Effects of Substituents on Synthetic Analogs of Chlorophylls. Part 1: Synthesis, Vibrational Properties and Excited-state Decay Characteristics
Abstract
Understanding the effects of substituents on the spectra of chlorins is essential for a wide variety of applications. Recent developments in synthetic methodology have made possible systematic studies of the properties of the chlorin macrocycle as a function of diverse types and patterns of substituents. In this paper, the spectral, vibrational and excited-state decay characteristics are examined for a set of synthetic chlorins. The chlorins bear substituents at the 5,10,15 (meso) positions or the 3,13 (β) positions (plus 10-mesityl in a series of compounds) and include 24 zinc chlorins, 18 free base (Fb) analogs and one Fb or zinc oxophorbine. The oxophorbine contains the keto-bearing isocyclic ring present in the natural photosynthetic pigments (e.g. chlorophyll a). The substituents cause no significant perturbation to the structure of the chlorin macrocycle, as evidenced by the vibrational properties investigated using resonance Raman spectroscopy. In contrast, the fluorescence properties are significantly altered due to the electronic effects of substituents. For example, the fluorescence wavelength maximum, quantum yield and lifetime for a zinc chlorin bearing 3,13-diacetyl and 10-mesityl groups (662 nm, 0.28, 6.0 ns) differ substantially from those of the parent unsubstituted chlorin (602 nm, 0.062, 1.7 ns). Each of these properties of the lowest singlet excited state can be progressively stepped between these two extremes by incorporating different substituents. These perturbations are associated with significant changes in the rate constants of the decay pathways of the lowest excited singlet state. In this regard, the zinc chlorins with the red-most fluorescence also have the greatest radiative decay rate constant and are expected to have the fastest nonradiative internal conversion to the ground state. Nonetheless, these complexes have the longest singlet excited-state lifetime. The Fb chlorins bearing the same substituents exhibit similar fluorescence properties. Such combinations of factors render the chlorins suitable for a range of applications that require tunable coverage of the solar spectrum, long-lived excited states and red-region fluorescence.
Introduction
Understanding the effects of substituents on the electronic and photophysical properties of organic chromophores has been of longstanding interest in the field of photochemistry. Although this topic has been exhaustively investigated for compounds such as simple arenes (e.g. benzene) that absorb in the ultraviolet region, substantially less is known about larger, structurally more complex chromophores. The chlorophylls represent a class of compounds of tremendous fundamental interest owing to their central role in photosynthesis. Prior studies that probed the effects of substituents on the photophysical properties of chlorophylls have typically relied on semisynthesis rather than de novo synthesis owing to the dearth of versatile de novo synthetic routes to chlorophylls and analogs thereof. The structures of chlorophylls a and b are shown in Chart 1. Examples of semisynthetic modifications of chlorophylls that result in alteration of the optical spectra include (1) reduction or deoxygenation of the keto group in the isocyclic ring; (2) hydrogenation or oxidation of the 3-vinyl group; (3) homologation of the 3-vinyl group; and (4) derivatization of the 7-formyl group in chlorophyll b (1–14). Such synthetic modifications of the naturally occurring chlorophylls have enabled identification of the effects of a number of specific groups on photophysical characteristics, but the presence of a full complement of β-substituents in chlorophylls and the complexity of the chemistry has precluded a comprehensive examination. In particular, no studies have heretofore been performed that span the range from a fully unsubstituted chlorin, to chlorins bearing one or a few substituents, to chlorins that contain the isocyclic ring characteristic of naturally occurring chlorophylls.
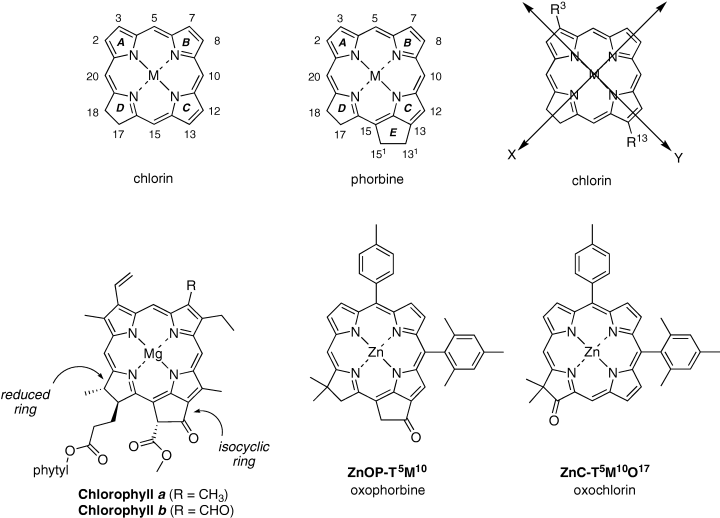
Structures, numbering system and transition-moment directions. A chlorin contains a reduced ring (D, top left). A phorbine is a chlorin that also contains an isocyclic ring (E, top middle). A 131-oxophorbine contains an oxo group at the 131-position, exemplified by chlorophylls a and b. Oxochlorins differ from 131-oxophorbines in the absence of the isocyclic ring and in the position of the keto group. The x- and y-axes of a chlorin (or 131-oxophorbine or oxochlorin) are both in plane. The x-axis contains the reduced ring. The y-axis contains the N atoms of the A and C rings, which contain the 3 and 13 substituents, respectively, and is the direction of the transition moment of the long-wavelength, Qy, absorption band. In the free base chlorins, the N–H protons are positioned on rings A and C (along the y-axis).
We recently developed a de novo synthesis of chlorins (15,16) and have been extending this synthetic route to probe the effects of substituents at defined locations (17–25). Each synthetic chlorin prepared in this manner bears a geminal dimethyl group in the reduced, pyrroline ring, which stabilizes the molecule to adventitious dehydrogenation leading to the porphyrin. Such chlorins are stable to routine handling in the laboratory. The chlorins available via this general route range from the fully unsubstituted chlorin to chlorins bearing one to three substituents at a variety of positions. Recently, we also gained access to 131-oxophorbines, which are chlorins that contain the isocyclic ring spanning the 13- and 15-positions (22). Chlorophylls a and b also contain the 131-oxophorbine skeleton. Chart 1 compares the structures of the chlorophylls and synthetic chlorin species, including a synthetic 131-oxophorbine and a synthetic oxochlorin (18) and also provides relevant nomenclature. Oxochlorins lack an isocyclic ring and also differ from oxophorbines in the position of the keto group.
A series of chlorins bearing a diverse range of substituents at the 3- and 13-positions were found to provide fine red region, spectral tuning of the long-wavelength absorption band (21). The substituents (i.e. auxochromes) include acetyl, triisopropylsilyl (TIPS) -ethynyl and vinyl at the 3-position and acetyl and TIPS-ethynyl at the 13-position. These chlorins, in conjunction with others prepared previously (15,18,20,23–25) and additional analogs prepared here, afford systematic sets of compounds that make possible an in-depth study of the electronic effects of diverse substituents arrayed about the chlorin macrocycle. The ability to synthesize chlorins with precise control over the number and type of substituents complements work in the semisynthesis of chlorophylls, where the nature of the substituents can be modified but a full set of six β-pyrrole substituents in the 131-oxophorbine framework is typically maintained. In this regard, we note that there is a large body of data spanning many decades about chlorophyll chemistry (26–30); however, little systematic spectral data are available. A review of the literature on substituent effects on spectral data will be presented elsewhere and is not cited herein.
In this paper and the companion article that follows (31), we describe studies of 24 zinc chlorins and 18 free base (Fb) chlorins, as well as a zinc oxophorbine and a Fb oxophorbine. The structures of the zinc chlorins are shown in Chart 2; the Fb chlorins are analogs of the zinc chelates. The range of chlorins examined encompasses the fully unsubstituted benchmark chlorin, chlorins with one to three meso-substituents and chlorins with diverse substituents at the 3- and/or 13-positions either with or without a 10-mesityl group (Chart 2). The substituents employed herein include acetyl (A), TIPS-ethynyl (E), pentafluorophenyl (F), mesityl (M), oxo (O), phenyl (P), p-tolyl (T) and vinyl (V) groups; the position of substitution is indicated by superscripts (e.g. 5, 10 or 15 [meso] positions and 3, 13 or 17 [β] positions). The parent framework is a chlorin (C) or oxophorbine (OP) in a Fb or zinc (Zn) chelate form.
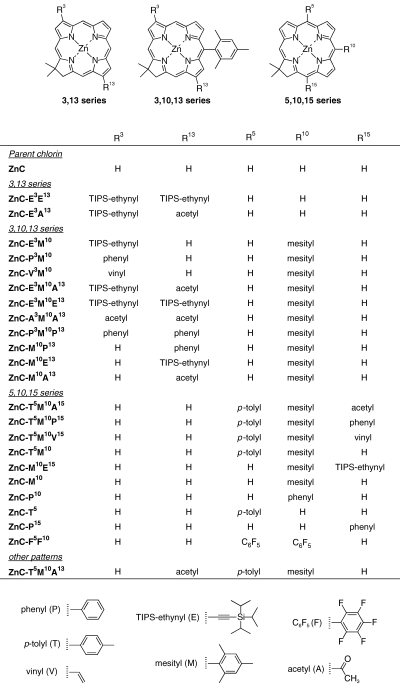
Structures and nomenclature for all Zn chlorins studied.
The present paper focuses on synthesis, vibrational properties, fluorescence characteristics (spectra, lifetimes and quantum yields) and excited-state decay properties of the compounds. The companion paper gives a detailed analysis of the static electronic properties, including optical absorption spectra, redox potentials and the characteristics of the frontier molecular orbitals (energies and electron-density distributions) as revealed by density functional theory calculations (31). The results presented in the two articles provide insights into the fundamental effects of diverse substituents on chlorin electronic attributes. In addition to gaining a fundamental understanding of substituent effects, the ability to tune the spectral and photophysical features of chlorophyll-like molecules in a rational manner is important for a wide variety of practical applications. Such applications range from artificial photosynthesis to photomedicine, where strong absorption in the red region of the spectrum is highly desirable.
Materials and methods
Synthesis. 1H NMR (400 MHz) spectra were collected at room temperature in CDCl3 unless noted otherwise. Absorption spectra were obtained in toluene at room temperature. Chlorins were analyzed by laser desorption mass spectrometry (LD-MS) in the absence of a matrix. Fast atom bombardment mass spectrometry (FAB-MS) data are reported for the molecule ion or protonated molecule ion. Silica gel (40 μm average particle size) was used for column chromatography. THF was freshly distilled from sodium/benzophenone as required. Toluene was distilled from CaH2. Anhydrous DMF and triethylamine (TEA) were used as obtained from commercial sources. All other solvents were reagent grade and were used as received. All palladium-coupling reactions were carried out using standard Schlenk-line techniques.
The syntheses of many of the chlorin compounds have been reported. The previously synthesized compounds include zinc chlorins ZnC (21), ZnC-M10 (21), ZnC-V3M10 (21), ZnC-E3M10 (21), ZnC-M10E13 (21), ZnC-M10A13 (21), ZnC-E3E13 (21), ZnC-E3A13 (21), ZnC-E3M10E13 (21), ZnC-E3M10A13 (21), ZnC-A3M10A13 (21), ZnC-T5M10 (15), ZnC-F5F10 (15), ZnC-T5 (23), ZnC-P10 (23), ZnC-T5M10P15 (20) and ZnC-T5M10A13 (22); Fb chlorins FbC (24), FbC-M10 (24), FbC-P10 (24), FbC-T5 (24), FbC-P15 (24), FbC-F5F10 (15), FbC-T5M10 (15), FbC-T5M10P15 (20) and FbC-T5M10A13 (22); oxophorbines ZnOP-T5M10 (22) and FbOP-T5M10 (22); and the oxochlorins ZnC-T5M10O17 (18) and FbC-T5M10O17 (18). The syntheses of the zinc chlorins ZnC-P15, ZnC-P3M10, ZnC-T5M10A15, ZnC-T5M10V15, ZnC-M10E15, ZnC-M10P13 and ZnC-P3M10P13 and Fb chlorins FbC-P3M10, FbC-M10P13, FbC-P3M10P13, FbC-M10E13, FbC-M10E15, FbC-T5M10A15, FbC-T5M10V15, FbC-A3M10A13 and FbC-E3M10E13 are described below. The chlorin precursors for these syntheses that are not mentioned above include ZnC-M10Br13 (21), ZnC-Br3M10 (21), ZnC-Br3M10Br13 (21), ZnC-Br3Br13 (21), FbC-T5M10Br15 (20), FbC-M10Br15 (24) and FbC-Br15 (24).
Demetalation. 3,13-Diacetyl-17,18-dihydro-10-mesityl-18,18-dimethylporphyrin (FbC-A3M10A13). A solution of ZnC-A3M10A13 (9.20 mg, 0.0152 mmol) in CH2Cl2 (1.0 mL) was treated dropwise with trifluoroacetic acid (TFA; 35.0 μL, 0.454 mmol) over a 2 min period. The solution was stirred at room temperature for 2 h. CH2Cl2 was added, and the organic layer was washed (saturated aqueous NaHCO3 and water), dried (Na2SO4) and filtered. The filtrate was concentrated to dryness. The resulting solid was chromatographed (silica, hexanes/CH2Cl2 [1:9]) to afford a purple solid (6.8 mg, 83%): 1H NMR (300 MHz) δ−1.27 (br s, 2H), 1.85 (s, 6H), 2.03 (s, 6H), 2.62 (s, 3H), 3.07 (s, 3H), 3.27 (s, 3H) 4.61 (s, 2H), 7.26 (s, 2H), 8.35 (d, J = 4.5 Hz, 1H), 8.82 (s, 1H), 8.87 (d, J = 4.5 Hz, 1H), 8.93 (s, 1H), 9.31 (s, 1H), 10.10 (s, 1H), 10.64 (s, 1H) LD-MS obsd. 543.4; FAB-MS obsd. 542.2655, calcd. 542.2682 (C35H34N4O2); λabs 432, 687 nm.
17,18-Dihydro-10-mesityl-18,18-dimethyl-13-[2-(triisopropylsilyl)ethynyl]porphyrin (FbC-M10E13). A solution of ZnC-M10E13 (6.20 mg, 0.00883 mmol) in CH2Cl2 (0.8 mL) was treated dropwise with TFA (20.4 μL, 0.265 mmol) over a 2 min period. The solution was stirred at room temperature for 3 h. CH2Cl2 was added, and the organic layer was washed (saturated aqueous NaHCO3 and water), dried (Na2SO4) and filtered. The filtrate was concentrated to dryness. The resulting solid was chromatographed (silica, hexanes then hexanes/CH2Cl2 [2:1]) to afford a purple solid (4.6 mg, 82%): 1H NMR δ−1.97 (br s, 1H), −1.68 (br s, 1H), 1.36–1.42 (m, 21H), 1.85 (s, 6H), 2.06 (s, 6H), 2.61 (s, 3H), 4.63 (s, 2H), 7.25 (s, 2H), 8.42 (d, J = 4.4 Hz, 1H), 8.64 (s, 1H), 8.84–8.86 (m, 2H), 8.90 (d, J = 4.4 Hz, 1H), 9.18 (d, J = 4.4 Hz, 1H), 9.26 (s, 1H), 9.73 (s, 1H), LD-MS obsd. 638.6; FAB-MS obsd. 638.3826, calcd. 638.3805 (C42H50N4Si); λabs 401, 418, 654 nm.
17,18-Dihydro-10-mesityl-18,18-dimethyl-3,13-bis[2-(triisopropylsilyl)ethynyl]porphyrin (FbC-E3M10E13). A solution of ZnC-E3M10E13 (11.5 mg, 0.0131 mmol) in CH2Cl2 (0.8 mL) was treated dropwise with TFA (22.0 μL, 0.393 mmol) over a 2 min period. The solution was stirred at room temperature for 2 h. CH2Cl2 was added, and the organic layer was washed (saturated aqueous NaHCO3 and water), dried (Na2SO4), filtered and concentrated to dryness. The resulting solid was chromatographed (silica, hexanes/CH2Cl2 [2:1]) to afford a purple solid (8.5 mg, 79%): 1H NMR (300 MHz) δ−1.85 (br s, 1H), −1.49 (br s, 1H), 1.36–1.42 (m, 42H), 1.85 (s, 6H), 2.04 (s, 6H), 2.61 (s, 3H), 4.60 (s, 2H), 7.25 (s, 2H), 8.39 (d, J = 4.5 Hz, 1H), 8.61 (s, 1H), 8.74 (s, 1H), 8.84 (d, J = 4.5 Hz, 1H), 8.94 (s, 1H), 9.22 (s, 1H), 9.92 (s, 1H), LD-MS obsd. 819.1; FAB-MS obsd. 819.5220, calcd. 819.5217 ([M + H]+, M = C53H70N4Si2); λabs 427, 672 nm.
Suzuki coupling. 17,18-Dihydro-10-mesityl-18,18-dimethyl-13-phenylporphyrin (FbC-M10P13). A solution of ZnC-M10Br13 (11.6 mg, 0.0193 mmol) in CH2Cl2 (1.0 mL) was treated dropwise with TFA (44.0 μL, 0.579 mmol) over a 2 min period. The solution was stirred at room temperature for 4 h. CH2Cl2 was added, and the organic layer was washed (saturated aqueous NaHCO3 and water), dried (Na2SO4) and filtered. The filtrate was concentrated to dryness. The resulting crude solid was used in the next step. Following a reported procedure (20), a mixture of the crude product, 4,4,5,5-tetramethyl-2-phenyl-[1,3,2]dioxaborolane (7.87 mg, 0.0386 mmol), anhydrous K2CO3 (40.0 mg, 0.289 mmol) and Pd(PPh3)4 (6.70 mg, 0.0152 mmol) was heated in toluene/DMF (3.0 mL, 2:1) using a Schlenk line. TLC analysis of the reaction mixture indicated incomplete consumption of the starting chlorin after 6 h; therefore, an additional amount of Pd(PPh3)4 (6.70 mg) and K2CO3 (40.0 mg) was added, and the reaction mixture was stirred for 14 h at 90°C. The reaction mixture was concentrated. The resulting residue was chromatographed (silica, hexanes then hexanes/CH2Cl2 [1:1]) to afford a green solid (6.2 mg, 60%): 1H NMR (300 MHz) δ−1.99 (br s, 2H), 1.85 (s, 6H), 2.06 (s, 6H), 2.60 (s, 3H), 4.61 (s, 2H), 7.25 (s, 2H), 7.55–7.63 (m, 1H), 7.70–7.80 (m, 2H), 8.14–8.32 (m, 2H), 8.46 (d, J = 4.2 Hz, 1H), 8.65 (s, 1H), 8.89 (s, 1H), 8.91 (d, J=4.8 Hz, 1H), 8.93 (d, J=4.2 Hz, 1H), 9.13 (s, 1H), 9.21 (d, J = 4.8 Hz, 1H), 9.80 (s, 1H); LD-MS obsd. 534.7; FAB-MS obsd. 534.2806, calcd. 534.2783 (C37H34 N4); λabs 413, 504, 594, 646 nm.
17,18-Dihydro-10-mesityl-18,18-dimethyl-3-phenylporphyrin (FbC-P3M10). A sample of ZnC-Br3M10 (5.0 mg, 0.0083 mmol) in CH2Cl2 (1.0 mL) was treated dropwise with TFA (19 μL, 0.0249 mmol) over a 2 min period. The solution was stirred at room temperature for 4 h. CH2Cl2 was added, and the organic layer was washed (saturated aqueous NaHCO3 and water), dried (Na2SO4) and filtered. The filtrate was concentrated to dryness. The resulting crude solid was used in the next step. Following a reported procedure (20), a mixture of the crude product, 4,4,5,5-tetramethyl-2-phenyl-[1,3,2]dioxaborolane (3.4 mg, 0.016 mmol), anhydrous K2CO3 (9.2 mg, 066 mmol) and Pd(PPh3)4 (2.9 mg, 0.0025 mmol) was heated in toluene/DMF (1.5 mL, 2:1) using a Schlenk line. TLC analysis of the reaction mixture indicated incomplete consumption of the starting chlorin after 6 h; therefore, an additional amount of Pd(PPh3)4 (2.9 mg) and K2CO3 (9.2 mg) was added, and the reaction mixture was stirred for 14 h at 90°C. The reaction mixture was concentrated. The resulting residue was chromatographed (silica, hexanes then hexanes/CH2Cl2 [1:1]) to afford a green solid (2.5 mg, 56%): 1H NMR δ−2.02 (br s, 1H), −1.74 (br s, 1H), 1.85 (s, 6H), 2.08 (s, 6H), 2.60 (s, 3H), 4.64 (s, 2H), 7.24 (s, 2H), 7.67–7.71 (m, 1H), 7.80–7.84 (m, 2H), 8.31–8.34 (m, 2H), 8.44 (d, J = 4.4 Hz, 1H), 8.63 (d, J =4.4 Hz, 1H), 8.76 (d, J = 4.4 Hz, 1H), 8.87 (d, J = 4.4 Hz, 1H), 8.90 (s, 1H), 8.97 (s, 1H+1H), 9.88 (s, 1H); LD-MS obsd. 534.5; FAB-MS obsd. 534.2799, calcd. 534.2783 (C37H34 N4); λabs 412, 504, 594, 644 nm.
17,18-Dihydro-10-mesityl-18,18-dimethyl-3,13-diphenylporphyrin (FbC-P3M10P13). A solution of ZnC-Br3M10Br13 (25.0 mg, 0.0367 mmol) in CH2Cl2 (1.0 mL) was treated dropwise with TFA (85.0 μL, 1.10 mmol) over a 2 min period. The solution was stirred at room temperature for 4 h. CH2Cl2 was added, and the organic layer was washed (saturated aqueous NaHCO3 and water), dried (Na2SO4) and filtered. The filtrate was concentrated to dryness. The resulting crude solid was used in the next step. A mixture of the crude product, 4,4,5,5-tetramethyl-2-phenyl-[1,3,2]dioxaborolane (44.9 mg, 0.220 mmol), anhydrous K2CO3 (76.0 mg, 0.550 mmol) and Pd(PPh3)4 (12.7 mg, 0.0110 mmol) was heated in toluene/DMF (4.5 mL, 2:1) using a Schlenk line. TLC analysis of the reaction mixture indicated incomplete consumption of the starting chlorin after 6 h; therefore, an additional amount of Pd(PPh3)4 (12.7 mg) and K2CO3 (76.0 mg) was added and the reaction mixture was stirred for 14 h at 90°C. The reaction mixture was concentrated. The resulting residue was chromatographed (silica, hexanes then hexanes/CH2Cl2 [1:3]) to afford a green solid (16.6 mg, 74%): 1H NMR (300 MHz) δ−1.83 (br s, 1H), −1.68 (br s, 1H), 1.90 (s, 6H), 2.07 (s, 6H), 2.60 (s, 3H), 4.60 (s, 2H), 7.25 (s, 2H), 7.57–7.63 (m, 1H), 7.66–7.76 (m, 3H), 7.80–7.85 (m, 2H), 8.13–8.17 (m, 2H), 8.31–8.34 (m, 2H), 8.44 (d, J = 4.5 Hz, 1H), 8.64 (s, 1H), 8.86 (d, J = 4.5 Hz, 1H), 8.90 (s, 1H), 8.97 (s, 1H), 9.11 (s, 1H), 9.88 (s, 1H); LD-MS obsd. 610.5; FAB-MS obsd. 610.3124, calcd. 610.3096 (C43H38N4); λabs 418, 507, 652 nm.
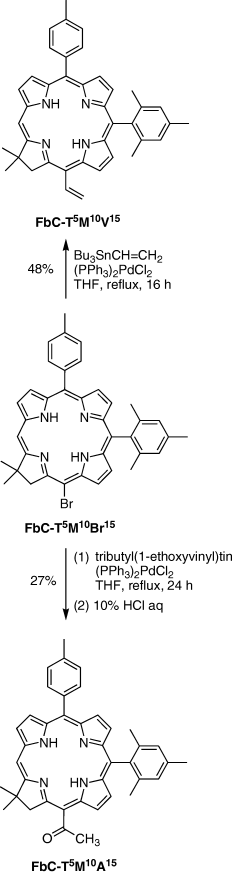
Stille coupling. 15-Acetyl-17,18-dihydro-10-mesityl-18,18-dimethyl-5-(4-methylphenyl)porphyrin (FbC-T5M10A15). Following a procedure for replacement of a bromo group with an acetyl group on a chlorin (21), a mixture of FbC-T5M10Br15 (62.8 mg, 0.100 mmol), tributyl(1-ethoxyvinyl)tin (68 μL, 0.20 mmol) and (PPh3)2PdCl2 (28.1 mg, 0.0400 mmol) was refluxed in THF (10 mL) for 24 h in a Schlenk flask. The reaction mixture was treated with 10% aqueous HCl (10 mL) at room temperature for 2 h. CH2Cl2 was added, and the organic layer was separated. The organic layer was washed with saturated aqueous NaHCO3, dried (Na2SO4) and concentrated to dryness. Chromatography (silica, hexanes/CH2Cl2 [1:2] then hexanes/CH2Cl2 [1:3]) afforded a reddish purple solid (15.9 mg, 27%): 1H NMR δ−1.53 to −1.47 (br, 1H), −1.13 to −1.07 (br, 1H), 1.84 (s, 6H), 2.03 (s, 6H), 2.60 (s, 3H), 2.67 (s, 3H), 3.25 (s, 3H), 4.49 (s, 2H), 7.20–7.23 (m, 2H), 7.49–7.54 (m, 2H), 7.98–8.03 (m, 2H), 8.28 (d, J = 4.3 Hz, 1H), 8.41 (d, J = 4.3 Hz, 1H), 8.56–8.60 (m, 1H), 8.64–8.67 (m, 1H), 8.74–8.82 (m, 2H), 8.80 (s, 1H); LD-MS obsd. 590.1; FAB-MS obsd. 590.3096, calcd. 590.3046 (C40H38N4O); λabs 408, 646 nm.
17,18-Dihydro-10-mesityl-18,18-dimethyl-5-(4-methylphenyl)-15-vinylporphyrin (FbC-T5M10V15). Following a procedure for replacement of a bromo group with a vinyl group on a chlorin (21), a mixture of FbC-T5M10Br15 (62.8 mg, 0.100 mmol), Bu3SnCH=CH2 (100 μL, 0.293 mmol) and (PPh3)2PdCl2 (10.5 mg, 0.0150 mmol) was refluxed in THF (10 mL) for 16 h in a Schlenk flask. The reaction mixture was concentrated and chromatographed (silica, hexanes/CH2Cl2 [1:1]) to afford a reddish purple solid (27.4 mg, 48%): 1H NMR δ−1.76 to −1.70 (br, 1H), −1.40 to −1.34 (br, 1H), 1.84 (s, 6H), 2.04 (s, 6H), 2.60 (s, 3H), 2.66 (s, 3H), 4.48 (s, 2H), 5.94 (dd, J =11.2, 2.0 Hz, 1H), 6.21 (dd, J = 17.3, 2.0 Hz, 1H), 7.20–7.26 (m, 2H), 7.48–7.52 (m, 2H), 7.99–8.04 (m, 2H), 8.22 (dd, J =17.3, 11.2 Hz, 1H), 8.30 (d, J =4.4 Hz, 1H), 8.44 (d, J =4.4 Hz, 1H), 8.53–8.56 (m, 1H), 8.74–8.76 (m, 1H), 8.78–8.80 (m, 1H), 8.82 (s, 1H), 8.98–9.01 (m, 1H); LD-MS obsd. 574.2; FAB-MS obsd. 575.3131, calcd. 574.3096 (C40H38 N4); λabs 419, 649 nm.
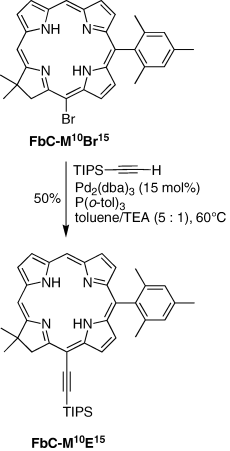
Sonogashira coupling. 17,18-Dihydro-18,18-dimethyl-10-mesityl-15-[2-(triisopropylsilyl)ethynyl]porphyrin (FbC-M10E15). Following a reported procedure for Sonogashira coupling with porphyrinic compounds (19,20,32), samples of FbC-M10Br15 (16 mg, 0.031 mmol), Pd2(dba)3 (4.3 mg, 0.0046 mmol, 15 mol%) and P(o-tol)3 (12 mg, 0.040 mmol) were weighed in a Schlenk flask. The flask was pump-purged with argon three times. A degassed mixture of toluene/triethylamine (5:1, 13 mL) was added, and the resulting reaction mixture was treated with (triisopropylsilyl)acetylene (21 μL, 0.093 mmol) and stirred at 60°C for 16 h. The reaction mixture was diluted with CH2Cl2 (∼50 mL) and filtered. The filtrate was concentrated. Column chromatography (silica, hexanes/CH2Cl2 [1:1]) afforded a trace amount of an unidentified colored byproduct (first fraction) and the title compound (second fraction, orange-brown). The latter was rechromatographed twice (first column: silica, hexanes/CH2Cl2 [3:2]; second column: silica, hexanes/CH2Cl2 [2:1]) to afford an orange-brown solid (10 mg, 50%): 1H NMR δ−1.62 to −1.50 (br, 1H), −1.50 to −1.42 (br, 1H), 1.36–1.40 (m, 21H), 1.84 (s, 6H), 2.04 (s, 6H), 2.59 (s, 3H), 4.70 (s, 2H), 7.23 (s, 2H), 8.38 (d, J =4.8 Hz, 1H), 8.58 (d, J =4.8 Hz, 1H), 8.79 (s, 1H), 8.80 (d, J =4.8 Hz, 1H), 8.83 (d, J =4.8 Hz, 1H), 9.11 (d, J =4.8 Hz, 1H), 9.21 (d, J =4.8 Hz, 1H), 9.67 (s, 1H); LD-MS obsd. 638.2; FAB-MS obsd. 639.3885, calcd. 639.3883 ([M + H]+, M = [C42H50N4Si]); λabs 413, 655 nm.
Metalation. Zn(II)-17,18-Dihydro-10-mesityl-18,18-dimethyl-13-phenylporphyrin (ZnC-M10P13). A solution of Zn(OAc)2·2H2O (31 mg, 0.14 mmol) in methanol (0.5 mL) was added to a solution of FbC-M10P13 (5.0 mg, 0.0093 mmol) in CHCl3 (2.0 mL) with stirring at room temperature. After 16 h, the reaction mixture was concentrated and filtered through a pad of silica (hexanes then hexanes/CH2Cl2 [1:2]). The eluate was concentrated to afford a green solid (4.6 mg, 82%): 1H NMR (300 MHz) δ 1.88 (s, 6H), 2.02 (s, 6H), 2.58 (s, 3H), 4.48 (s, 2H), 7.21 (s, 2H), 7.51–7.56 (m, 1H), 7.63–7.70 (m, 2H), 8.02–8.06 (m, 2H), 8.37 (d, J =4.2 Hz, 1H), 8.54 (s, 1H), 8.61 (s, 1H), 8.76 (d, J =4.2 Hz, 1H), 8.80–8.84 (m, 2H), 9.08 (d, J =4.2 Hz, 1H), 9.60 (s, 1H); LD-MS obsd. 596.3; FAB-MS obsd. 596.1895, calcd. 596.1918 (C37H32N4Zn); λabs 408, 615 nm.
Zn(II)-17,18-Dihydro-10-mesityl-18,18-dimethyl-3-phenylporphyrin (ZnC-P3M10). A solution of Zn(OAc)2·2H2O (12.3 mg, 0.0561 mmol) in methanol (0.5 mL) was added to a solution of FbC-P3M10 (2.0 mg, 0.0037 mmol) in CHCl3 (2.0 mL) with stirring at room temperature for 16 h. Standard workup and filtration through a pad of silica (hexanes then hexanes/CH2Cl2 [1:1]) afforded a green solid (1.9 mg, 86%): 1H NMR (300 MHz) δ 1.85 (s, 6H), 2.04 (s, 6H), 2.58 (s, 3H), 4.52 (s, 2H), 7.21 (s, 2H), 7.62–7.66 (m, 1H), 7.75–7.80 (m, 2H), 8.20–8.22 (m, 2H), 8.33 (d, J =4.5 Hz, 1H), 8.53 (d, J =4.5 Hz, 1H), 8.57 (s, 1H), 8.59–8.60 (m, 1H), 8.65 (s, 1H), 8.77 (d, J = 4.5 Hz, 1H), 8.78 (s, 1H), 9.66 (s, 1H); LD-MS obsd. 596.3; FAB-MS obsd. 596.1819, calcd. 596.1918 (C37H32N4Zn); λabs 409, 613 nm.
Zn(II)-17,18-Dihydro-10-mesityl-18,18-dimethyl-3,13-diphenylporphyrin (ZnC-P3M10P13). A solution of Zn(OAc)2·2H2O (60.0 mg, 0.250 mmol) in methanol (1.0 mL) was added to a solution of FbC-P3M10P13 (10.2 mg, 0.0167 mmol) in CHCl3 (4.0 mL) with stirring at room temperature for 16 h. Standard workup and filtration through a pad of silica (hexanes then hexanes/CH2Cl2 [1:1]) afforded a green solid (10.6 mg, 96%): 1H NMR (300 MHz) δ 1.90 (s, 6H), 2.04 (s, 6H), 2.58 (s, 3H), 4.50 (s, 2H), 7.22 (s, 2H), 7.52–7.57 (m, 1H), 7.61–7.70 (m, 3H), 7.75–7.80 (m, 2H), 8.04–8.08 (m, 2H), 8.20–8.24 (m, 2H), 8.35 (d, J =4.2 Hz, 1H), 8.54 (s, 1H), 8.61 (s, 1H), 8.76–8.80 (m, 3H), 9.67 (s, 1H); LD-MS obsd. 672.4; FAB-MS obsd. 672.2244, calcd. 672.2231 (C43H36N4Zn); λabs 414, 623 nm.
Zn(II)-15-Acetyl-17,18-dihydro-10-mesityl-18,18-dimethyl-5-(4-methylphenyl)porphyrin (ZnC-T5M10A15). A solution of FbC-T5M10A15 (11.8 mg, 0.0200 mmol) in CH2Cl2 (4 mL) was treated with Zn(OAc)2·2H2O (87.8 mg, 0.400 mmol) in methanol (0.8 mL) at room temperature for 8 h. Standard workup and chromatography (silica, CH2Cl2) afforded a bluish green solid (6.3 mg, 48%): 1H NMR δ 1.84 (s, 6H), 1.98 (s, 6H), 2.57 (s, 3H), 2.65 (s, 3H), 3.14 (s, 3H), 4.32 (s, 2H), 7.19 (s, 2H), 7.47 (d, J =7.6 Hz, 2H), 7.92 (d, J =7.6 Hz, 2H), 8.17 (d, J =4.4 Hz, 1H), 8.29 (d, J =4.4 Hz, 1H), 8.42–8.45 (m, 2H), 8.48 (s, 1H), 8.57 (d, J =4.4 Hz, 1H), 8.62 (d, J =4.4 Hz, 1H); LD-MS obsd. 651.8; FAB-MS obsd. 652.2199, calcd. 652.2181 (C40H36N4OZn); λabs 414, 613 nm.
Zn(II)-17,18-Dihydro-10-mesityl-18,18-dimethyl-5-(4-methylphenyl)-15-vinylporphyrin (ZnC-T5M10V15). A solution of FbC-T5M10V15 (11.5 mg, 0.0200 mmol) in CH2Cl2 (4 mL) was treated with Zn(OAc)2·2H2O (87.8 mg, 0.400 mmol) in methanol (0.8 mL) at room temperature for 8 h. Standard workup and chromatography (silica, CH2Cl2) afforded a bluish green solid (7.8 mg, 60%): 1H NMR δ 1.85 (s, 6H), 2.00 (s, 6H), 2.57 (s, 3H), 2.65 (s, 3H), 4.41 (s, 2H), 5.79 (dd, J =17.5, 1.9 Hz, 1H), 6.14 (dd, J =11.0, 1.9 Hz, 1H), 7.19 (s, 2H), 7.47 (d, J =7.9 Hz, 2H), 7.95 (d, J =7.9 Hz, 2H), 8.13–8.17 (m, 1H), 8.20 (d, J =4.5 Hz, 1H), 8.34 (d, J =4.5 Hz, 1H), 8.46 (d, J =4.5 Hz, 1H), 8.53 (s, 1H), 8.61 (d, J =4.5 Hz, 1H), 8.65 (d, J =4.5 Hz, 1H), 8.88 (d, J =4.5 Hz, 1H); LD-MS obsd. 635.8; FAB-MS obsd. 636.2203, calcd. 636.2231 (C40H36N4Zn); λabs 418, 615 nm.
Zn(II)-17,18-Dihydro-18,18-dimethyl-10-mesityl-15-[2-(triisopropylsilyl)ethynyl]porphyrin (ZnC-M10E15). A solution of FbC-M10E15 (8.1 mg, 0.012 mmol) in CH2Cl2/MeOH (2.5 mL, 3:2) was treated with Zn(OAc)2·2H2O (48 mg, 0.22 mmol). The resulting mixture was stirred at room temperature for 3 h, concentrated and chromatographed (silica, hexanes/CH2Cl2 [1:1]). The first fraction (traces of starting material) and the second fraction (unidentified byproduct) were discarded and the third fraction (blue) was rechromatographed under identical conditions to afford a green solid (4.3 mg, 51%): 1H NMR δ 1.35–1.36 (m, 21H), 1.84 (s, 6H), 2.01 (s, 6H), 2.58 (s, 3H), 7.19 (s, 2H), 4.60 (s, 2H), 8.27 (d, J =4.4 Hz, 1H), 8.46 (d, J =4.4 Hz, 1H), 8.50 (s, 1H), 8.65 (d, J =4.4 Hz, 1H), 8.72 (d, J =4.4 Hz, 1H), 8.99 (d, J =4.4 Hz, 1H), 9.07 (d, J =4.4 Hz, 1H), 9.47 (s, 1H); LD-MS obsd. 700.2; FAB-MS obsd. 700.2927, calcd. 700.2940 (C42H48N4SiZn); λabs 420, 618 nm.
Zn(II)-17,18-Dihydro-18,18-dimethyl-15-phenylporphyrin (ZnC-P15). A solution of FbC-P15 (9.0 mg, 0.022 mmol) in CH2Cl2/MeOH (5 mL, 3:2) was treated with Zn(OAc)2·2H2O (95 mg, 0.43 mmol) with stirring at room temperature for 1 h. Standard workup and chromatography (silica, hexanes/CH2Cl2 [1:1]) afforded a green solid (7 mg, 80%): 1H NMR δ 1.95 (s, 6H), 4.17 (s, 2H), 7.68–7.73 (m, 3H), 7.88–7.91 (m, 2H), 8.25 (d, J =4.4 Hz, 1H), 8.65 (s, 1H), 8.77 (d, J =4.4 Hz, 1H), 8.80–8.82 (m, 2H), 8.94 (d, J =4.4 Hz, 1H), 9.04 (d, J =4.4 Hz, 1H), 9.46 (s, 1H), 9.52 (s, 1H); LD-MS obsd. 477.7; FAB-MS obsd. 478.1155, calcd. 478.1136 ([M + H]+, M = [C28H22N4Zn]); λabs 403, 607 nm.
Absorption and fluorescence spectroscopy. Static absorption (Varian Cary 100) and fluorescence (Spex Fluorolog Tau 2) measurements were performed as described previously, typically for very dilute solutions of the compounds in toluene (33,34). Fluorescence lifetimes were obtained using a phase modulation technique (33). Argon-purged solutions with an absorbance of ≤0.10 at the Soret-band λexc were used for the fluorescence spectral and lifetime measurements. For fluorescence spectra, the excitation and detection monochromators typically had a band pass of 1.8 and 3.7 nm, respectively, and spectra were obtained using 0.2 nm data intervals. The emission spectra were corrected for detection-system spectral response. Fluorescence quantum yields were determined for argon-purged solutions of the chlorin relative to chlorophyll a in benzene (Φf = 0.325 [35]) and were corrected for solvent refractive index. Samples for fluorescence polarization studies were prepared by dissolving a small aliquot of a concentrated toluene solution of the compound into a heated toluene solution of polyvinylacetate (Mw 167 000) in a 1 cm path glass cuvette and allowing the toluene to evaporate at room temperature and the matrix to solidify. A database of absorption and fluorescence spectra for a number of chlorins has been compiled (36).
For calculation of the integrated-intensity ratio of the Soret (B) and Qy absorption manifolds ( ), the Soret region was typically integrated from the red of the origin maximum (∼450 nm) to ∼350 nm in order to encompass the Bx(0,0), By(0,0), Bx(1,0) and By(1,0) features. Similarly, integration of the Qy manifold encompassed the (0,0) band and the (1,0) feature(s), but not any (2,0) contributions. For each compound, the integration range for both the B and Qy manifolds was chosen to attempt to best capture the associated oscillator strength while minimizing contributions from absorption to higher-energy electronic states (e.g. the Qx bands in the case of the Qy manifold).
), the Soret region was typically integrated from the red of the origin maximum (∼450 nm) to ∼350 nm in order to encompass the Bx(0,0), By(0,0), Bx(1,0) and By(1,0) features. Similarly, integration of the Qy manifold encompassed the (0,0) band and the (1,0) feature(s), but not any (2,0) contributions. For each compound, the integration range for both the B and Qy manifolds was chosen to attempt to best capture the associated oscillator strength while minimizing contributions from absorption to higher-energy electronic states (e.g. the Qx bands in the case of the Qy manifold).
Resonance Raman spectroscopy. Resonance Raman (RR) spectra were acquired for both solution and solid film samples of all the zinc complexes. The solution measurements were made on samples dissolved in CH2Cl2; the sample cell was a sealed 4 mm i.d. NMR tube, which was spun to mitigate photodecomposition. The film measurements were made on samples deposited on a copper tip that was mounted in an evacuated chamber. All of the RR spectra were acquired at ambient temperature. The spectra of the solution samples were obtained using only Soret excitation because the intrinsic fluorescence from the Qy state of the solution samples precludes obtaining good quality RR spectra. The spectra of the solid samples were obtained using both Soret and Qy-state excitation. Qy-state excitation RR spectra can be obtained for the films owing to quenching of the fluorescence emission (37–39).
The RR spectra were acquired with a triple spectrograph (Spex 1877) equipped with a holographically etched 1200 or 2400 groove/mm grating in the first or third stage. The excitation wavelengths were provided by the discrete outputs of a krypton ion (Coherent Innova 200-K3), an argon ion (Coherent Innova 400-15 UV) or a dye (Coherent CR-590) laser. The dye laser was pumped with an argon ion (Coherent Innova 90-5 UV) laser. The scattered light was collected in a 90° configuration using a 50 mm f/1.4 Canon camera lens. A UV-enhanced charge-coupled device (CCD) was used as the detector (Princeton Instruments LN/CCD equipped with an EEV 1152-UV chip). The data acquisition times ranged from ∼0.5 h (180 × 10 s frames) to ∼5 h (180 × 100 s frames or 60 × 300 s frames), depending on the sample and excitation wavelength. Cosmic spikes were removed prior to addition of the datasets. The laser power at the samples was ∼5–15 mW, depending on the excitation wavelength. The spectral resolution was ∼2.5 cm−1. The frequencies were calibrated using the known frequencies of indene, fenchone and 50/50 toluene/acetonitrile.
Results and discussion
Synthetic chlorins
The zinc chlorins examined herein are shown in Chart 2. For 18 of the compounds, the Fb analogs were also investigated. Each chlorin bears a geminal dimethyl moiety in the reduced, pyrroline ring. The geminal dimethyl structural motif precludes adventitious dehydrogenation of the chlorin to give the corresponding porphyrin, a prevalent problem with many other types of synthetic chlorins. (Such dehydrogenation is believed to be less problematic with chlorophylls owing to the trans-configuration of the vicinal alkyl substituents [18-methyl, 17-propionate] in the reduced ring of chlorophyll, which would result in steric hindrance upon planarization caused by dehydrogenation.) Most of the chlorins have been prepared previously. Additional chlorins were prepared for the spectroscopic studies described herein and in the companion paper (31). Each new synthetic chlorin was characterized by absorption spectroscopy, 1H NMR spectroscopy and mass spectrometry (LD-MS and FAB-MS).
Chlorins bearing a phenyl group at the 3- and/or 13-position were prepared by Suzuki coupling of the corresponding bromochlorins (Scheme 1). Suzuki coupling of ZnC-Br3M10 or ZnC-M10Br13 with 4,4,5,5-tetramethyl-2-phenyl-[1,3,2]dioxaborolane under conditions (30 mol% of Pd(PPh3)4 and K2CO3 in toluene/DMF [2:1]) for use with chlorins (20) gave an inseparable mixture composed of the desired product and the starting chlorin. Therefore, the Suzuki coupling reaction using the corresponding Fb chlorin was employed. Thus, demetalation of chlorin ZnC-Br3M10 with TFA in CH2Cl2 at room temperature afforded the crude Fb chlorin, which was subjected to Suzuki coupling with 4,4,5,5-tetramethyl-2-phenyl-[1,3,2]dioxaborolane in the presence of 30 mol% of Pd(PPh3)4 and K2CO3. In this manner, FbC-P3M10 was obtained in 56% overall yield. The synthesis of 13-phenylchlorin FbC-M10P13 or 3,13-diphenylchlorin FbC-P13M10P13 was carried out in the same manner. Thus, demetalation of ZnC-M10Br13 or ZnC-Br13M10Br13 followed by Suzuki coupling of the corresponding crude product with 4,4,5,5-tetramethyl-2-phenyl-[1,3,2]dioxaborolane afforded FbC-M10P13 or FbC-P3M10P13 in 60% or 74% yield, respectively. This method provides a facile and regioselective synthesis of chlorins bearing phenyl groups at the 3- and/or 13-positions.
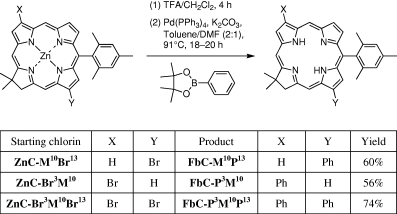
Chlorins bearing an acetyl group or vinyl group at the 15-position were prepared by Stille coupling in the same manner as employed for preparing the 13-acetyl or 3-vinyl substituted chlorins (23). Thus, Pd-mediated coupling of 15-bromochlorin FbC-T5M10Br15 with tributyl(1-ethoxyvinyl)tin followed by acidic hydrolysis afforded the 15-acetylchlorin (FbC-T5M10A15). Pd-mediated coupling of the same 15-bromochlorin with tributylvinyltin afforded FbC-T5M10V15 (Scheme 2).
A chlorin bearing an ethynyl group at the 15-position was prepared by Sonogashira coupling. The Sonogashira reaction was carried out using a copper-free method that was developed for use with porphyrinic compounds (32). Thus, reaction of FbC-M10Br15 with triisopropylsilylacetylene in the presence of Pd2(dba)3 (15 mol%) and tri(o-tolyl)phosphine in toluene/triethylamine (5:1) afforded the corresponding 15-[(2-triisopropylsilyl)ethynyl]chlorin FbC-M10E15 in 50% yield (Scheme 3).
Three chlorins available as the zinc chelate were demetalated. Thus, exposure of ZnC-M10E13, ZnC-E3M10E13 or ZnC-A3M10A13 to TFA in CH2Cl2 at room temperature gave the Fb 13-TIPS-ethynylchlorin FbC-M10E13, 3,13-bis(TIPS-ethynyl)chlorin FbC-E3M10E13 or 3,13-diacetylchlorin FbC-A3M10A13 in 82%, 79% or 83% yield, respectively. Seven chlorins available as the Fb were metalated with zinc acetate at room temperature to give the zinc chelate. The zinc chlorins prepared in this manner include ZnC-P15 (80%), ZnC-P3M10 (86%), ZnC-M10P13 (82%), ZnC-M10E15 (51%), ZnC-P3M10P13 (96%), ZnC-T5M10V15 (60%) and ZnC-T5M10A15 (48%).
Resonance Raman spectra
The vibrational characteristics of all of the zinc complexes were investigated using RR spectroscopy. The high-frequency regions of the RR spectra of selected zinc complexes are shown in 1-3. The complexes for which RR spectra are shown include the parent unsubstituted ZnC, several β-vinyl-, ethynyl- and acetyl-substituted zinc chlorins and the zinc oxophorbine. Spectra acquired for solution and solid film samples using excitation in the near-UV Soret (B) absorption band (390–440 nm; cfFig. 4) are shown in 1, 2, respectively. Spectra of solid film samples acquired using excitation in the Qy absorption band (600–670 nm) are shown in Fig. 3. As noted in the Materials and Methods, Qy-excitation spectra of solution samples cannot be obtained owing to the large intrinsic fluorescence from these samples.
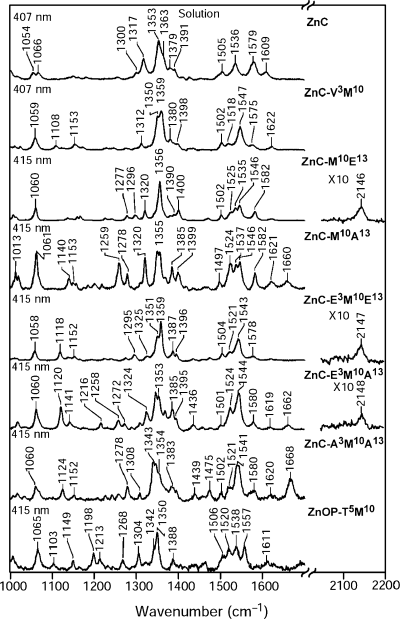
Soret-excitation RR spectra of selected zinc chlorins in solution.

Soret-excitation RR spectra of selected zinc chlorins in solid films.
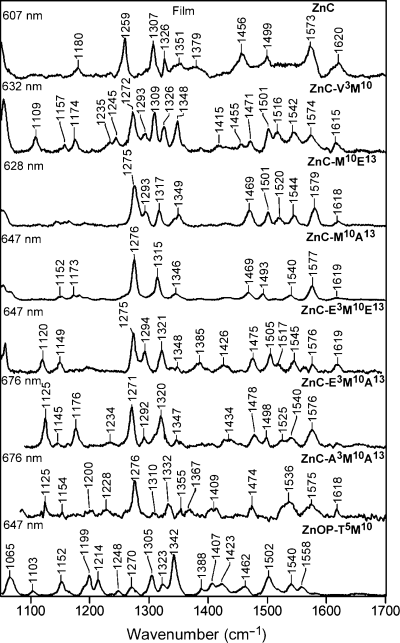
Qy-excitation RR spectra of selected zinc chlorins in solid films.
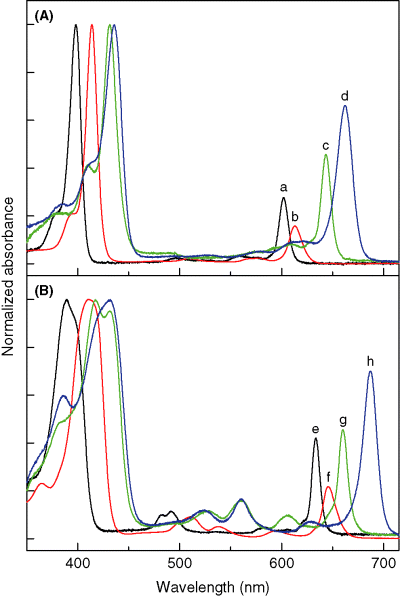
Room temperature electronic ground-state absorption spectra in toluene of (A) zinc chelates ZnC (a, black), ZnC-T5M10A15 (b, red), ZnOP-T5M10 (c, green) and ZnC-A3M10A13 (d, blue) and (B) free base compounds FbC (e, black), FbC-T5M10A15 (f, red), FbOP-T5M10 (g, green) and FbC-A3M10A13 (h, blue).
The Soret and Qy-excitation RR spectra for all the complexes are extremely rich, as is typical of chlorins, reflecting the lowered symmetry (versus a porphyrin) that results from reduction of one of the pyrrole rings (40,41). The detailed interpretation of the RR spectra of the various zinc chlorins is beyond the scope of the current paper. Consequently, the features that are most relevant to the studies are reported herein.
One important feature of the RR spectra is that the vibrational characteristics of the solution and solid samples are generally similar to one another (cf1, 2). Only minor shifts are observed in the frequencies of ring skeletal modes in the solution versus solid, indicating that the basic structure of the chlorin macrocycle is similar in the two media. Likewise, only minor shifts are observed in the frequencies of modes that are characteristics of the substituent groups. These include the C=O stretching modes of the acetyl groups (∼1665 cm−1) and the C≡C stretching modes of the ethynyl groups (∼2147 cm−1).
A second important feature of the RR spectra is that intensity enhancement of the modes due to the substituents is quite weak in general. Indeed, no bands could be clearly identified that could be attributed to the C=C stretch of the vinyl group (expected near 1620 cm−1) or the C=O stretch of the oxophorbine (expected near 1690 cm−1) (40–42). Interestingly, the modes due to the C=O and C≡C stretches of the acetyl and ethynyl groups undergo the largest RR intensity enhancement with Soret excitation. These modes exhibit negligible intensity with Qy excitation despite the fact that the substituents lie along the Qy axis of the molecule (Chart 1). This pattern of RR intensity enhancement has been previously observed for chlorins and chlorophyll model complexes, but is not clearly understood (42).
In summary, the incorporation of substituents in the synthetic chlorins studied here results in no major changes in macrocycle structure as revealed by the skeletal modes. The effects on the structural/electronic properties as assayed by vibrational characteristics are consistent with those found previously for chlorophylls and a number of synthetic chlorins.
Electronic absorption and fluorescence spectra
Figure 4A shows absorption spectra for three zinc chlorins and the zinc oxophorbine in toluene. Figure 4B gives spectra for the Fb analogs. Figure 5A and B give the corresponding fluorescence spectra. The four zinc complexes are the unsubstituted chlorin ZnC (black spectra), chlorin ZnC-T5M10A15 that contains p-tolyl, mesityl and acetyl groups at three meso-positions (red), chlorin ZnC-A3M10A13 that contains acetyl groups at the 3- and 13-positions along with a 10-mesityl group (blue) and oxophorbine ZnOP-T5M10 (green). Each absorption spectrum in Fig. 4 is normalized to the maximum of the strong near-UV Soret band, B(0,0), which contains underlying x- and y-polarized components. The Bx(0,0) and By(0,0) components are somewhat better resolved but remain substantially overlapped for the Fb compounds (Fig. 4B). The long-wavelength absorption band, Qy(0,0), of each compound lies in the range 600–690 nm, with one or more resolved and weaker Qy(1,0) vibronic components roughly 900–1300 cm−1 to higher energy. The weak Qx features lie between the B and Qy bands. Each fluorescence spectrum shown in Fig. 5 has approximate mirror symmetry to the Qy absorption contour for that compound. The fluorescence is dominated by a strong Qy(0,0) feature, with one or more weaker Qy(0,1) components at lower energy derived (43) from Franck–Condon and Herzberg–Teller active vibrational modes.
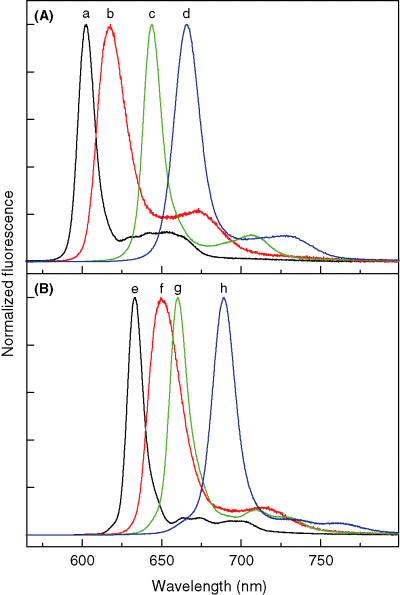
Room temperature fluorescence spectra in toluene of (A) zinc chelates ZnC (a, black), ZnC-T5M10A15 (b, red), ZnOP-T5M10 (c, green) and ZnC-A3M10A13 (d, blue) and (B) free base compounds FbC (e, black), FbC-T5M10A15 (f, red), FbOP-T5M10 (g, green) and FbC-A3M10A13 (h, blue).
Fluorescence-excitation polarization spectra were acquired for the four Fb compounds whose absorption and fluorescence spectra are shown in 4, 5, namely FbC, FbC-T5M10A15, FbOP-T5M10 and FbC-A3M10A13. The polarization spectra generally exhibit a small negative polarization in the vicinity of the Qx(0,0) band (e.g.∼560 nm in spectra g and h in Fig. 4B), consistent with this absorption having a polarization perpendicular to the Qy fluorescence. The polarization spectra show a small positive (0–0.05) polarization across the Soret region, in general agreement with spectra that have been obtained for chlorophyll a and other chlorins (44,45). These findings are consistent with substantially overlapped (or mixed) x- and y-polarized components. These results, like previous experimental and theoretical work (44–48), leave open the question of the relative positions of Bx(0,0) and By(0,0) bands, the possible contribution of other states and the extent to which the relative energies depend on substituent effects.
The substituents have a marked effect on the positions of the B and Qy(0,0) absorption maxima and their relative peak intensities. These effects are described in detail in the companion paper (31), where key trends are analyzed in conjunction with redox properties and molecular-orbital characteristics. We are most interested here in the wavelengths (energies) of the Qy absorption and fluorescence transitions, as well as the oscillator strength of the Qy absorption. These properties are related (via the Einstein coefficients for absorption/emission and the energy-gap law for nonradiative decay) to the decay characteristics of the lowest excited singlet state, namely the Qy state. For example, the Qy absorption intensity is proportional to the radiative (spontaneous fluorescence) rate constant kf.
The wavelengths of the Qy(0,0) absorption and fluorescence bands and the relative intensities at the B and Qy absorption maxima (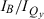 ) are listed in Table 1. The peak-intensity ratio
) are listed in Table 1. The peak-intensity ratio 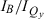 has been used extensively for characterizing chlorophyll pigments, particularly where measurements of molar absorption (extinction) coefficients are often not possible or cannot be obtained with the precision necessary to track other properties (49). This simple ratio is a useful first-order gauge of the variation of Qy intensity among a series of complexes. Indeed, in our prior characterization of 3,13-substituted chlorins (21), we noted the discrepancy between the systematic trend of increasing Qy absorption (relative to B absorption) with redshift and the lack of apparent trends on the basis of the measured molar absorption coefficients (determined with tiny quantities of material).
has been used extensively for characterizing chlorophyll pigments, particularly where measurements of molar absorption (extinction) coefficients are often not possible or cannot be obtained with the precision necessary to track other properties (49). This simple ratio is a useful first-order gauge of the variation of Qy intensity among a series of complexes. Indeed, in our prior characterization of 3,13-substituted chlorins (21), we noted the discrepancy between the systematic trend of increasing Qy absorption (relative to B absorption) with redshift and the lack of apparent trends on the basis of the measured molar absorption coefficients (determined with tiny quantities of material).
| Compound† | Q y (0,0) absorbance (nm) | Q y (0,0) emission (nm) | 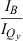 ‡ ‡ |
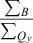 § § |
Φf | τ f (ns) | (kf)−1 (ns) |
|---|---|---|---|---|---|---|---|
| Zn chlorin | |||||||
| ZnC | 602 | 602 | 3.4 | 4.1 | 0.062 | 1.7 | 27 |
| ZnC-T 5 | 605 | 606 | 4.2 | 4.2 | 0.077 | 1.7 | 22 |
| ZnC-P 10 | 605 | 606 | 4.1 | 4.7 | 0.065 | 1.6 | 24 |
| ZnC-M 10 | 606 | 606 | 4.3 | 4.4 | 0.061 | 1.6 | 26 |
| ZnC-P 15 | 607 | 607 | 3.6 | 4.1 | 0.068 | 1.7 | 25 |
| ZnC-T 5 M 10 | 608 | 609 | 5.0 | 6.0 | 0.058 | 1.6 | 28 |
| ZnC-T 5 M 10 P 15 | 613 | 614 | 5.1 | 5.1 | 0.066 | 1.6 | 24 |
| ZnC-P 3 M 10 | 613 | 616 | 3.8 | 4.0 | 0.088 | 1.8 | 20 |
| ZnC-T 5 M 10 A 15 | 613 | 617 | 6.2 | 5.9 | 0.051 | 2.4 | 47 |
| ZnC-T 5 M 10 V 15 | 615 | 618 | 6.0 | 5.6 | 0.041 | 2.1 | 51 |
| ZnC-F 5 F 10 | 615 | 617 | 4.7 | 5.1 | 0.047 | 1.2 | 26 |
| ZnC-M 10 P 13 | 615 | 617 | 3.9 | 3.8 | 0.095 | 1.9 | 20 |
| ZnC-M 10 E 15 | 618 | 619 | 4.5 | 4.5 | 0.090 | 2.2 | 24 |
| ZnC-V 3 M 10 | 620 | 622 | 3.4 | 3.6 | 0.078 | 2.0 | 26 |
| ZnC-P 3 M 10 P 13 | 623 | 627 | 3.0 | 3.3 | 0.13 | 2.2 | 17 |
| ZnC-M 10 E 13 | 625 | 626 | 2.2 | 3.0 | 0.16 | 3.1 | 19 |
| ZnC-E 3 M 10 | 627 | 628 | 2.7 | 3.7 | 0.12 | 2.3 | 19 |
| ZnC-M 10 A 13 | 632 | 633 | 2.2 | 3.1 | 0.22 | 5.2 | 24 |
| ZnC-T 5 M 10 A 13 | 634 | 638 | 2.9 | 4.0 | 0.23 | 5.3 | 23 |
| ZnC-E 3 E 13 | 645 | 645 | 1.5 | 2.2 | 0.18 | 3.1 | 17 |
| ZnC-E 3 M 10 E 13 | 646 | 646 | 1.6 | 2.5 | 0.24 | 4.3 | 18 |
| ZnC-E 3 M 10 A 13 | 652 | 654 | 1.5 | 2.4 | 0.26 | 5.0 | 19 |
| ZnC-E 3 A 13 | 655 | 657 | 1.2 | 2.0 | 0.22 | 4.1 | 19 |
| ZnC-A 3 M 10 A 13 | 662 | 666 | 1.5 | 2.2 | 0.28 | 6 | 21 |
| Zn oxochlorin∥ | |||||||
| ZnC-T 5 M 10 O 17 | 609 | 609 | 5.4 | – | 0.04 | 0.9 | 22 |
| Zn oxophorbine | |||||||
| ZnOP-T 5 M 10 | 643 | 644 | 2.2 | 4.0 | 0.26 | 6.1 | 24 |
| Fb chlorin | |||||||
| FbC | 633 | 633 | 2.4 | 5.2 | 0.20 | 9 | 46 |
| FbC-T 5 | 636 | 636 | 2.9 | 8.1 | 0.22 | 10 | 45 |
| FbC-M 10 | 637 | 637 | 2.7 | 7.2 | 0.22 | 10 | 45 |
| FbC-P 10 | 636 | 636 | 2.8 | 7.8 | 0.20 | 9.7 | 48 |
| FbC-P 15 | 638 | 638 | 2.7 | 6.8 | 0.17 | 9.4 | 55 |
| FbC-T 5 M 10 | 640 | 640 | 3.4 | 9.2 | 0.22 | 10.7 | 50 |
| FbC-P 3 M 10 | 643 | 644 | 2.7 | 6.6 | 0.21 | 8.7 | 42 |
| FbC-F 5 F 10 | 645 | 645 | 3.2 | 6.7 | 0.18 | 7.9 | 45 |
| FbC-M 10 P 13 | 645 | 647 | 2.6 | 6.1 | 0.22 | 8.8 | 40 |
| FbC-T 5 M 10 P 15 | 645 | 645 | 3.9 | 9.5 | 0.27 | 11 | 42 |
| FbC-T 5 M 10 A 15 | 646 | 650 | 4.6 | 8.5 | 0.11 | 6.0 | 56 |
| FbC-T 5 M 10 V 15 | 649 | 652 | 5.9 | 10.0 | 0.12 | 12.8 | 103 |
| FbC-P 3 M 10 P 13 | 652 | 654 | 2.6 | 5.3 | 0.24 | 8.2 | 34 |
| FbC-M 10 E 13 | 654 | 654 | 1.6 | 5.0 | 0.29 | 9.1 | 31 |
| FbC-M 10 E 15 | 655 | 655 | 3.8 | 7.5 | 0.24 | 11.6 | 48 |
| FbC-T 5 M 10 A 13 | 661 | 662 | 2.5 | 6.3 | 0.26 | 10 | 38 |
| FbC-E 3 M 10 E 13 | 672 | 672 | 1.2 | 3.8 | 0.33 | 8.7 | 26 |
| FbC-A 3 M 10 A 13 | 687 | 689 | 1.4 | 3.8 | 0.24 | 7.5 | 31 |
| Fb oxochlorin∥ | |||||||
| FbC-T 5 M 10 O 17 | 643 | 643 | 8.6 | – | 0.13 | 8.9 | 682 |
| Fb oxophorbine | |||||||
| FbOP-T 5 M 10 | 660 | 660 | 2.1 | 5.6 | 0.33 | 13.8 | 42 |
| Reference | |||||||
| Chlorophyll a | 666 | 671 | 1.3 | 1.6 | 0.325¶ | 6.3# | 19 |
- *All samples were measured at room temperature in toluene except for chlorophyll a, which was in benzene. †For nomenclature, see text and Chart 1., Chart 2.. Substituent abbreviations: acetyl (A), TIPS-ethynyl (E), pentafluorophenyl (F), mesityl (M), oxo (O), phenyl (P), p-tolyl (T) and vinyl (V) groups. ‡Ratio of the peak intensities of the B and Qy(0,0) bands. §Ratio of the integrated intensities of the B (Bx plus By components) and Qy absorption manifolds, which includes the (0,0) and (1,0) features. ∥Reference 18. ¶Reference 35. #Values of 6.4 ns and 6.05 ns for chlorophyll a in toluene were measured here and in reference 61, respectively.
Table 1 also lists the integrated intensity ratio of the Soret and Qy manifolds (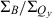 ), which will be useful here for comparisons where relative oscillator strengths are important, particularly for compounds that differ in metalation state (e.g. Fb versus zinc chelates). The integrated-intensity ratio better accounts for the fact that the Bx(0,0) and By(0,0) components of the Soret absorption are markedly split in the Fb chlorins, greatly lowering the intensity at the maximum compared with the zinc chelates. The extent of overlap of the Bx(0,0) and By(0,0) components also depends on the substituent pattern. Additionally, part of the oscillator strength in each manifold rests in vibronic components that contribute little to the peak intensity. This characteristic of the spectra applies to both Fb chlorins and zinc chelates. The ranges of integration and limitations in
), which will be useful here for comparisons where relative oscillator strengths are important, particularly for compounds that differ in metalation state (e.g. Fb versus zinc chelates). The integrated-intensity ratio better accounts for the fact that the Bx(0,0) and By(0,0) components of the Soret absorption are markedly split in the Fb chlorins, greatly lowering the intensity at the maximum compared with the zinc chelates. The extent of overlap of the Bx(0,0) and By(0,0) components also depends on the substituent pattern. Additionally, part of the oscillator strength in each manifold rests in vibronic components that contribute little to the peak intensity. This characteristic of the spectra applies to both Fb chlorins and zinc chelates. The ranges of integration and limitations in 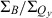 are described in the Materials and Methods. Below, we obtain an additional measure of the Qy oscillator strength via the radiative rate constant kf derived from fluorescence quantum yields and lifetimes.
are described in the Materials and Methods. Below, we obtain an additional measure of the Qy oscillator strength via the radiative rate constant kf derived from fluorescence quantum yields and lifetimes.
The absorption spectra of the four zinc chelates in Fig. 4A show that the Qy(0,0) band redshifts and the 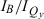 peak-intensity ratio and
peak-intensity ratio and 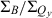 integrated-intensity ratio vary along the series ZnC (Qy = 602 nm,
integrated-intensity ratio vary along the series ZnC (Qy = 602 nm, 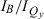 = 3.4,
= 3.4, 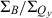 = 4.1); ZnC-T5M10A15 (613 nm, 6.2, 5.9); ZnOP-T5M10 (643 nm, 2.2, 4.0); and ZnC-A3M10A13 (662 nm, 1.5, 2.2). The Fb analogs have Qy(0,0) maxima at longer wavelengths than the zinc chelates but show the same redshift: FbC (Qy = 633 nm,
= 4.1); ZnC-T5M10A15 (613 nm, 6.2, 5.9); ZnOP-T5M10 (643 nm, 2.2, 4.0); and ZnC-A3M10A13 (662 nm, 1.5, 2.2). The Fb analogs have Qy(0,0) maxima at longer wavelengths than the zinc chelates but show the same redshift: FbC (Qy = 633 nm, 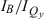 = 2.4,
= 2.4, 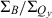 = 5.2); FbC-T5M10A15 (646 nm, 4.6, 8.5); FbOP-T5M10 (660 nm, 2.1, 5.6); and FbC-A3M10A13 (687 nm, 1.4, 3.8). Thus, substituents at the 5,10,15 positions or 3,13 positions of a chlorin, or the formation of the oxophorbine, result in a redshift in the Qy(0,0) absorption band. Fig. 5 shows accompanying redshifts in the Qy(0,0) fluorescence maximum. These data in conjunction with those in Table 1 show that, in parallel with the redshift, the addition of 5,10,15 substituents generally results in a decrease in the relative peak (and integrated) intensity of the Qy band, whereas the addition of 3,13 substituents or the formation of the oxophorbine is generally accompanied by an increase in the relative Qy intensity. These observations and comparison of the specific effects of 3,13 versus 5,10,15 substituents on the absorption characteristics are analyzed in detail in the companion paper (31). For our purposes here, these spectral data provide insights into how the substituent pattern alters the relationship between the energy of the lowest excited singlet state and the radiative excited-state decay, which is related to the oscillator strength of the associated absorption transition. This relationship is discussed below.
= 5.2); FbC-T5M10A15 (646 nm, 4.6, 8.5); FbOP-T5M10 (660 nm, 2.1, 5.6); and FbC-A3M10A13 (687 nm, 1.4, 3.8). Thus, substituents at the 5,10,15 positions or 3,13 positions of a chlorin, or the formation of the oxophorbine, result in a redshift in the Qy(0,0) absorption band. Fig. 5 shows accompanying redshifts in the Qy(0,0) fluorescence maximum. These data in conjunction with those in Table 1 show that, in parallel with the redshift, the addition of 5,10,15 substituents generally results in a decrease in the relative peak (and integrated) intensity of the Qy band, whereas the addition of 3,13 substituents or the formation of the oxophorbine is generally accompanied by an increase in the relative Qy intensity. These observations and comparison of the specific effects of 3,13 versus 5,10,15 substituents on the absorption characteristics are analyzed in detail in the companion paper (31). For our purposes here, these spectral data provide insights into how the substituent pattern alters the relationship between the energy of the lowest excited singlet state and the radiative excited-state decay, which is related to the oscillator strength of the associated absorption transition. This relationship is discussed below.
Fluorescence quantum yields
The fluorescence quantum yields of 10 zinc chlorins containing one to three substituents at the 5,10,15-positions lie in the range 0.041–0.090, with an average of 0.069 (Table 1). This average value is comparable with Φf = 0.062 for the unsubstituted chlorin ZnC, indicating little effect of meso-substituents on the fluorescence intensity. On the other hand, six zinc chlorins having one substituent at the 3- or 13-positions (and a 10-mesityl group) have Φf in the range 0.078–0.22. The average of 0.13 is about two-fold that of the meso-substituted compounds. An even greater average fluorescence yield of 0.22 is obtained for a set of six zinc chlorins having both 3 and 13 substituents (with or without 10-mesityl), which have Φf ranging from 0.13 to 0.28. This latter set has fluorescence properties comparable with the zinc analog of chlorophyll a (Φf ∼0.23 [50]).
The general comparison above, along with closer inspection of the data in Table 1, indicate a trend of increasing fluorescence yield with increasing oscillator strength of the Qy absorption band (relative to the Soret band). The most luminous zinc chlorin (ZnC-A3M10A13), and that with the most intense (and most redshifted) Qy band, has acetyl groups at both the 3- and 13-positions (and 10-mesityl). The quantum yields of the 18 Fb chlorins show similar general trends, but with less variation as a function of substituent pattern (average Φf = 0.22 ± 0.06).
The quantum yields of the zinc oxophorbine ZnOP-T5M10 (Φf = 0.26) and Fb oxophorbine FbOP-T5M10 (Φf = 0.33) are each near or at the upper end of the range of values for the respective 3,13-substituted zinc and Fb chlorins. Thus, the oxophorbines have fluorescence yields comparable with those of chlorophyll a (∼0.33) (35) and its zinc analog. In comparison, the oxochlorins are much less fluorescent (Chart 1). For example, zinc oxochlorin ZnC-T5M10O17 has a quite low fluorescence quantum yield (Φf = 0.040) (18). The lower fluorescence yields for oxochlorins compared with oxophorbines correlate with the less intense (and less redshifted) Qy band. All of the above comparisons and trends for chlorins, oxophorbines and oxochlorins are traced below to a common dependence of Φf and the Qy-band intensity on the radiative probability.
Fluorescence lifetimes
The excited singlet-state lifetime of each compound was determined by fluorescence methods (Table 1). The lifetimes are in the approximate range of 1–6 ns for the zinc chlorins and 6–13 ns for the Fb analogs. The oxophorbines have lifetimes of 6.1 (zinc) and 13.8 ns (Fb) that are at the upper ends of the ranges for the zinc and Fb chlorins, respectively. Closer examination of the data reveals several systematic differences in fluorescence lifetime with substituent pattern. Consider first the zinc chlorins. The six zinc chlorins bearing one to three aryl (p-tolyl, mesityl and phenyl) rings at the 5,10,15 positions all have lifetimes of 1.6–1.7 ns. For example, ZnC-M10 and ZnC-T5M10P15 both have 1.6 ns lifetimes. The incorporation of an auxochrome (acetyl/ethynyl/vinyl) at the 15 position causes a small increase in the lifetime, with the effect of acetyl being greater than vinyl. In particular, the lifetime of ZnC-T5M10V15 and ZnC-T5M10A15 are 2.1 and 2.4 ns, respectively.
Substituents at the 3 and 13 positions give rise to longer lifetimes than 5,10,15 substituents. This effect is modest for aryl rings, but is quite pronounced for vinyl, ethynyl and acetyl groups. For example, the presence of a 13-acetyl group in ZnC-T5M10A13 (5.3 ns) more than doubles the fluorescence lifetime compared with a 15-acetyl group in the chlorin ZnC-T5M10A15 (2.4 ns) that has otherwise the same substituents. The chlorins containing 3,13-substituents (also generally bearing a 10-mesityl group) show a systematic variation in fluorescence lifetime with the type and number of substituent as follows: mono- and bis-aryl (1.8–2.2 ns) < mono- and bis-ethynyl (2.3–4.3 ns) < mixed ethynyl and acetyl (4.1–5.0 ns) < mono- and di-acetyl (5.2–6.0 ns). At the lower end of this range, the incorporation of a single 3 or 13-phenyl ring in ZnC-P3M10 and ZnC-M10P13 (1.8–1.9 ns) and two phenyl rings in ZnC-P3M10P13 (2.2 ns) increases the lifetime modestly compared with ZnC-M10 and ZnC-T5M10P15 (1.6 ns). At the top of the range, the enhanced effect of acetyl versus ethynyl groups is conveyed by the trend ZnC-E3M10E13 (4.3 ns) < ZnC-E3M10A13 (5.0 ns) < ZnC-A3M10A13 (6.0 ns).
Even longer lifetimes are observed for the Fb chlorins. However, there is a less pronounced variation with substituents compared with the zinc chelates, and some of the trends are reversed. For example, six compounds that have one to three (nonfluorinated) meso-aryl rings, including FbC-M10 and FbC-T5M10P15 have an average lifetime of 9.9 ns (range 9.2–11 ns). This value is longer than the average lifetime of 8.6 ns (range 8.2–8.8 ns) for three chlorins including FbC-P3M10P13 that have a 3-phenyl or 3,13-diphenyl groups (along with 10-mesityl). This trend is reversed compared with the zinc chelates, where 3,13 substituents give longer lifetimes than 5,10,15 groups. The effect of acetyl, ethynyl or vinyl groups is also less pronounced for the Fb chlorins. For example, the average lifetime is essentially the same for three Fb chlorins with 3,13 groups (8.4 ns), namely FbC-M10E13 (9.1 ns), FbC-E3M10E13 (8.7 ns) and ZnC-A3M10A13 (7.5 ns). Indeed, a 13-acetyl group appears to result in a slightly shorter lifetime than an ethynyl group, which is opposite (and less pronounced) of the effect observed for the zinc chelates. The direction of the effect of acetyl, ethynyl or vinyl groups at the 15-position of the Fb chlorins is less clear, with FbC-T5M10V15 (12.8 ns) having a lifetime modestly longer than analogs with aryl substituents, including FbC-T5M10P15 (11.0 ns), while the lifetime for FbC-T5M10A15 (6.0 ns) is unusually short by comparison. For both Fb and zinc chlorins, the addition of pentafluorophenyl rings at the 5,10-positions results in a decrease in excited-state lifetime. This effect parallels those observed previously for porphyrin analogs, consistent with the heavy atom effect on the rate of singlet to triplet interconversion (51).
In summary, the fluorescence lifetime of the zinc chlorins can be tuned from 1.6 to 6.0 ns by using (nonfluorinated) aryl rings and acetyl, ethynyl or vinyl groups at appropriate positions, and the complexes with longest lifetimes have the most redshifted absorption bands. Even longer lifetimes (>10 ns) can be achieved with the Fb chlorins, whose longest-wavelength absorption features lie even farther to the red than the zinc chelates. The zinc and Fb oxophorbines have lifetimes at the upper ends of the ranges for the respective chlorins. Thus, by tailoring the substituent pattern and choosing the metalation state (zinc or Fb), considerable latitude in excited-state lifetime and position of the Qy absorption band can be obtained.
Radiative rate
The fluorescence lifetime and quantum yield are related by the rate constants for radiative (fluorescence) decay (kf) and internal conversion (kic) of the excited singlet state to the ground state and intersystem crossing (kisc) to the triplet excited state by the formulas τ = 1/(kf + kic + kisc) and Φf = kf/(kf + kic + kisc). These equations and the measured values of these quantities for each compound can be combined to obtain the radiative rate via the expression kf = Φf/τ. The radiative lifetime (in nanoseconds), the inverse of kf, is tabulated in Table 1.
Consider first the effect of meso-substituents alone. The value of kf does not change appreciably (≤10%) upon the addition of up to three aryl groups at the 5,10,15 positions for either zinc or Fb chlorins. For these compounds, the Qy maximum undergoes only a small net redshift: 602 nm for ZnC to 613 nm for ZnC-T5M10P15 and 633 nm for FbC to 645 nm for FbC-T5M10P15. The effect of a 15 acetyl, ethynyl or vinyl substituent is quite interesting. For both zinc and Fb chlorins, compared with compounds with otherwise the same meso-substituent, a 15-acetyl group gives a 5–6 nm redshift and a ∼25% average decrease in kf; a 15-vinyl group gives a 7–9 nm redshift and ∼50% average decrease in kf. A 15-ethynyl group gives a larger (12–18 nm) redshift and a relatively small change in kf.
Consider next the effects of substituents at the 3,13 positions (generally in conjunction with a 10-mesityl group). The data show that in progressing from the unsubstituted parent zinc chlorin (ZnC; Qy at 602 nm) to the zinc chlorin having the most redshifted Qy band, namely with 3,13-diacetyl and 10-mesityl substituents (ZnC-A3M10A13, Qy at 662 nm) there is a ∼30% increase in kf. Similarly, there is a ∼50% increase in kf for the Fb analog FbC-A3M10A13 (Qy at 687 nm) compared with that of FbC (Qy at 633 nm).
The radiative rate kf for (spontaneous) fluorescence and the oscillator strength for (stimulated) absorption to the lowest excited singlet state (Qy) are intimately related in the expressions for the Einstein coefficients. In particular, 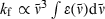 , where the integration over the molar absorption (extinction) coefficient covers the wavenumber (
, where the integration over the molar absorption (extinction) coefficient covers the wavenumber (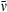 ) range of the vibronic absorption transitions from the ground to lowest excited singlet state (i.e. Qy(0,0) plus Qy(1,0), etc.). This simple analysis ignores the fact that the absorption and emission transitions do not occur at the same wavelength and obey an approximate mirror-symmetry relationship. Fig. 6 indicates that there is a general correlation between kf and
) range of the vibronic absorption transitions from the ground to lowest excited singlet state (i.e. Qy(0,0) plus Qy(1,0), etc.). This simple analysis ignores the fact that the absorption and emission transitions do not occur at the same wavelength and obey an approximate mirror-symmetry relationship. Fig. 6 indicates that there is a general correlation between kf and 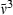 times the
times the 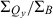 integrated absorption-intensity ratio (the inverse of the value listed in Table 1), which is a measure of the total Qy intensity relative to that of the Soret. The trend spans the zinc and Fb chlorins and the associated oxophorbines. Similar relationships between kf and the peak-intensity ratio
integrated absorption-intensity ratio (the inverse of the value listed in Table 1), which is a measure of the total Qy intensity relative to that of the Soret. The trend spans the zinc and Fb chlorins and the associated oxophorbines. Similar relationships between kf and the peak-intensity ratio 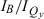 are seen individually for zinc chelates and Fb chlorins, but not when the two types of complexes are grouped together on the same plot. This latter relationship is so because the variation in Soret (B) peak intensity does not track the oscillator strength (integrated intensity) across both sets of compounds due to differences in the net contributions of the x- and y-polarized Soret components to the peak intensity (i.e. substantial splitting for Fb chlorins), as described above.
are seen individually for zinc chelates and Fb chlorins, but not when the two types of complexes are grouped together on the same plot. This latter relationship is so because the variation in Soret (B) peak intensity does not track the oscillator strength (integrated intensity) across both sets of compounds due to differences in the net contributions of the x- and y-polarized Soret components to the peak intensity (i.e. substantial splitting for Fb chlorins), as described above.

Plot of the 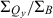 integrated-absorption-intensity ratio multiplied by the cube of the wavenumber (
integrated-absorption-intensity ratio multiplied by the cube of the wavenumber (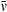 ) position of the Qy(0,0) band versus the radiative rate constant kf derived from the fluorescence quantum yield and lifetime data.
) position of the Qy(0,0) band versus the radiative rate constant kf derived from the fluorescence quantum yield and lifetime data.
The kf values derived from the fluorescence yield and lifetime data, like the 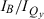 and
and 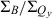 relative-intensity ratios, indicate that the oscillator strength of the Qy transition increases as it is shifted to the red by 3,13-substituents, for both zinc and Fb chlorins (Table 1). This effect is illustrated by the spectra in Fig. 4A for ZnC-A3M10A13 (blue, d) versus for ZnC (black, a) and in Fig. 4B for FbC-A3M10A13 (blue, h) versus for FbC (black, e). On the other hand, the 5,10,15 substituents tend to result in a decrease in Qy intensity as the band redshifts, particularly as reflected in the
relative-intensity ratios, indicate that the oscillator strength of the Qy transition increases as it is shifted to the red by 3,13-substituents, for both zinc and Fb chlorins (Table 1). This effect is illustrated by the spectra in Fig. 4A for ZnC-A3M10A13 (blue, d) versus for ZnC (black, a) and in Fig. 4B for FbC-A3M10A13 (blue, h) versus for FbC (black, e). On the other hand, the 5,10,15 substituents tend to result in a decrease in Qy intensity as the band redshifts, particularly as reflected in the 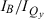 and
and 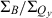 relative-intensity ratios. This effect is illustrated by the spectra in Fig. 4A for ZnC-T5M10A15 (red, b) versus for ZnC (black, a) and in Fig. 4B for FbC-T5M10A15 (red, f) versus for FbC (black, e). Although this trend is generally not as evident in the kf values, it is apparent for both zinc and Fb chlorins bearing three meso-substituents including 15-vinyl and 15-acetyl groups (Table 1).
relative-intensity ratios. This effect is illustrated by the spectra in Fig. 4A for ZnC-T5M10A15 (red, b) versus for ZnC (black, a) and in Fig. 4B for FbC-T5M10A15 (red, f) versus for FbC (black, e). Although this trend is generally not as evident in the kf values, it is apparent for both zinc and Fb chlorins bearing three meso-substituents including 15-vinyl and 15-acetyl groups (Table 1).
Effects of substituents on excited-state decay pathways
The excited singlet-state decays by fluorescence, intersystem crossing and internal conversion. The internal conversion rate (kic) is expected to increase as the energy of the lowest excited singlet state decreases due to an improved Franck–Condon (vibrational overlap) factor, which defines the energy-gap law for nonradiative decay. The data in Table 1 show that the zinc chlorins with the lowest excited singlet states (the most redshifted Qy bands) have the largest relative Qy intensity and kf values. These compounds generally have 3,13 substituents, particularly vinyl, ethynyl or acetyl groups. Together, an increase in kic and kf would predict a decrease in the lifetime of the lowest excited singlet state. However, we find that the lifetime actually increases among this series of zinc chlorins (by a factor of ∼3.5), as does the fluorescence yield (Table 1). For example, τ increases from 1.7 ns for ZnC to 6.0 ns for ZnC-A3M10A13 and Φf increases from 0.062 to 0.28. These findings imply a commensurate decrease in the rate constant (kisc) for intersystem crossing to the excited triplet state.
This trend in photodynamics of the synthetic chlorins examined herein follows the trends in the decay characteristics of the excited singlet state along the series porphyrin→chlorin→bacteriochlorin (52–60). The average and typical range are as follows using data for Fb and closed-shell metal chelates (e.g. Zn and Mg) (18,19,46,51–61):
- •
porphyrins: Φf∼0.05 (0.03–0.16), Φisc∼0.85 (0.7–0.9) and Φic∼0.10 (0.05–0.2);
- •
chlorins: Φf∼0.20 (0.1–0.3), Φisc∼0.70 (0.6–0.8) and Φic∼0.10 (0.1–0.2);
- •
bacteriochlorins: Φf∼0.15 (0.1–0.3), Φisc∼0.50 (0.3–0.8) and Φic∼0.35 (0.3–0.4).
Thus, the trends with progressive reduction of the parent macrocycle include (1) a decrease in kisc and Φisc; (2) an increase in the Qy wavelength and oscillator strength; and (3) an increase in kic and Φic (consistent with the Franck–Condon effect described above). These parallels suggest that the peripheral substituents on chlorins, particularly 3,13 substituents, give rise to diminished spin-orbit coupling as is observed upon progressive reduction of the parent macrocycle.
Conclusions
The availability of new synthetic methods has opened the door to systematic studies of substituent effects in chlorin macrocycles. Previously, limited studies only could be carried out by modification of the intact chlorophyll ring. Understanding the effects of substituents on spectral properties of chlorins is of fundamental interest and may provide a foundation for the rational design of tailored red-absorbing pigments. The key observation reported herein is that changes in the inherent radiative and nonradiative rate constants positively reinforce each other in a manner that endows the new synthetic chlorins with photophysical properties that are well suited for a number of applications. In particular, the new architectures bearing 3 and 13 substituents combine a redshift in the long-wavelength absorption band, an increase in the intensity of the long-wavelength absorption band, a redshift in the wavelength of the fluorescence maximum, only a small decrease in fluorescence lifetimes for the Fb chlorins (to 8–10 ns) and an actual increase in the fluorescence lifetime for zinc chlorins (to 4–6 ns). These characteristics are obtained with no significant effects on the structure of the macrocycle as evidenced by the vibrational properties of the complexes. Applications of such chlorins may include artificial photosynthesis in model systems, solar energy conversion in molecular-based photovoltaic cells and medical applications ranging from flow-cytometric fluorescent labels to photodynamic therapy.
Acknowledgements— This research was supported by grants from the Division of Chemical Sciences, Office of Basic Energy Sciences, Office of Energy Research, US Department of Energy to DH (DE-FG02-05ER15661), DFB (DE-FG02-05ER15660) and JSL (DE-FG02-96ER14632). Mass spectra were obtained at the Mass Spectrometry Laboratory for Biotechnology at North Carolina State University. Partial funding for the NCSU Facility was obtained from the North Carolina Biotechnology Center and the NSF.




