater than that of DNA base photo-oxidation under the conditions of these samples. Thus, HX could be protecting DNA from base photo-oxidation. Further work on this subject is in progress.
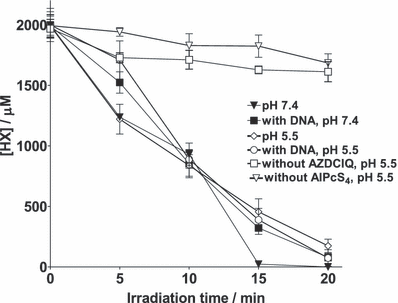
HX consumption after photolysis in samples containing 2.0 mm HX in 20 mm phosphate buffer in the presence or absence of 875 μm AZDClQ, in the presence or absence of AlPcS4 (A = 1 at 675 nm) and in the presence or absence of 1 mg mL−1 calf-thymus DNA at pH 7.4 or 5.5.
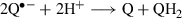 (1)
(1)Semiquinone formation
Photolysis of a nitrogen-saturated sample containing 2 mm HX, 875 μm AZDClQ and AlPcS4 (A = 1 at 675 nm) in 20 mm phosphate buffer at pH 7.4 produces the corresponding semiquinone, AZDClQ•−, as detected by EPR spectrometry (Fig. 3a). The semiquinone concentration shown in this figure is the equilibrium concentration because its intensity did not change appreciably after three or four scans of the spectrum. Due to the large disproportionation constant of this semiquinone, i.e. 1.5 × 1017 (Eq. 1) (25), the corresponding hydroquinone should have also formed through the semiquinone disproportionation. As indicated above, the hydroquinone species is also postulated as DNA alkylator (12). As the initial quinone concentrations are the same in nonirradiated and irradiated samples, the fact that more semiquinone is detected in irradiated samples is evidence that more reduced quinone species are being produced in those samples. At the more acidic pH of 5.5, a much smaller concentration of semiquinone is generated due to increased semiquinone disproportionation at acidic pH values (Eq. 1). Therefore, only data at pH 7.4 are reported here. Much smaller semiquinone concentrations are obtained if either AlPcS4 or HX is not included in the sample undergoing photolysis (Fig. 3b). This basal semiquinone production could be ascribed to autosensitization of the quinone (or direct dark reduction by HX of the quinone), in the absence of AlPcS4, activated by background room light, or from dye-photosensitized auto-oxidation in the absence of HX. Thus, as expected from the enhanced HX photo-oxidation in the presence of quinone, more semiquinone is produced from electrons provided by HX photo-oxidation than by other routes.
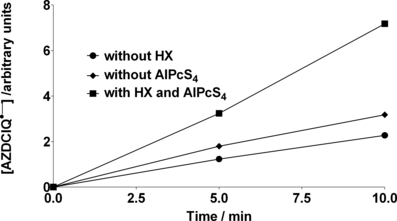
Relative AZDClQ•− concentration produced as a function of irradiation time of anaerobic samples containing 875 μm AZDClQ, 2.0 mm HX, AlPcS4 (A = 1 at 675 nm) and 20 mm phosphate buffer at pH 7.4 and without HX or AlPcS4.
Quinone covalent binding to DNA
Absorption spectroscopic analysis of N2-saturated samples, prepared and photolyzed as indicated above, show the presence of covalently bound quinone to DNA, at pH 5.5, if AZDClQ and AlPcS4 are both present in the photolyzed sample, and more if HX is also present (Fig. 4). In a previous work, it was found that no adduct was detected at pH 7.4 after photolysis of samples containing AlPcS4, AZDClQ and DNA (15). Therefore, pH 7.4 was not used in the present work to detect covalent adducts. As indicated above, this type of quinone is activated by both protonation and reduction to become a DNA-alkylating agent. The bound AZDClQ wavelength maximum (364 nm) is red-shifted relative to that of the corresponding unreduced parent quinone (339 nm). A similar red-shift in absorption wavelength maximum has been reported for 2,5-bis(1-aziridinyl)-1,4-benzoquinone upon reduction and alkylation of calf-thymus DNA (24). The latter was ascribed to the formation of products with open aziridine rings upon quinone reduction (24,35). If the absorbance maximum of the bound quinone above 300 nm is divided by the absorbance of DNA at 260 nm, the absorbance of quinone per unit DNA absorbance, AQ, is obtained. The number of bound quinone molecules per 1000 bases can be estimated if AQ is multiplied by the ratio of the extinction coefficient of DNA to that of the quinone (6.6 × 103/1.35 × 104) times 1000. The latter is a measure of the relative abundance of bound quinone among different sample conditions (Fig. 4b). The presence of HX almost always induced the formation of a small amount of covalent adducts in the absence of the dye, most probably due to HX oxidation photosensitized by the quinone after background room light absorption, or direct reduction of AZDClQ by HX under dark conditions. This observation of DNA covalent adducts, combined with the fact that bound quinone is observed at pH 5.5 but not at pH 7.4 (16), strongly suggest that the bound species is the aziridinyl quinone bound to DNA through its opened aziridinyl group. The increased alkylation of DNA by reduced aziridinylquinone at a lower pH than 7.4 indicates that protonation of the aziridine ring is an essential step for alkylation, which is in agreement with a high reactivity of protonated aziridines toward nucleophilic species (36). As binding of this type of quinone involves DNA cross-linking (10,15,23), this type of damage should be difficult for a cell to repair (37). The fact that more adducts are detected in samples irradiated for 5 min as compared with those irradiated for 30 min suggests that the decomposition of the quinone-DNA covalent adduct can also be photosensitized. Thus, the irradiation period needs to be optimized to maximize the net amount of quinone-DNA adduct.
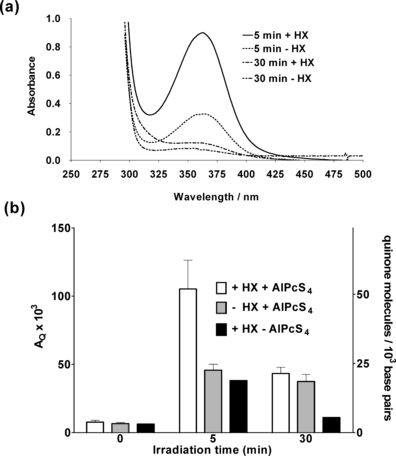
(a) Absorption spectra highlighting the quinone absorption band of N2-saturated samples with the same composition as that stated in Fig. 3a (but at pH 5.5) containing (+HX) or not containing (−HX) hypoxanthine, which have been photolyzed for the indicated time period followed by Sephadex spun filtration, solvent extraction and DNA precipitation and redissolution. (b) Estimated relative fractions of covalently bound quinone per unit amount of DNA (AQ) and number of bound quinone molecules per 1000 DNA base pairs after photolysis of samples with composition as described in Fig. 3a (pH 5.5) and in the presence (+) or absence (−) of HX or AlPcS4. These fractions were determined after Sephadex spun filtration, solvent extraction and DNA precipitation and redissolution of photolyzed samples. Results shown are averages of three determinations.
Possible mechanism
A possible set of reactions involved in the system under study here is shown in Scheme 1, where photo-oxidation of both HX and X are postulated. This scheme is, of course, presented as a hypothesis for further work as the proposed intermediates have not been characterized yet in this system. That will be a matter for a near future study. Two electrons are transferred in both HX and X oxidations (38). In contrast to monoxygenase systems, XO generates rather than consumes reducing equivalents during catalysis and utilizes water, as opposed to O2, as the source of oxygen for substrate hydroxylation (39). Swaraga et al. (30) proposed that X can be oxidized in two stepwise reactions, by two equivalents of SO4•−, to produce UA. According to that work, the nucleophilic attack of water at the C8 position in the X cation radical occurs followed by further SO4•− oxidation to produce UA. The radical cation of another purine, i.e. guanine, undergoes hydration depending on the environment (40). In analogy, we propose that water-derived substrate hydroxylation of HX and X cation radicals also occurs in the present work, as oxygen has been excluded from samples. Structures of the postulated hydroxylated intermediates [HX-OH]• and [X-OH]• are also shown in Scheme 1, where the [X-OH]• structure is taken from Ref. (30). Photoactivated water-derived hydroxylation has also been reported for other substrates. For example, quinone photohydroxylation, in many cases, is postulated to involve the reaction of the quinone triplet state, or cation radical with water to produce the hydroxylating intermediate (41–45). The photosensitized hydroxylation by water of 5,5-dimethylpyrroline-N-oxide has also been reported (42,46). Once the DNA adducts are formed by the reaction of DNA with the quinone-reduced species, oxidation of the resulting adducts when exposed to air during Sephadex spun exclusion produces the observed quinone-DNA adducts.
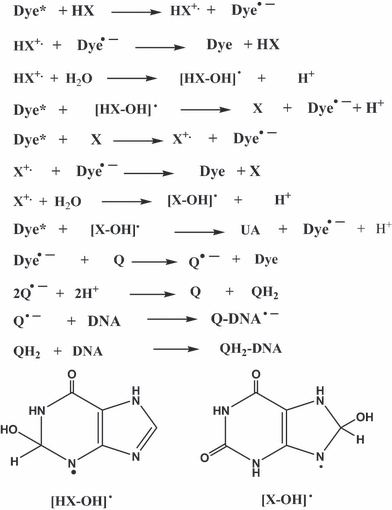
Possible mechanism in accordance to observations reported in this work.
In summary, the photosensitized oxidation, under anaerobic conditions, of HX and X to form X and UA is reported. Observations in this work suggest that a PDT modality in which a red–light-absorbing dye is photolyzed in the presence of HX could result in larger yields of activated bioreductive quinone in hypoxic regions of tumors, and thus an improvement in the therapy.
Acknowledgments
Acknowledgements— The authors express appreciation for grants P20 RR-016470 and S06-GM008216 from NIH for financial support of this work and Professor Nancy L. Oleinick from Case Western Reserve University and Professor Rafael Arce from University of Puerto Rico for advice and critical reading of the manuscript.




