Ultraviolet B Irradiation Affects Resistance of Rainbow Trout (Oncorhynchus mykiss) Against Bacterium Yersinia ruckeri and Trematode Diplostomum spathaceum
Abstract
Ultraviolet B (UVB) radiation is known to have various effects on the immune system of fish, but the effect on the actual disease resistance has remained largely unknown. Here we studied the effect of UVB on the resistance of rainbow trout (Oncorhynchus mykiss) against a bacterium Yersinia ruckeri, the causative agent of enteric red mouth disease, and a trematode parasite Diplostomum spathaceum, which causes cataracts in fish. The fish were exposed to UVB irradiation seven times in 14 days, and inoculated intraperitoneally with Y. ruckeri on day 5 after the first irradiation. On day 2 postinfection (p.i.), the number of viable bacteria in the kidney was lower in UVB-exposed than in unexposed fish. However, on day 8 p.i., UVB-irradiated fish had not been able to clear remaining Y. ruckeri effectively, and had a slightly higher bacterial load than controls. A similar, although not significant, effect was seen in the bacterial numbers in spleen. In the other experiment, fish were exposed to UVB for six consecutive days and then exposed to D. spathaceum. A significantly higher number of parasites was detected in the eyes of irradiated fish, indicating reduced resistance against the pathogen. Furthermore, UVB-irradiation altered the immunological and hematological parameters of fish, which also verified the immunomodulatory potential of UVB in the present study.
Introduction
Solar ultraviolet B (UVB) radiation is an environmental factor affecting both terrestrial and aquatic animals. One of the harmful effects of excess UVB-exposure is the compromised functioning of the immune system, which may lead to increased susceptibility to diseases. UVB-induced immunomodulation has been described in cyprinid fish, roach (Rutilus rutilus) (1–4) and common carp (Cyprinus carpio) (5,6), and recently also in rainbow trout (Oncorhynchus mykiss) (6). Irradiation affects both specific and nonspecific branches of fish immune system, including modulations in the functioning of phagocytes, natural cytotoxic cells as well as lymphocytes (1–6). However, there is very little experimental information about the effects of UVB on the disease resistance of fish. UVB-exposed rainbow trout skin has been shown to be susceptible to fungal and myxobacterial infections (7–9), but the effects of radiation on infections occurring outside the actual site of exposure are so far unknown.
Harmful effects of excess UVB radiation on mammalian immune function and disease resistance have been established, and include lowered resistance against bacterial, viral and parasitic infections (10). Impaired resistance against bacteria Mycobacterium bovis bacillus Calmette-Querin (BCG) (11,12) and Mycobacterium lepraemurium (13) has been observed in rodents after a chronic low-dose UVB-exposure, as well as a single high-dose irradiation. Exposure also enhances Listeria monocytogenes infection in rats (14). UVB has similar effects on viral infections, and increased amount of herpes simplex virus-induced lesions in rats (15) as well as increased mortality of influenza virus-infected mice have been reported (16). Information about the UVB-effects on the resistance against parasites is, however, more contradictory. Irradiation is known to enhance parasitic worm Trichinella spiralis infection in rats (17,18), and increase the susceptibility of mice to malarial infection (19). Instead, a helminth parasite Schistosoma mansoni infection was not significantly affected by UVB-exposure, when doses suppressing the contact-hypersensitivity response in experimental animals were used (20). Further, pathogenesis in cutaneous leishmaniasis was suppressed in mice exposed to immunosuppressive UVB-doses (21).
The aim of the present work was to investigate the effect of UVB exposure on the ability of rainbow trout to manage bacterial and parasitic infections. The infections were carried out using two pathogens, bacterium Yersinia ruckeri (Finnish strain) and trematode Diplostomum spathaceum. These parasitic pathogens are common disease-causing agents both in wild and farmed fish (22–26), and essentially provided us with two different types of disease models for the purpose of this study: a directly transmitted microparasite and a multicellular metazoan parasite with a complex life cycle. The UVB-doses (43 mJ cm−2 erythemally weighted) of this study correspond to the magnitude of radiation reaching the Earth’s surface today; daily CIE-weighted UVB-doses measured in southern Finland during May in 1998–2000 varied around 200 mJ cm−2 (27). We expected to observe decreased disease resistance in UVB-exposed fish, appearing as a higher disease incidence in these fish. Further, selected immune parameters were simultaneously monitored to attest the immunomodulatory potential of the UVB-doses.
Materials and Methods
Fish and experimental design. Two separate experiments were performed in the present study, which investigated the effects of UVB on disease resistance against Y. ruckeri (Exp. I) and D. spathaceum (Exp. II). Juvenile rainbow trout (O. mykiss) were obtained from a commercial fish farm and the average weight of the fish was 42 ± 1.7 g (±SE) in Exp. I, and 11 ± 0.3 g in Exp. II. Exposure to UVB was conducted indoors in 60 L flow-through tanks filled with aerated groundwater (15 ± 1°C). The fish were fed daily with commercial dry food pellets, and were allowed to adapt to experimental conditions for at least a week before the treatment.
Both experiments included three treatment groups: Unexposed fish (no light), fish exposed to UV-depleted irradiation (UV-wavelengths filtered out from the radiation emitted by UVB-lamps) and UVB-exposed fish. The first two groups were considered as controls for the UVB treatment, but exposure with UV-depleted radiation also allowed us to assess the impact of stress on immune function possibly caused by changes in illumination when the lamps are turned on for irradiation. Each treatment consisted of two or three replicate aquaria. In Exp. I, eight fish in each aquarium were exposed to UVB-irradiation (150 mJ cm−2 unweighted) seven times in 14 days. All 48 fish were infected with bacterium Y. ruckeri on day 5 after the first irradiation. The level of bacterial infection in fish was determined on days 2 and 8 postinfection (p.i.), after the fish had received a total UVB dose of 600 and 1050 mJ cm−2, respectively. In Exp. II, 15 fish in each tank were irradiated daily with UVB (150 mJ cm−2) for 6 days receiving a total UVB dose of 900 mJ cm−2. On day 7, 1 day after the last irradiation, 120 fish were exposed to D. spathaceum to study their resistance against this pathogen, and 86 fish were sampled for immune parameters.
Irradiation. The fish were exposed to UVB for 15 min with two unfiltered TL 40 W/12 lamps (Philips Lightning, Rosendal, The Netherlands). To prevent overexposure of fish near the water surface, vertical movement of the fish was restricted to a lower part of the aquaria with a UV-penetrating plastic grid during the exposure. The underwater spectrum was measured with Hamamatsu PMA-11 model C5965 spectrograph (Hamamatsu Photonics, Hamamatsu City, Japan), as described earlier (6). The ultraviolet radiation penetrating the water was measured with a UVX Digital Radiometer (Ultraviolet Products, Inc., San Gabriel, CA) equipped with polyethylene plastic waterproofed sensor, UVX-31. The spectrograph and the radiometer were calibrated with an Optronic-750 spectroradiometer (Optronic Laboratories, Inc., Orlando, FL). In air, TL12 lamps emitted less than 2% ultraviolet C (UVC), 53% UVB and 45% ultraviolet A (UVA). No UVC-radiation was detected in underwater measurements in the tank, and the irradiation consisted of 46% UVB and 54% UVA. When erythemally weighted the underwater dose was composed of 99% UVB and 1% UVA. UVB-dose received by fish in a single irradiation was 150 mJ cm−2 given at a mean radiant intensity of 74 μW cm−2. When erythemally weighted (28), the dose corresponds to 43 mJ cm−2 UVB. In control exposures UV-wavelengths were filtered out with a screen made of glass and polyacrylic sheets, and unexposed fish were kept in the shaded aquaria. To minimize the effects of factors other than spectral treatment, both unexposed and exposed fish were subjected to the similar handling during the experiment.
Host resistance model with Yersinia. Yersinia ruckeri bacterium is the causative agent of salmonid enteric red mouth disease (26). In this study, we used a Y. ruckeri strain that has been isolated in Finland (25). This is a well-characterized strain, which is an endemic pathogen in Finland, and is able to infect hosts whose condition or resistance is lowered, e.g. because of stress or primary infection (23,25). The incidence of Y. ruckeri in organs and tissues of fish during different stages of infection is also well documented (23). Before the experiment, the bacterium was once passed through rainbow trout. Bacteria for the infection were then isolated from the liver, subcultured on tryptone soy agar, and identified as Y. ruckeri by determining the biochemical properties (API 20 E; bioMérieux sa, Lyon, France). For preparing the stock suspension, the isolate was cultured as a suspension for 18 h at room temperature (RT), washed twice and resuspended in phosphate-buffered saline. The stock suspension was stored in small aliquots at −24°C until use. The concentration of viable Y. ruckeri in a thawed stock suspension was 2.4 × 1010 colony-forming units mL−1 (CFU mL−1). The fish were infected by injecting the bacterial suspension (1 × 107 CFU) into the peritoneal cavity.
For determining Y. ruckeri infection the spleen was carefully removed from each fish, and the surface of the organ was disinfected by immersing in 70% ethanol for 1 min. The spleen was then rinsed thoroughly with sterile saline. The surface of the empty body cavity of fish was disinfected with 70% ethanol for 2 min, and rinsed with sterile saline. The posterior kidney tissue was then aseptically sampled from under the membrane lining the body cavity. Tissue samples were weighed and stored frozen until determining the Y. ruckeri infection. Thawed samples of spleen and head kidney were aseptically homogenized against nylon net (80 mesh) in 5 mL saline. The series of dilutions (1:1–1:300) of tissue homogenates were cultured (48 h, RT) in duplicates on the SW bacterial agar dishes (29), and the Y. ruckeri colonies on the agar were counted. Disinfection method was verified effective as only on the average of 1 CFU mg−1 viable Y. ruckeri was detected in spleen, and 3 CFU mg−1 in kidney sampled 5 min after the infection.
Host resistance model with D. spathaceum. Eye flukes of the genus Diplostomum are ubiquitous parasites of wild freshwater fish (22,24). The disease, parasitic cataracts, is caused by the development of larval parasites (metacercariae) in the lens (22). Infection of fish with D. spathaceum was performed according to methods described in Karvonen et al. (30) on day 1 after the last irradiation (Exp. II). Briefly, parasite cercariae were obtained from naturally infected snails (Lymnaea stagnalis) collected earlier from earth ponds of a commercial fish farm. Each snail was placed in a glass jar and allowed to produce cercaria for 3 h at RT. Cercarial suspensions from the snails were then combined, and the number of cercaria was estimated by counting the number of cercariae under a microscope in 1 mL samples. The infection dose of 500 cercariae, less than 4 h old, was then presented to each fish (n = 120) placed individually in aerated 1 L containers. After 30 min of exposure, the fish were transferred back to the experimental aquaria and maintained there until dissection of the eyes. The D. spathaceum cercariae migrate to the lens of fish in 24 h, and therefore the eye lenses from each fish were dissected and studied under a microscope for the number of established metacercariae after 2 days from exposure. Fish in the experiment had a low-level initial D. spathaceum infection (mean parasite load per fish 0.6 ± 0.08) already at the fish farm. The newly established metacercariae (originating from the experiment) were distinguished from fully developed ones (originating from the fish farm) according to their size and morphology (31,32). Diplostomum spathaceum infection induces immune responses in fish (22), and the infection acquired at the fish farm would probably result in a decrease in the infection level during the secondary challenge of the fish (31). In the present study the average of 28% of cercariae presented to fish in experimental infection were established in the lenses. The individual fish were assigned randomly to different treatments, and fish with old, fully developed metacercariae (the sign of an earlier infection at fish farm) were found equally in all treatment groups. The infection level between the fish with or without the old metacercariae was not statistically different in the treatment groups.
Blood sample. Blood samples were taken to determine the blood chemistry and hematology on day 1 after the last irradiation (Exp. II). Fish were anesthetized with 0.01% MS-222 (Sigma Chemical Co., St Louis, MO) and blood sample was drawn to heparinized capillary tubes from the caudal vein. Whole blood was used for the hematological assays, and the capillaries were centrifuged (10 400 g, 5 min) for the separation of plasma. Plasma samples were stored frozen (−70°C) until use. The sampling then continued with isolating the head kidney leukocytes for tests measuring the functioning of cells (Exp. II).
Isolation of head kidney leukocytes. The head kidney was removed and homogenized against a nylon net (80 mesh) in heparinized incubation medium: RPMI-1640 medium supplemented with 3% Ultroser G serum substitute (Gibco), 0.5 mg mL−1 sodium pyruvate, 0.05 mm 2-mercaptoethanol, 20 mm NaCl and 10 mm HEPES, pH 7.4. The head kidney leukocytes were isolated with a two-step Percoll-gradient (Pharmacia LKB Biotechnology AB, Uppsala, Sweden) and collected from the 1.040–80 g mL−1 interface. The cells were washed twice and resuspended in culture medium: incubation medium supplemented with 2 mm l-glutamine, 100 IU mL−1 penicillin, 100 mg mL−1 streptomycin and 2 mm NaHCO3. Cells were counted by trypan blue exclusion in a hemocytometer (viability >95%) and adjusted to the desired concentration.
Hematological parameters, plasma protein, cortisol and lysozyme. For differential blood leukocyte counts, thin blood smears were prepared on objective glass from fresh heparinized blood (6). The smears were stained (Diff-Quick, Baxter Diagnostic AG, Düdingen, Germany), and a total of at least 200 leukocytes were counted and classified as lymphocytes, thrombocytes, granulocytes, monocytes or unidentified cells based on morphology and staining properties under a light microscope. Hematocrits were measured in heparinized 75 mm hematocrit tubes. The plasma protein concentration was determined using Bio-Rad Protein Assay Kit with bovine serum albumin as a calibrator, and the cortisol concentration using a radioimmunoassay kit (GammaCoatTM Cortisol; INCSTAR Co., Stillwater, MN). Plasma lysozyme activity was determined with a turbidometric assay (33,34), as described earlier (6). Plasma (2.5 μL) in 100 μL of 0.15 m phosphate buffer, pH 6.2, and 100 μL Micrococcus lysodeicticus (Sigma Chemical Co.) suspension, 1 mg mL−1 in phosphate buffer, were added to microtiter plates. The decrease in the optical density of bacterial suspension was monitored with a microplate reader (Victor2TM, 1420 Multilabel counter; Wallac, Turku, Finland) at 450 nm for 30 min.
Plasma immunoglobulin. The plasma IgM concentration of rainbow trout was measured with a modification of ELISA (enzyme-linked immunosorbent assay) (6,35) using polyclonal antitrout Ig antibody (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD). The assay was standardized with a pooled rainbow trout serum collected from five fish, and the pooled serum was given the concentration of 1000 artificial units per milliliter (U mL−1).
Respiratory burst by phagocytes. Phorbol 12-myristate 13-acetate (PMA)-stimulated respiratory burst by head kidney leukocytes was determined with the luminol-enhanced chemiluminescence (CL) method (2,36) at 25°C. Cells (2.5 × 105) were suspended in culture medium, luminol (10−4 m) was added, and adding PMA (2 μg mL−1) started the reaction. The CL was measured with a microplate luminometer (Victor2TM, 1420 Multilabel counter; Wallac). The peak value of CL in counts per second (cps) and the peak time (min) were determined. One to two peaks were determined for each CL response curves, and the higher peak value was considered as the actual peak of the respiratory burst.
Activity of natural cytotoxic cells (NCC). The NCC activity of head kidney leukocytes against K562 target cells was determined with a 51chromium release assay, as described earlier (2,6). Briefly, K562 cells labeled with sodium 51chromate (Amersham International plc.) were added to 96-well microtiter plates (Nunc Co.), 104 cells/well. Thereafter, leukocytes (1 × 106) were added into wells in triplicates producing the effector:target cell ratio (100:1). After gentle centrifugation (50 g, 2 min) the plates were incubated overnight at 18°C under 5% CO2 atmosphere. The supernatant from each well was harvested after centrifugation and the radioactivity was measured. The percent cytotoxicity was calculated from the equation (values represent the mean 51Cr-release of triplicate wells): % Cytotoxicity = 100 × (experimental cpm − spontaneous cpm)/(maximal cpm − spontaneous cpm). Spontaneous-release values were obtained from targets incubated in the absence of effector cells, and for maximal release-values sodium dodecyl sulfate (final concentration 2.5%) was added to the wells to lyse targets.
Statistical analysis. The data were analyzed for statistically significant differences using the Mann–Whitney U-test. The level of statistical significance was set at P ≤ 0.05. The data were standardized with the daily controls for testing the effect of treatment on the immune parameters in the case of day-to-day intra-assay variation in measured response levels.
Results
Experimental infection of fish with Y. ruckeri and D. spathaceum
The number of viable Y. ruckeri bacteria in the kidney of UVB-exposed fish on day 2 p.i. was lower than that in the unexposed control group (PC vs UVB = 0.023) or the group exposed to UV-depleted radiation (PUV− vs UVB = 0.031). On day 8 p.i., higher numbers of bacteria were detected in UVB-irradiated fish than in unexposed fish or those exposed to UV-depleted radiation, but the difference between the treatment groups did not reach statistical significance. More than 94% of the Y. ruckeri, detected on day 2 p.i., were deleted by day 8 p.i. from the kidney in both control groups (PC < 0.001, PUV− < 0.001). However, in the UVB-treated fish, the reduction in viable bacteria was only 56%, and the bacterial loads on days 2 and 8 p.i. were not statistically different (Fig. 1). The treatments also had a similar effect on bacterial loads in the spleen, but there was no statistically significant difference between the groups either on day 2 or day 8 p.i. The reduction in the number of bacteria between sampling points was highest in untreated controls (P < 0.001) and lowest in UVB-treated fish (P = 0.017), as noted in the kidney (Fig. 1). Fish exposed to UVB for 2 weeks showed, on visual inspection, thinning of the mucus layer on the dorsal skin. Fish in the other treatment groups of this experiment had skin with normal appearance.
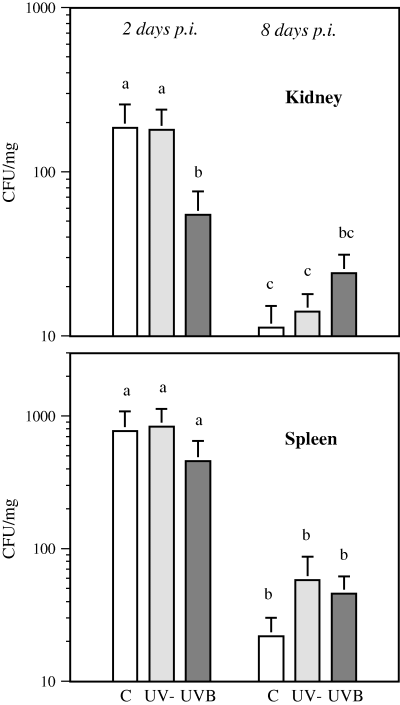
Yersinia ruckeri infection in the rainbow trout kidney and spleen on days 2 and 8 postinfection (p.i.). Results, expressed as colony forming units in mg tissue (CFU mg−1, mean + SE), are presented for unexposed control fish (C), fish exposed to UV-depleted irradiation (UV−) and to UVB radiation (UVB). n = 16 for each treatment. Identical letters over the bars indicate no statistically significant difference (P > 0.05) between the treatment groups.
In the D. spathaceum model, fish exposed to UVB irradiation had more severe infection than the untreated controls (PC vs UVB = 0.008) or the fish irradiated with UV-depleted radiation (PUV− vs UVB = 0.033) (Fig. 2). In this experiment, approximately one-third of the UVB-exposed fish had decreased amount of mucus on the dorsal skin. The infection level of the fish with the thinner mucus layer was not statistically different from that of the other fish in the same treatment group.
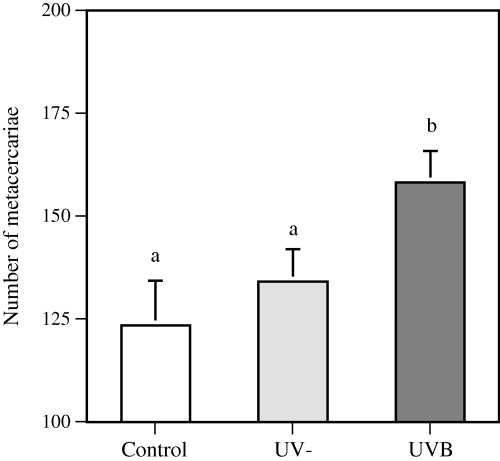
Number of Diplostomum spathaceum metacercariae established in the rainbow trout eye lenses. Results are expressed as the number of metacercariae (mean + SE) microscopically detected in the lenses of the fish eyes. n = 30, 45 and 45 for the controls, fish exposed to UV-depleted irradiation and fish exposed to UVB treatment, respectively. Identical letters over the bars indicate no statistically significant difference (P > 0.05) between the treatment groups.
Hematology and blood chemistry
In leukocyte differential counts a decreased percentage of lymphocytes (PC vs UVB < 0.001, PUV− vs UVB < 0.001), and an increased proportion of granulocytes (PC vs UVB < 0.001, PUV− vs UVB < 0.001), monocytes (PC vs UVB = 0.027, PUV− vs UVB = 0.003) and thrombocytes (PC vs UVB = 0.033, PUV− vs UVB < 0.001) was found in UVB-exposed fish (Table 1). The percentage of granulocytes increased also in fish exposed to UV-depleted radiation (PC vs UV− = 0.002), but in much less quantity than in the UVB-exposed group. Plasma cortisol concentration increased by 130% in fish exposed to UVB compared with untreated fish; however, the difference did not reach the level of statistical significance. UVB irradiation had no effect on plasma immunoglobulin concentration, or lysozyme activity. Blood hematocrit value decreased in the fish exposed to UVB (PC vs UVB = 0.001, PUV− vs UVB = 0.010), and plasma protein concentration decreased in both irradiated groups, fish exposed to UVB (PC vs UVB < 0.001) and UV-depleted radiation (PC vs UV− = 0.044).
| Control (n = 18) | UV− (n = 33) | UVB (n = 30) | |
|---|---|---|---|
| Plasma protein (mg mL−1) | 29 ± 1a | 27 ± 1b | 21 ± 1c |
| Plasma immunoglobulin (U mL−1) | 1884 ± 118a | 1792 ± 82a | 1680 ± 59a |
| Plasma lysozyme (ΔOD × 10−3) | 144 ± 12a | 135 ± 8a | 139 ± 7a |
| Plasma cortisol (ng mL−1) | 12 ± 2a | 15 ± 4a | 28 ± 8a |
| Hematocrit (%) | 52 ± 1a | 51 ± 1a | 47 ± 1b |
| Differential blood cell counts (%) | |||
| Lymphocytes | 56 ± 4a | 58 ± 3a | 32 ± 2b |
| Thrombocytes | 41 ± 4a | 36 ± 3a | 52 ± 4b |
| Granulocytes | 3 ± 1a | 6 ± 1b | 14 ± 2c |
| Monocytes | 0.1 ± 0.1a | 0.1 ± 0.1a | 0.5 ± 0.1b |
| Unidentified cells | 0.6 ± 0.2a | 0.7 ± 0.1a | 0.8 ± 0.2a |
| Head kidney NCC (%) | 19 ± 2a | 21 ± 1a | 20 ± 1a |
- Values represent the mean ± SE of each group. Identical letters indicate no statistically significant difference (P > 0.05) between the treatment groups.
Respiratory burst and NCC activity
Respiratory burst activity of the head kidney phagocytes was lower in UVB-exposed fish when compared with fish exposed to UV-depleted radiation (PUV− vs UVB = 0.042), but did not differ statistically from the untreated fish. The time of the peak response in CL was shorter in UVB-exposed fish than in controls (PUV− vs UVB = 0.001, PC vs UVB = 0.011) (Fig. 3). Majority of the fish expressed CL response with the maximum value in the first of the two peak responses, and the fish expressing the highest CL values in the second peak were distributed evenly among the treatment groups. UVB irradiation had no effect on the natural cytotoxic activity of head kidney leukocytes (Table 1).
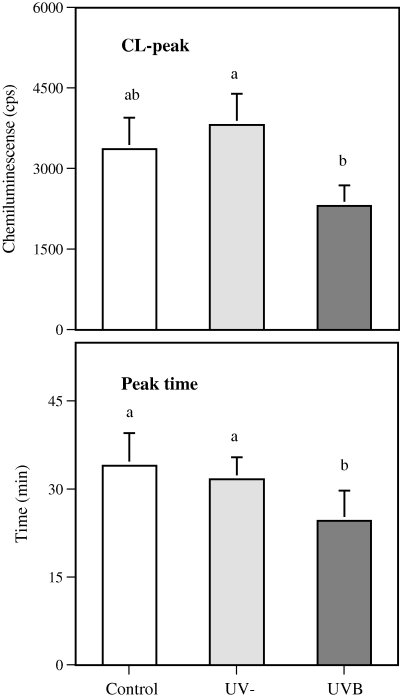
Effect of exposure to UVB irradiation and UV-depleted irradiation on respiratory burst activity of rainbow trout head kidney leukocytes. The bars represent PMA-stimulated peak chemiluminescence (cps, mean + SE) and peak time (min, mean + SE) of the reaction. n = 20, 35 and 31 for the control, UV-depleted radiation and UVB radiation, respectively. Identical letters over the bars indicate no statistically significant difference (P > 0.05) between the treatment groups.
Discussion
In the present study, the immunomodulatory doses of UVB radiation induced changes in disease resistance of rainbow trout. Protection against Y. ruckeri was suppressed in UVB-exposed fish at the end of the 2 week UVB-exposure and 8 days p.i., while a transient enhancement in clearance of bacteria was detected on day 2 p.i. UVB-exposed rainbow trout showed also suppressed resistance against the parasite D. spathaceum after a 6 day irradiation, concomitant with alterations in the immune function parameters. The results are among the first to demonstrate the negative effects of UVB on disease resistance of fish, and are consistent with earlier findings conducted using other immunosuppressive (37–39) or immunoenhancing treatments (40–43).
The functioning of phagocytes is one possible mechanism associated with resistance to Y. ruckeri and D. spathaceum. Fish are known to be protected from these pathogens by means of cooperating innate and adaptive immune functions (22,44–47). Specific anti-Yersinia antibodies have a strong opsonic effect resulting in increased phagocyte uptake of bacteria (45), and synergistic interaction between the activated macrophages and specific antibodies is proposed to be involved in protection against D. spathaceum (47). Furthermore, the ability of Y. ruckeri to elicit a reduced respiratory burst reaction in host cells has been suggested as one of the mechanisms used by the bacterium to resist phagocytic killing (46,48). The oxygen radicals produced in respiratory burst are larvicidal as well, and induce D. spathaceum mortality, but probably are not the major mode of diplostomule killing in rainbow trout resident macrophages (22). In the present study, suppressed head kidney respiratory burst activity and decreased CL peak times were detected in UVB-exposed fish. The decreased peak times were not only connected to lowered peak values, but may be, at least partly, induced by UVB-treatment. Considering the importance of phagocytes in resistance against Y. ruckeri and D. spathaceum, the ability of UVB to induce systemic suppression in functioning of these cells may be related to the impaired disease resistance observed in this work.
UVB-exposure of six consecutive days enhanced the immunological potential in the blood of uninfected rainbow trout by increasing the number of circulating granulocytes. Also in an earlier study, UVB-induced increase in the percentage of carp blood granulocytes was found after 1 week irradiation, but thereafter the cell numbers returned to normal level despite the ongoing exposure of fish (5). Phagocytic macrophages and neutrophils play a major role in clearing the pathogens from the intraperitoneal cavity after an intraperitoneal injection of bacteria into fish (44), and the lower number of Y. ruckeri in the tissues of UVB-exposed rainbow trout after 1 week irradiation (2 days p.i.) may be caused by alterations in the numbers of circulating phagocytes capable of infiltrating into the tissues. After 2 weeks, however, the UVB-exposed fish carried a higher number of bacteria in the kidney than the unexposed fish, suggesting reduced ability to eliminate pathogens from the tissue. The clearance of the bacteria from spleen and kidney was parallel, although an earlier study with mycobacteria-infected mice has shown that the effects of UVB may also be tissue specific (11).
The suppressed resistance against D. spathaceum was detected in UVB-exposed fish. The increase in the amount of circulating phagocytes clearly was not adequate to compensate this negative effect, and although NCC cells have also been proposed to participate in defense against parasitic diseases in fish (49), we did not find any connection between the cytotoxic activity of head kidney leukocytes and disease resistance. In addition to compromised immune functions, direct UVB-induced changes in the fish skin could also increase the penetration of D. spathaceum cercaria. Histological studies on the skin of brown trout (Salmo trutta) have revealed a loss of cell layers following UVB-irradiation, leading to the disappearance of mucous cells from the epidermis (50). Reduction in mucus secreting goblet cells has also been detected in cyprinid and salmonid fish after exposure to UVB-radiation (51). However, the UVB-induced decrease in the amount of mucus on the fish skin had no effect on the D. spathaceum infection in the present study. The detected changes in the mucus layer were probably too minor to notably affect the parasite invasion, particularly as the gills are the most actively employed route of invasion by D. spathaceum.
Parameters of innate humoral immunity—alternative pathway of complement, anti-microbial proteins, especially lysozyme, and acute phase proteins have been proposed as effectors of resistance against both bacteria (44) and D. spathaceum (22). In addition, the release of certain cytokines and inflammation reaction are acting in defense against bacteria (44), and neutral proteases are known to participate in the anti-diplostomule activity in rainbow trout (22). Suppressed plasma lysozyme activity in rainbow trout exposed to a single UVB dose of 1000 mJ cm−2 has been demonstrated (6), but in the present study, UVB-irradiation had no effect on this parameter. Plasma total protein, however, was decreased in UVB-exposed fish, and to a lesser extent, in fish exposed to UV-depleted radiation. This alteration, together with decreased blood hematocrit, is considered as an indication of lowered condition of the fish.
UVB-induced immunomodulation is suggested to be transmitted partly via the cortisol-mediated stress response, as increased plasma cortisol concentration and blood granulophilia are often detected in irradiated fish (3,5). In this work, exposure of fish to UV-depleted radiation was used to distinguish the effects of UVB-treatment from those possibly caused by visually perceived changes in illumination when the lamps are turned on for irradiation. However, UV-depleted radiation had no effect on disease resistance of fish and induced only minor alterations in other studied parameters. The moderate increase in circulating granulocytes was the only indication of cortisol-mediated stress response in this treatment group. Furthermore, all the changes noted in fish exposed to UV-depleted radiation were more pronounced in UVB-exposed fish. Thus, visible light may induce a slight stress reaction in fish, but UVB wavelengths clearly were the major agent affecting the irradiated fish in the present work. Similar results have also been obtained from earlier studies comparing the effects of visual light and UVB on the immune functions of fish (3,6).
Taken together, the results of the present study show that the harmful effects of UVB on fish immune system extend not only to immune parameters but also to the actual disease resistance, suggesting that exposure to excess UVB radiation presents a clear risk for fish health. This emphasizes the need for further research on the effects of long-term exposure of aquatic animals to UVB in their natural environments.
Acknowledgments
Acknowledgements— Dr. Lotta-Riina Suomalainen is acknowledged for the consultation in establishing and identifying the Y. ruckeri cultures. We also thank M.Sc. Johanna Rinne for the assistance in sampling and laboratory analysis. Funding from the Academy of Finland, Finnish Cultural Foundation and Foundation of Maj and Tor Nessling supported this work.




