Genotoxicity of Combined Exposure to Polycyclic Aromatic Hydrocarbons and UVA—A Mechanistic Study
Abstract
Solar UV radiation and benzo[a]pyrene (BaP) are two carcinogenic agents. When combined, their deleterious properties are synergistic. In order to get insights into the underlying processes, we carried out a mechanistic study within isolated DNA photosensitized to UVA radiation by either BaP, its diol epoxide metabolite (BPDE) or the tetraol arising from the hydrolysis of this last molecule. Measurement of the level of the oxidized base 8-oxo-7,8-dihydroguanine revealed that BaP is a poor sensitizer while BPDE and tetraol are more potent ones. None of these compounds was found to photosensitize formation of cyclobutane pyrimidine dimers through triplet energy transfer. On the basis of the distribution of oxidized DNA bases, we could show that photosensitization of DNA by BPDE involves electron abstraction (Type I) while tetraol acts mainly through singlet oxygen production (Type II). Under our experimental conditions, Type I was the major photosensitization process, which shows the lack of involvement of tetraol in the observed photo-oxidation reaction. Finally, we could show that the adducts, resulting from the alkylation of DNA by BPDE, are very potent sensitizers. Indeed, they are located in the close vicinity of the double helix and thus perfectly placed to induce oxidation reactions.
Introduction
Exposure to solar UV radiation is a major cause of skin cancer (1). The underlying genotoxic processes are roughly identified (2). In the UVB range (280–320 nm), photons are directly absorbed by DNA bases and induce dimerization of adjacent pyrimidines. The resulting dimeric photoproducts are the main mutagenic DNA lesions as shown by the predominance of mutations at bipyrimidine sites in human skin tumors. UVA radiation (320–400 nm) also exhibits mutagenic and tumorogenic properties, although to a lesser extent than UVB. The genotoxic effects of UVA photons are mostly mediated by photosensitization reactions. In these pathways, endogenous chromophores, still poorly characterized (3), absorb UV energy and induce DNA damage through oxidative processes. Pyrimidine dimers are also produced upon UVA irradiation (4), likely through a triplet energy transfer process that remains though to be definitively established.
The genotoxic properties of solar radiation can be worsened by co-exposure with various chemicals. Such compounds, that are often active upon illumination with UVA or visible radiation, trigger deleterious pathways similar to those of endogenous chromophores, although with a higher efficiency. Interestingly, phototoxicity can be sometimes beneficial as illustrated by the cancer treatment strategies involving photodynamic therapy or by psoralen-UVA phototherapy. In both types of treatments, phototoxicity cannot be dissociated from genotoxicity because occurrence of secondary tumors is a major concern.
Polycyclic aromatic hydrocarbons (PAHs), which are produced upon incomplete combustion of organic matter, represent another class of phototoxic and photogenotoxic compounds (5). PAHs such as benzo[a]pyrene (BaP) exhibit genotoxic properties of their own (6,7), without light activation. These compounds are actively metabolized and converted into chemically active electrophiles that form adducts within DNA (8). BaP gives rise to BaP-diol epoxide (BPDE) that adds to guanine bases as well as, to a lesser extent, to adenine and cytosine. Several diastereoisomeric adducts are produced from racemic (±)-anti-BPDE that include the (±)-trans-anti and (±)-cis-anti derivatives. The mutagenic and carcinogenic properties of BaP have been documented by a number of studies and this chemical is classified as a recognized carcinogen (6,7). In addition, evidence is accumulating for an increase in the carcinogenic properties of BaP upon combined exposure of skin with sunlight (9) and in particular its UVA portion (10). At the cellular level, evidence is obtained for a strong mutagenicity of the combination of BaP with UVA (11,12).
From a mechanistic point of view, no definitive explanation of the DNA damaging pathways triggered by the combined exposure to BaP and UV light is available. Production of hydrogen peroxide has been reported (13). This finding would be in agreement with increased damage measured by the Comet assay (14–16). Indeed, this last technique mostly visualizes strand breaks induced by hydroxyl radicals, a byproduct of H2O2. The production of •OH is the styling of this OK or should the dot be enlarged and bolded as I've seen in other articles? Have highlighted all instances could also account for the observation of double-strand breaks (17–19). Using quenchers of reactive oxygen species, implication of singlet oxygen was also proposed (18,19). In another study, the photosensitization properties of BaP metabolites were also shown (20). When isolated plasmid was exposed to UVB in the presence of BPDE, addition of superoxide dismutase was found to decrease the extent of DNA damage, suggesting involvement of the superoxide anion (21). Interestingly, the same study showed that UVB and UVC radiations enhance the mutagenicity of BPDE adducts to DNA. Conversion of BPDE adducts into more mutagenic lesions was proposed together with an increase in BPDE adduct mutagenicity resulting from the presence of UVB- and UVC-induced damage (21). However, photosensitization was not considered.
In order to obtain more accurate data on BaP-mediated photosensitization of DNA to UVA, we used the distribution of oxidized bases as a probe for the major oxidative pathways. A number of studies have reported data on the formation of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo) upon BaP photosensitization of cells and isolated DNA (10,15,20). Unfortunately, this oxidized base is produced by a wide variety of oxidants and cannot provide useful mechanistic information alone (22). Therefore, we also measured the induction of thymine oxidation products within isolated DNA photosensitized by either BaP or its metabolite BPDE in order to compare their efficiency. In addition, we determined the photo-oxidative properties of BPDE bound to DNA and of its hydrolysis product tetraol. Using this strategy, we were able to show that the most damaging BaP derivative was BPDE bound to guanine bases that induced electron abstraction within DNA.
Materials and methods
Chemicals. BaP and (±)-anti-BPDE were purchased from the National Cancer Institute (Bethesda, MD). Acetophenone, methylene blue, riboflavin, nuclease P1 (penicillium citrium), phosphodiesterase II, phosphodiesterase I (Crotalus adamentus venom) and alkaline phosphatase were obtained from Sigma (St Quentin-Fallavier, France). High-grade calf thymus (Sigma) was used as received. Solvents used were of analytical grade. Deionized water was prepared with a Millipore Milli-Q water purification system (Molsheim, France). Oxidized nucleosides were synthesized according to previously reported procedures (23).
UVA irradiation of DNA. An aqueous solution of DNA (0.1 mg mL−1) was prepared in 0.1 m NaCl. Concentrated solutions of photosensitizers either in water (riboflavin, methylene blue) or in ethanol (acetophenone, BaP, BPDE, tetraol) were added to reach the expected final concentration. In some experiments, a concentrated solution of BPDE was prepared in a 1:1 mixture of water/methanol and left at room temperature for various periods of time before being added to the DNA samples. In all cases, samples were left in the dark for 1 h at room temperature prior to irradiation. Aliquot fractions of 1 mL were then placed in a spectrophotometer quartz cell (surface 1 × 1 cm, volume 3 mL). Samples were exposed from above under stirring to the UVA radiation emitted by a Waldman UVA 700L irradiator fitted with a high pressure lamp MSR 700 (700 W) (Waldman, Villingen-Schwenningen, Germany) with an emission spectrum providing mostly photons of wavelength higher than 330 nm. The irradiance was 40 mW cm−2. After irradiation, DNA was precipitated by the addition of cold ethanol (2.5 mL) in order to eliminate soluble organic molecules. The obtained DNA pellet was suspended in 100 μL of water and kept frozen until analysis.
Quantification of DNA damage. DNA was digested into nucleosides in two steps. First, incubation was carried out at pH 6 for 2 h at 37°C in the presence of nuclease P1, phosphodiesterase II and DNase II. The TRIS buffer was then added to reach pH 7.5 and incubation was resumed for 2 h after addition of phosphodiesterase I and alkaline phosphatase. The resulting solution was centrifuged in order to remove insoluble particles, and the liquid phase was transferred into HPLC injection vials. Three types of analyses were performed.
For the sole quantification of 8-oxodGuo, HPLC with electrochemical detection was used. The separation was performed on an Uptisphere ODB octadecylsilyl silica gel column (250 × 4.6 mm, 5 μm particle size) with an isocratic eluent (25 mm potassium phosphate, 8% methanol). The coulometric detection was provided by a Coulochem II detector equipped with a 5011 cell (ESA, Chelmsford, MA) with the potential of the two electrodes set at 200 and 450 mV, respectively. Normal nucleosides were quantified by a UV detector at the output of the HPLC column that was set at 254 nm.
Simultaneous determination of the frequency of 8-oxodGuo and thymine oxidation products was carried out by HPLC coupled to tandem mass spectrometry (23). HPLC separations were performed on a 150 × 2 mm octadecylsilyl silica gel (3 μm particle size) column (Uptisphere, Interchim, Montluçon, France) connected to an Agilent Series 1100 HPLC system. A gradient of acetonitrile in a 2 mm aqueous solution of ammonium formate was used. Ions of the DNA lesions of interest were produced in the electrospray ionization probe of an API 3000 triple quadrupole mass spectrometer (SCIEX/Perkin-Elmer, Thornhill, Canada). cis and trans diastereoisomers of thymidine glycols (ThdGly), 5-(hydroxymethyl)-2′-deoxyuridine (5-HMdUrd), 5-formyl-2′-deoxyuridine (5-FordUrd) and 8-oxodGuo were quantified either in the positive or negative ionization mode using MS/MS transitions reported elsewhere (23). The retention times of the oxidized nucleosides were the following (in min): trans ThdGly 3.4 and 3.9; cis ThdGly 6.3; 5-HMdUrd 13.8; 5-FordUrd 19.4; 8-oxodGuo 27.3. An external calibration with authentic standards was used. The amount of analyzed DNA was calculated from the area of the 2′-deoxyguanosine peak (19.8 min) displayed in the HPLC elution profile recorded on a UV spectrometer (λ = 285 nm) placed at the outlet of the column.
Adducts of BPDE to 2′-deoxyguanosine (BPDE-dGuo) were quantified using a recently developed method (24). The same HPLC-MS/MS system was used. The only modification was the particle size of the column that was 5 μm instead of 3. The elution gradient was linear during 30 min from 0% to 100% of acetonitrile in 2 mm ammonium formate, at a flow rate of 0.2 mL min−1. Detection of DNA adducts was achieved in the positive ionization mode using the multiple reaction monitoring mode. Monitored fragmentations were 570.4→257.2, 570.4→454.2 and 570.4→285.2. The peak corresponding to the BPDE-dGuo adducts appeared at a retention time of 17.1 min. An external calibration based on analysis of authentic standards was used. The amount of analyzed DNA was calculated from the area of the thymidine peak recorded on the UV detector placed before the entrance of the mass spectrometer.
The level of damage in DNA was expressed in number of lesions per million bases. In all cases, statistical significance of differences in either the frequency of lesions or yield of damage (calculated by linear regression with respect to the dose) was checked by using a student test.
Results
BaP is a poor photosensitizer
First, we evaluated the photosensitization potential of BaP in the UVA range. To this end, its ability to photosensitize DNA was compared with that of other known photosensitizers. Differences in absorption spectra of the photosensitizers might slightly modulate their respective response. However, the UVA source used exhibited a broad spectrum and thus was able to excite all studied compounds. Samples of isolated DNA were exposed to UVA in the presence of oxygen. Linear dose dependence was observed (Fig. 1), with the exception of riboflavin that is extremely photo-oxidizing and likely induces secondary photo-oxidation of 8-oxodGuo. In order to quantify the photosensitizing efficiency, the ability of a sensitizer to induce the formation of 8-oxodGuo was calculated as a function of the dose (Table 1). Photosensitization of isolated DNA by BaP was found to be very low. In the dose range applied, the difference with respect to samples exposed to UVA only was hardly detectable.

Dose-course formation of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo) in isolated DNA samples exposed to increasing fluence of UVA radiation in the presence of various photosensitizers (10 μm). Results represent the average ± standard deviation of three independent experiments.
| Photosensitizer | Control | BaP | Methylene blue | Acetophenone | Riboflavin |
|---|---|---|---|---|---|
| Yield | 0.56 ± 0.14 | 0.65 ± 0.07 | 4.19 ± 0.19* | 6.31 ± 1.05* | 134 ± 10.3* |
- 8-oxodGuo = 8-oxo-7,8-dihydro-2′-deoxyguanosine. The yields expressed in lesions per 106 bases per J cm−2 (and the standard error) were calculated by linear regression of the data shown on Fig. 1. For riboflavin, the point at the highest UVA fluence was omitted (differences with respect to control were statistically different at *P < 0.01).
BPDE is a better photosensitizer than BaP
Because BaP is efficiently metabolized in cells, we then studied the ability of its diol epoxide metabolite to induce the formation of 8-oxodGuo upon exposure to UVA radiation. For this purpose, DNA was incubated for 1 h with either BaP or BPDE and then exposed to increasing fluences of UVA radiation. As discussed above, only a limited amount of DNA oxidation was observed with BaP (Fig. 2) or in the control samples. In contrast, an almost five-fold larger amount of damage was observed for BPDE.
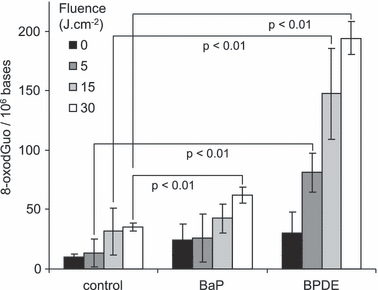
Dose-course formation of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo) within isolated DNA exposed to UVA radiation in the absence or presence of 10 μm benzo[a]pyrene (BaP) or its diol epoxide metabolite (BPDE). Results represent the average ± standard deviation of three independent experiments.
Tetraol and BPDE exhibit different photosensitization properties
BPDE is often described as being unstable in water. Therefore, we also investigated the photosensitizing properties of the resulting tetraol hydrolysis product. In order to get insights into the contribution of each compound, we first quantified the formation of a series of oxidized DNA bases produced upon photosensitization by either BDPE or pure tetraol. A first observation was that tetraol was more efficient than BPDE at inducing 8-oxodGuo within isolated DNA (Table 2). In addition, the distribution of oxidative lesions was found to differ for the two compounds. Photosensitization with tetraol leads to the almost exclusive formation of 8-oxodGuo. When BPDE was used, 8-oxodGuo was also predominant. However, other lesions arising from the oxidation of thymine were also produced, representing 30% of the amount of 8-oxodGuo. In order to check for a possible role of hydroxyl radicals, BPDE-mediated photosensitization was repeated in the presence of increasing amounts of the •OH radical scavenger Tris. No decrease in the amount of thymine damage was observed (Fig. 3).
| 8-oxodGuo | 5-HMdUrd | 5-FordUrd | ThdGly | |
|---|---|---|---|---|
| Control | 59 ± 10 (66.4 ± 4.3) | 3.2 ± 0.8 (3.7 ± 1.2) | 9.0 ± 1.9 (10.2 ± 2.6) | 17.4 ± 0.9* (19.7 ± 1.3) |
| BPDE | 170 ± 28* (63.8 ± 0.6) | 26.3 ± 4.9** (9.9 ± 0.2) | 25.4 ± 5.9** (9.5 ± 0.9) | 44.9 ± 8.2* (16.9 ± 0.7) |
| tetraol | 976 ± 168* (92.8 ± 0.8) | 13.2 ± 1.4* (1.3 ± 0.2) | 19.7 ± 4.3** (1.9 ± 0.3) | 41.6 ± 1.3* (4.0 ± 0.6) |
- 8-oxodGuo = 8-oxo-7,8-dihydro-2′-deoxyguanosine; BPDE = the diol epoxide metabolite of BaP; 5-HMdUrd = 5-(hydroxymethyl)-2′-deoxyuridine; 5-FordUrd = 5-formyl-2′-deoxyuridine; ThdGly = thymidine glycol. Results are expressed in lesions per 106 normal bases. Values in brackets are the proportion of a given oxidized base among the quantified lesions. Results represent the average ± standard deviation of three independent experiments. Differences with respect to control were statistically significant at *P < 0.01 and **P < 0.02.
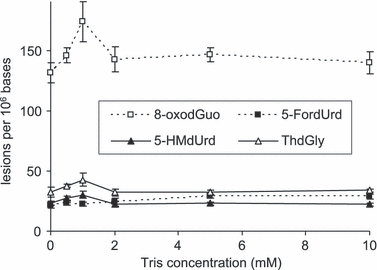
Frequency of oxidized bases in isolated DNA exposed to 30 J m−2 UVA in the presence of 10 μm BPDE and increasing concentration of Tris. Results represent the average ± standard deviation of three independent experiments.
Incubation in water poorly impacts BPDE-mediated photosensitization
In order to get further insights into the contribution of tetraol to the photosensitization of DNA by BPDE, we monitored the effect of water on the ability of BPDE to induce 8-oxodGuo and other oxidized bases upon UVA irradiation. To this end, isolated DNA was incubated with 10 μm BPDE for 1 h. Another set of samples was incubated with 10 μm BPDE that was preincubated for 1 h in a 1:1 mixture of water and methanol. All samples were then exposed to increasing doses of UVA (0–30 J cm−2). The yield of formation of oxidized bases was similar with BDPE and “hydrolyzed” BPDE (Fig. 4).
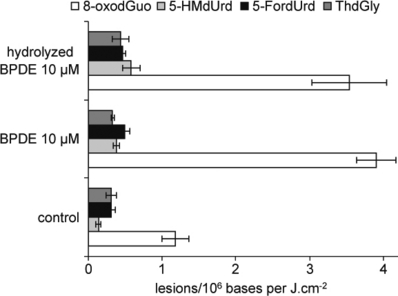
Influence of the diol epoxide metabolite of BaP (BPDE) “hydrolysis” on the yield of oxidized DNA bases. Samples were exposed to UVA either in absence of sensitizer or with either 10 μm BPDE or 10 μm BPDE preliminarily hydrolyzed for 1 h in a water/methanol mixture. The results represent the slope of the dose-course formation (±standard error) between 0 and 30 J cm−2 UVA. Statistically significant differences (P < 0.01) for all lesions except ThdGly were observed between control-irradiated samples on the one hand and samples exposed to UVA in the presence of both BPDE and “hydrolyzed” BPDE on the other hand. No significant differences were found between the yields of DNA damage induced by UVA in the presence of BPDE and “hydrolyzed” BPDE.
Formation of DNA adducts in the BPDE photosensitization experiments
In a subsequent experiment, we quantified the level of BDPE adducts to DNA after increasing period of hydrolysis time of this last compound in water. Emphasis was placed on BPDE-N2-dGuo that is expected to be the major adduct. A first goal was to compare the level of alkylation product with that of oxidized bases in DNA. In addition, the time-course study would reflect the rate of hydrolysis of BPDE as revealed by its ability to add to guanine within DNA. A slow decrease in the level of DNA adducts was observed when the BPDE hydrolysis time increased (Fig. 5). The BPDE half-life time determined in this way was 5.2 h. We also checked the stability of adducts upon exposure to UVA radiation. A decrease of 2 % per J cm−2 was observed within DNA incubated for 1 h with 10 μm BPDE.

Effect of the diol epoxide metabolite of BaP (BPDE) hydrolysis on the yield of adducts to DNA. A solution of isolated DNA was incubated with BPDE preliminarily left in a water/methanol mixture for increasing periods of time. The results represent the average ± standard deviation of three independent experiments.
DNA adducts to BPDE are major photosensitizing species
In order to estimate the contribution of free BPDE to the DNA photosensitization process, we extracted the BPDE-treated DNA solution by ethyl acetate prior to UVA exposure in order to remove any unbound soluble aromatic molecules. In spite of this treatment, very efficient photosensitization of DNA was observed, showing that BPDE bound to DNA was actually the most damaging species in the system. Based on the yields of 8-oxodGuo (Fig. 6), we could estimate that approximately 50% of the oxidized bases were due to these adducts present in DNA, although they represent only 2% of the total amount of initially applied BPDE. The amount of thymidine oxidation products (ThdGly, HMdUrd and FordUrd) was also quantified. The combined yield of these lesions was 90 ± 7 and 47 ± 4 lesions per 106 bases in samples photosensitized by BPDE and “extracted” BPDE, respectively.
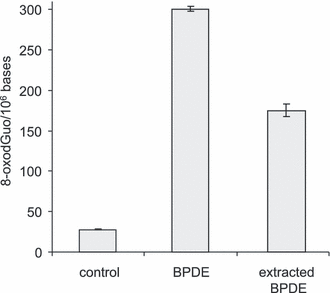
Effect of ethyl acetate extraction on the photosensitization of DNA. A sample of isolated DNA was incubated for 1 h with 10 μm BPDE. Half of the solution was exposed to 30 J cm−2 UVA. The other half was extracted by ethyl acetate that was removed under vacuum before irradiation of DNA. The results represent the average ± standard deviation of three independent experiments. Statistically significant differences (P < 0.01) were observed between the three values.
Discussion
Combined exposure to sunlight and PAH has been shown to potentiate the carcinogenic effects of both agents (5). Several mechanistic studies have been devoted to unraveling the underlying mechanisms, with emphasis placed on BaP, one of the most common carcinogenic PAHs. Work was carried out either in cultured cells or in isolated DNA to determine the molecular processes leading to DNA damage upon co-exposure to BaP and UV, and in particular UVA that was found to be the most active part of solar light (10). Induction of oxidative stress is likely to be the major consequence of combined exposure to PAH and UVA, as shown by redox-sensitive fluorescent probes (16). Therefore, several studies tried to determine the favored photo-oxidative pathway but yielded contradictory results, singlet oxygen being the most frequently proposed reactive oxygen species involved (18–21). These apparently contradictory results were mostly based on the use of scavengers. A drawback of this approach is that scavengers are not always specific. For instance, sodium azide often used to deactivate singlet oxygen might also deactivate triplet excited state of sensitizers. Therefore, we reinvestigated this topic in the present work by using the distribution of DNA lesions as a probe for the different photosensitization mechanisms.
Photosensitized oxidation of DNA mostly takes place through two pathways from the triplet excited state of the sensitizer (25). A first one involves the generation of singlet oxygen after energy transfer to molecular oxygen (Type II photosensitization). In DNA, singlet oxygen specifically reacts with guanine to yield 8-oxodGuo as the sole DNA lesion (26,27). Formation of strand breaks have been reported in plasmids but this more likely results from secondary oxidation of 8-oxodGuo leading to the formation of brittle lesions. Evidence for this mechanism is provided by a kinetic study showing that two molecules of singlet oxygen are required to induce one break in DNA (28). Another possible photo-oxidative pathway is referred to as Type I and involves electron abstraction from DNA by the excited sensitizer. Because guanine bases represent sites with the lowest oxidation potential and because charge can migrate through DNA, Type I photosensitization leads to predominant formation of guanine damage (29–31). This distribution of oxidative lesion has been found in numerous studies using hot piperidine cleavage as a DNA lesion revealing agent. A similar trend was observed using chromatographic methods, although some adenine, thymine and cytosine damage could be observed in lower yield than 8-oxodGuo (32,33). A last possible oxidative pathway involves hydroxyl radicals arising from superoxide anions produced upon Type I photosensitization. Hydroxyl radicals are highly reactive and unspecific. They are able to cleave the DNA backbone (34) and to efficiently oxidize any of the four normal bases (35). A nonoxidative photosensitization process leading to the formation of pyrimidine cyclobutane dimers has been reported for some compounds (36). This will not be discussed further because such dimeric DNA photoproducts could not be found in any of our experiments using HPLC-MS/MS analysis (4) (data not shown).
With such mechanistic information in mind, we undertook the study of the photosensitization of isolated DNA by BaP and some of its derivatives likely to be found in cells. First, we compared the ability of BaP to photo-oxidize DNA with that of other well-known sensitizers (32). We focused on the formation of 8-oxodGuo that is expected to be a major lesion through both Type I and Type II photosensitization pathways. The result of this comparison showed that BaP is a very poor sensitizer, ca 10-fold less efficient than methylene blue and acetophenone, and more than 200 times less potent than riboflavin. Evidence has been obtained, using S9 mix treated-BaP, that BaP metabolites also exhibit photosensitizing properties (20). Accordingly, we found that BPDE, one of the major and of the most reactive metabolites of BaP, more efficiently induced the formation of 8-oxodGuo within DNA upon exposure to UVA. A factor of 5 in the yield of 8-oxodGuo was found when DNA was photosensitized by BPDE with respect to BaP.
However, BPDE is not stable in an aqueous solution of DNA. Indeed, BPDE may be converted into its tetraol derivative upon hydrolysis of the epoxide function. Using authentic compound, we observed that tetraol-mediated UVA photosensitization of DNA leads to the overwhelming formation of 8-oxodGuo (more than 90% of the quantified oxidized nucleosides) with much lower amounts of thymine damage, strongly suggesting the occurrence of a Type II photosensitization process (26). In contrast, photosensitization of DNA by BPDE yielded thymine oxidation products in addition to 8-oxodGuo. This could be due to a one-electron oxidation process that, as shown upon biphotonic ionization (33), not only damages guanine but also other bases to a lesser extent. However, damage to thymine could also be explained by a contribution of •OH radicals. In order to rule out the formation of •OH radical, we repeated the BPDE photosensitization experiments in the presence of a scavenger for this last reactive oxygen species. Tris was used because it was found to protect DNA against hydroxyl radicals produced upon radiolysis of water by gamma rays and heavy ions, without affecting the relative yield of the bases (37). This last observation indicates that Tris, unlike commonly used DMSO (38,39), does not generate interfering secondary species. BPDE-mediated UVA photosensitization of DNA led to the same distribution of oxidized bases with or without Tris. The presence of oxidized thymines in addition to predominant formation of 8-oxodGuo and in the absence of •OH strongly suggests that Type I, rather than Type II, photosensitization is involved. In addition, this experiment showed that tetraol, a typical Type II sensitizer, is not involved in BPDE photosensitization. Accordingly, hydrolysis of BPDE for 1 h before mixing with DNA hardly affects the photosensitization process. In addition, the degradation of BPDE in water monitored by its loss of ability at adding to DNA was found to be slow (t1/2 > 5 h).
As mentioned above, BPDE, one of the major metabolites of BaP in vivo, is able to add to DNA bases. Although adducts were described for adenine and cytosine, guanine is the major modification site within BPDE-treated DNA (15). On this nucleobase, addition can take place either at the N7 position to yield unstable depurinating adduct or at the exocyclic N2 amino group. The four (±)-trans-anti and (±)-cis-anti adducts arising from this last reaction are stable and are proposed to be the main BPDE-induced DNA lesions. Accordingly, large amounts of BPDE-dGuo adducts were produced under our experimental conditions. As the polycyclic aromatic moiety of these adducts are UVA chromophores located in the close vicinity of the DNA double helix, it was tempting to suggest that they could trigger photosensitization reaction. This was confirmed by the observation that extraction of unreacted BPDE, and possibly tetraol and depurinated unstable adducts, prior to UVA irradiation only decreases DNA photo-oxidation by less than 50%. This experiment first rules out a major contribution of tetraol to the photosensitization process because extraction does not modify the ratio between the yields of the thymine and guanine lesions. As shown above, tetraol photosensitization leads mostly to the production of 8-oxodGuo. Extraction of tetraol from the medium should thus lower the proportion of 8-oxodGuo among oxidized nucleosides, which it did not as the combined yield of thymidine oxidation products decreased in approximately the same proportion as 8-oxodGuo. After extraction, the only remaining BPDE derivatives left to photosensitize DNA are the covalent adducts to the bases that can thus be estimated to provide more the half of oxidative damage in the BPDE experiments. As 10 μm BPDE was used in these experiments and the amount of BPDE-dGuo corresponds to approximately 0.2 μm, it may be concluded that adducts are almost 2 orders of magnitude more efficient than their BaP parent molecule at damaging DNA through photosensitization to UVA radiation.
In that respect, the location of the pyrene ring within DNA is an interesting issue. NMR studies have shown that for the major isomers (+)- and (−)-trans-anti BPDE-dGuo adducts, the polycyclic aromatic moiety is bound to the minor groove but not stacked with the bases (40,41). In contrast, the pyrene ring of the minor (+)-cis-anti adduct is intercalated (42). It would be interesting to determine the influence of the stereochemistry of the adducts on their photosensitization properties because an intercalated photosensitizer is more likely to abstract electrons from the surrounding bases. This was not possible in the present work because a mixture of the four isomers was produced. It should also be mentioned that a supercoiled DNA state favors the formation of intercalating cis-anti adducts and the stacking of trans-anti adducts with bases (43). It would thus be of interest to determine whether DNA in chromatin, which is more compact than isolated DNA, is more prone to photosensitization mediated by BPDE adducts.
Interestingly, BPDE-dGuo adducts were also found to be slowly degraded upon UVA irradiation, in a yield similar to that of formation of oxidized bases (350 adducts lost vs 250–300 oxidized bases produced per 106 normal nucleotides after 30 J cm−2 in 10 μm BPDE-treated DNA). This concordance may show that adducts behave more like a reagent than a catalytic photosensitizer. More extensive study of the photochemistry of BPDE adducts within DNA would be interesting. Results have been recently published but they involve biphotonic excitation that leads to the formation of the radical cation rather than the excited BPDE moiety (44).
In summary (Fig. 7), our present results show that BaP is a poor sensitizer. In cells, it is metabolized into its diol epoxide BPDE that is a more efficient photosensitizer, acting mostly through Type I photosensitization. BPDE may also be hydrolyzed into tetraol that was found to be a very efficient Type II photosensitizer. Although the formation of tetraol is slow in aqueous solution, it could contribute to cellular damage if the rate of elimination of BPDE by Phase II enzymes is low enough to permit accumulation of tetraol. A second possibility for BPDE is to react with DNA, although this is not a favored process in cells because of the compaction of DNA in the nucleus and the competition with other cellular components. However, formation of BPDE adducts may be a major process in the photo-genotoxicity of BaP because adducts are very efficient at degrading surrounding DNA bases. Such a process combined with the long half-life of adducts in skin (45,46) should be investigated in a whole cutaneous context.
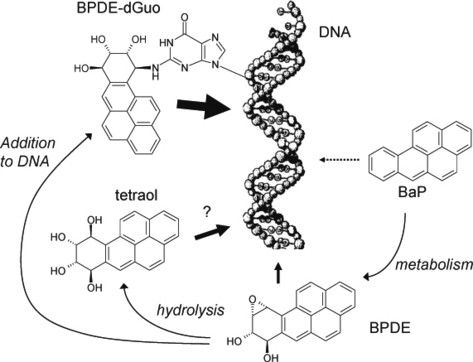
Proposed photosensitization pathways for benzo[a]pyrene (BaP) and its derivatives (the diol epoxide metabolite of BaP [BPDE]). BaP itself is a poor photosensitizer. However, it is metabolized into the more photoactive BPDE that might damage DNA through Type I photosensitization. Hydrolysis of BPDE slowly releases tetraol. Although the photogenotoxic properties of this compound could not explain the results of the present experiments, tetraol is an efficient Type II photosensitizer and might play a role in a cellular context. Finally, BPDE bound to DNA is very efficient at inducing photosensitized one-electron abstraction and oxidizing nucleobases.




