Effect of Photosensitizer Dose on Fluence Rate Responses to Photodynamic Therapy†
This invited paper is part of the Symposium-in-Print: Photodynamic Therapy.
Abstract
Photodynamic therapy (PDT) regimens that conserve tumor oxygenation are typically more efficacious, but require longer treatment times. This makes them clinically unfavorable. In this report, the inverse pairing of fluence rate and photosensitizer dose is investigated as a means of controlling oxygen depletion and benefiting therapeutic response to PDT under conditions of constant treatment time. Studies were performed for Photofrin-PDT of radiation-induced fibrosarcoma tumors over fluence rate and drug dose ranges of 25–225 mW cm−2 and 2.5–10 mg kg−1, respectively, for 30 min of treatment. Tumor response was similar among all inverse regimens tested, and, in general, tumor hemoglobin oxygen saturation (SO2) was well conserved during PDT, although the highest fluence rate regimen (225 mW × 2.5 mg) did lead to a modest but significant reduction in SO2. Regardless, significant direct tumor cell kill (>1 log) was detected during 225 mW × 2.5 mg PDT, and minimal normal tissue toxicity was found. PDT effect on tumor oxygenation was highly associated with tumor response at 225 mW × 2.5 mg, as well as in all other regimens tested. These data suggest that high fluence rate PDT can be carried out under oxygen-conserving, efficacious conditions at low photosensitizer dose. Clinical confirmation and application of these results will be possible through use of minimally invasive oxygen and photosensitizer monitoring technologies, which are currently under development.
Introduction
The therapeutic benefits of conserving tumor oxygenation during photodynamic therapy (PDT) are well known. Treatment regimens that lead to less oxygen depletion during illumination are more efficient (1). Preclinical studies demonstrate oxygen-conserving protocols to result in more tumor damage, longer tumor growth delays and/or a higher percentage of tumor cures (2–5). Normal tissue toxicity is also reduced during oxygen-conserving PDT under the condition that total dose is reduced to compensate for the enhanced efficiency of the oxygen-conserving regimen (6). Clinical investigation confirms that treatment at lower fluence rate, which is associated with better oxygen maintenance during PDT, leads to a greater number of complete responses (7).
Among the factors affecting oxygen depletion during PDT are light delivery method (continuous vs fractionated), intensity of light delivery (fluence rate), tissue photosensitizer level, photosensitizer type and tumor microenvironment (intracapillary spacing, hemoglobin oxygen saturation, etc.) (8–11). Hyperfractionated illumination, i.e. rapid cycles of alternating illumination and dark periods, facilitates the recovery of tissue oxygenation during the dark periods. Lower fluence rates favor oxygen maintenance during PDT by reducing the singlet oxygen production rate and thus the rate of ground state oxygen consumption. Replenishment of tissue oxygen through the blood flow can more effectively keep pace with oxygen consumption during low compared with high fluence rate PDT. As with fluence rate, lower tissue photosensitizer concentration enables better oxygen maintenance by reducing the amount of singlet oxygen produced per unit of time. Photosensitizer type affects oxygen consumption through drug photophysical characteristics; drugs with higher extinction coefficients at the treatment wavelength or higher quantum yields for singlet oxygen production will cause more rapid oxygen depletion. Finally, tumor microenvironment contributes to oxygenation during PDT through its effects on oxygen delivery.
In translational research, most investigations have focused on manipulation of light delivery, either through use of lower fluence rate or fractionated illumination, to control oxygen depletion during PDT (2,4,12,13). A negative aspect of these methods is the need to increase treatment times for delivery of an equally effective PDT dose under oxygen-conserving conditions (6). In clinical applications, especially those in which PDT is performed intraoperatively, long treatment times are unacceptable. For this reason, it is not uncommon for higher fluence rates to be employed in clinical trials; lowering fluence rate or fractionating the dose is not considered an option, even for the purpose of oxygen conservation (1). Importantly though, clinical trials also routinely use lower photosensitizer doses than preclinical studies, and in theory lower tumor drug levels could counterbalance the oxygen-consuming properties of high fluence rate (14,15).
Despite its pertinence to clinical PDT, few studies have investigated the PDT effect of fluence rate manipulation in combination with photosensitizer dose adjustment. In this report, we investigate how rationally designed inverse pairing of fluence rate and photosensitizer dose affects tumor oxygenation and therapeutic response to PDT under conditions of constant treatment time. These investigations have two primary purposes: (1) to evaluate whether pairing of increasing fluence rate with decreasing photosensitizer dose will provide efficacious response in the presence of oxygen conservation during PDT and (2) to determine if PDT effect on tumor oxygenation is directly correlated with response across the inversely paired treatment regimens.
Materials and methods
Tumor model and PDT. Radiation-induced fibrosarcoma (RIF) tumors were propagated on the shoulders of 9- to 11-week-old C3H mice (NCI-Frederick, Frederick, MD or Taconic; Germantown, NY) by the intradermal injection of 3 × 105 cells. Animals received PDT or control treatment ∼7–9 days later when tumors reached a volume appropriate for each experimental endpoint; tumors of volume ≤100 mm3 were used in tumor-response studies, whereas tumors ∼100–175 mm3 in volume were used for investigations requiring tumor excision, e.g. in vivo/in vitro clonogenic assay, in order to ensure sufficient sample for analysis. The photosensitizer Photofrin (Axcan Pharma Inc., Mont-Saint-Hilaire, QC, Canada) was administered via tail vein at ∼24 h prior to illumination at doses specified in the Results. Tumor concentration of photosensitizer was measured by spectrofluorometric assay, following a procedure previously published (16). Illumination of a shaved and depilated (Nair®) treatment field was performed using a KTP Yag pumped dye module (Laserscope, San Jose, CA) tuned to produce 630 nm of light. Light was delivered through microlens-tipped fibers (CardioFocus, Norton, MA) to produce an illumination area of 1.0–1.1 cm diameter, depending on tumor size. Laser output was measured with a power meter (Coherent, Auburn, CA) and adjusted as needed to produce fluence rates in the range of 25–225 mW cm−2 as specified in the Results. Treatment fluence was varied as required to maintain a constant treatment time of 30 min at all fluence rates. Controls included animals receiving only light (no photosensitizer), only Photofrin (no light) and untreated (neither photosensitizer nor light). During PDT- or control-treatment, mice were anesthetized by inhalation of isoflurane in medical air, delivered through a nose cone (VetEquip anesthesia machine, Pleasanton, CA).
Tumor-response assay. PDT was performed over a 1 cm diameter area centered on RIF tumors of ≤100 mm3 in volume. After PDT- or control-treatment, mice were followed daily to determine the number of days after treatment until tumor volume equaled or exceeded 400 mm3 (time-to-400 mm3). Tumor volume was measured in two orthogonal directions and calculated using the formula volume = diameter × width2 × 3.14/6. A cure was defined as no sign of tumor regrowth at 90 days after PDT; cures were treated as censored data points for the purpose of statistical analysis.
In vivo optical (diffuse reflectance) spectroscopy. A continuous wave broadband reflectance spectroscopy system was used to measure tumor optical properties and from these optical properties, tumor total hemoglobin concentration (THC) and hemoglobin oxygen saturation (SO2) were calculated. Optical measurements were performed immediately before and immediately after PDT in each animal with ∼10–20 spatially distributed measurements (acquisition time of 100 ms/measurement) collected at each timepoint. The sampling incorporated tissue up to ∼2 mm in depth, thus optical measurements were well averaged within a tumor. The instrumentation, theory and application of the spectroscopy system has been described in detail in previous reports (17–20). Briefly, the system consists of a 250 W quartz tungsten halogen lamp (Cuda Fiberoptics, Jacksonville, FL), a handheld surface contact fiber-optic probe, a monochromator (Acton Research, Acton, MA) to disperse light from the detection fibers and a liquid nitrogen-cooled CCD camera (Roper Scientific, Trenton, NJ) to image the reflectance spectra from multiple detection fibers simultaneously. Spectra were collected in the 600–800 nm wavelength range and calibrated based on measurements in a 6 inch diameter integrating sphere (LabSphere Inc., North Sutton, NH) (18,19,21). Data were fit by an algorithm based on the diffusion equation with the restriction that μs′ = Aλ−B and μa = Σciεi(λ), where λ is the wavelength and ci and εi are the concentration and extinction coefficient of the ith chromophore, respectively. Primary chromophores considered were oxyhemoglobin (HbO2), deoxyhemoglobin (Hb), Photofrin and water; the extinction coefficients of HbO2, Hb and water were obtained from the literature (22) and the extinction coefficient of Photofrin was obtained by direct measurement using an absorption spectrometer (Ocean Optics, Dunedin, FL). The diffuse reflectance algorithm simultaneously fit data from all wavelengths and source-detector separations to analytical solutions of the photon diffusion equation for a semi-infinite turbid medium (23–30), in order to extract A, B, cPhotofrin, cwater, cHbO2 and cHb. From these quantities, we calculated tumor THC (THC = cHbO2 + cHb) and tissue hemoglobin oxygen saturation (SO2 = cHbO2/THC). Relative-SO2 was calculated as the ratio of SO2 after to before PDT in the same tumor. Although cPhotofrin and cwater were extracted outputs in the fitting process, the absorption coefficients of Photofrin and water are relatively small compared with those of HbO2 and Hb in tissue; we have found previously that the extracted cHbO2 and cHb are quite insensitive to the extracted quantities for Photofrin and water (17).
In vivo/in vitro clonogenic assay. PDT was performed over a 1.1 cm diameter area centered on RIF tumors of ∼100–175 mm3 in volume. At times immediately, 5 and 17 h after PDT animals were killed by CO2 inhalation and tumors were excised and enzymatically digested to a single cell suspension. This involved placing the weighed and minced tumor sample in a trypsinizing flask containing 3000 U deoxyribonuclease (Sigma-Aldrich, St. Louis, MO), 2000 U collagenase (Sigma-Aldrich) and 3 mg protease (Sigma-Aldrich) dissolved in 12 mL of Hank’s Balanced Salt Solution. Samples were spun at low speed for 30 min at 37°C, with an interruption after 15 min in order to mix the suspension by pipette. The digested sample was passed through a cell strainer, centrifuged, resuspended in complete media (defined below) and counted. Cells were plated on 100 mm tissue culture dishes in triplicate at specific concentrations. The plating media, i.e. complete media, consisted of Minimum Essential Media Alpha (Gibco, Grand Island, NY) supplemented with 10% fetal calf serum (Atlanta Biologicals, Lawrenceville, GA), 100 U/mL penicillin G sodium (Gibco), 100 μg mL−1 streptomycin sulfate (Gibco) and 300 μM of l-glutamine (Gibco). After ∼10 days of incubation (37°C at 5% CO2) for colony growth, cells were fixed and stained using methylene blue (2.5 mg mL−1) dissolved in 30% alcohol. Colonies were counted and averaged over triplicate plates. The number of clonogenic cells/g was calculated as the number of cells per g of tumor times the ratio of the number of colonies counted to the number plated.
Normal tissue toxicity. Anesthetized mice received PDT over a 1 cm area on their depilated hindfoot. After PDT- or control-treatment, mouse foot response was scored using the scale listed in Table 1. Foot response was followed daily for 7 days and then five times a week thereafter until all feet returned to normal. A score of 2, which would require animal euthanasia, was not found for any of the mice treated in these investigations.
| Numerical score* | Appearance |
|---|---|
| 0 | No apparent difference from normal |
| 0.1 | Very slight edema and/or erythema |
| 0.3 | Slight edema and/or erythema |
| 0.5 | Moderate edema and/or erythema |
| 0.75 | Large edema and/or erythema |
| 1 | Large edema and/or erythema with exudate |
| 1.2 | Erythema with slight scaly or crusty appearance |
| 1.5 | Erythema with definite scaly or crusty appearance |
| 1.65 | Slight damage to toes |
| 1.75 | Definite damage and/or slight fusion of toes |
| 2.0† | Most toes fused but general shape unchanged |
- *Scale is continuous thus intermediate scores are also possible. †At scores of two mice are killed.
Statistics. Survival data were fitted with a Cox-regression model. Where needed, pair-wise comparisons of tumor responses between selected treatment regimens (hazard ratios) were made using the Wald test. The association between tumor response and PDT-induced change in tumor oxygenation was evaluated by plotting time-to-400 m3vs relative-SO2 and fitting the data with a linear model; the strength of the association was assessed by the correlation coefficient (r2) with statistical significance given by a Wald test comparing the estimated slope to a slope of 0. A sign-rank test was used to test for differences between the normalized fraction of SO2 (relative-SO2) or the normalized difference in THC and the null hypothesis, i.e. a value of 1 or 0, respectively. A Wilcoxon rank-sum test was used for all other comparisons. A P-value of <0.05 was considered significant for all but the Cox-regression analysis. In this case, a Bonferroni correction was incorporated for the multiple comparisons, which resulted in significance at P < 0.008.
Results
Tumor response to inverse fluence rate × photosensitizer dosing
We began these investigations by determining the therapeutic consequences of pairing increasing fluence rate with decreasing photosensitizer dose. A low PDT fluence rate of 25 mW cm−2 and a high PDT fluence rate of 225 mW cm−2 were chosen as three-fold lower and higher than our typical preclinical fluence rate of 75 mW cm−2. This nine-fold range in fluence rate adequately covers those most commonly employed in preclinical and clinical PDT. Photosensitizing conditions to pair with these fluence rates were chosen as those that produced an ∼nine-fold difference in RIF drug concentration. The measured RIF Photofrin concentrations resulting from 10, 3 and 2.5 mg kg−1 animal injections were 13 ± 3.7, 2.0 ± 0.3 and 1.3 ± 0.08 ng mg−1, respectively. Based on these data, a low drug dose of 2.5 mg kg−1 and a high drug dose of 10 mg kg−1 were chosen for investigation. A drug dose of 2.5 mg kg−1 was paired with 225 mW cm−2 and a drug dose of 10 mg kg−1 was paired with 25 mW cm−2.
A tumor-response assay was used to compare the therapeutic efficacy of the designed fluence rate and photosensitizer dose pairs for treatments of equivalent length (30 min). Kaplan–Meier curves (Fig. 1a) demonstrate that PDT at a fluence rate of 225 mW cm−2 with a drug dose of 2.5 mg kg−1 (225 mW × 2.5 mg) produced a similar effect to PDT at a fluence rate of 25 mW cm−2 with a drug dose of 10 mg kg−1 (25 mW × 10 mg). After 25 mW × 10 mg PDT, median time of tumor regrowth to 400 mm3 (time-to-400 mm3) was 12.5 days and 1 cure occurred among 10 animals treated. After 225 mW × 2.5 mg PDT, median time-to-400 mm3 was 12.5 days and no cures (n = 10) were found. Lowering the photosensitizer dose at either of the fluence rates led to substantial reductions in treatment efficacy. At 225 mW cm−2 reducing the drug dose to 1 mg kg−1 resulted in a median time-to-400 mm3 of 7 days (1 cure of n = 7). At 25 mW cm−2 lowering the drug dose to 5 mg kg−1 resulted in a median time-to-400 mm3 of 5 days (0 cures of n = 9). Tumor responses to PDT at 225 mW × 1 mg and 25 mW × 5 mg were no different from the growth of size-matched untreated RIF tumors (median time-to-400 mm3 of 7 days). Light controls at 225 mW cm−2 and drug controls with 10 mg kg−1 Photofrin also produced short regrowth times (median time-to-400 mm3 of 4 and 3 days, respectively). On the other hand, tumor responses at 225 mW × 2.5 mg and 25 mW × 10 mg were both significantly different from the untreated controls (P < 0.001 for both).
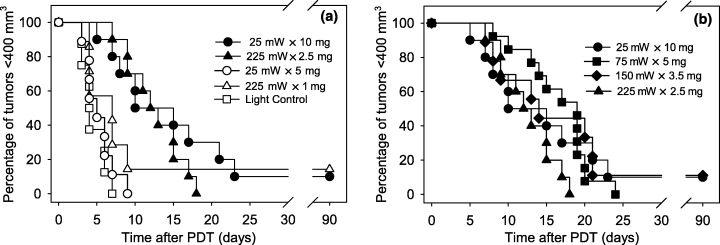
Kaplan–Meier curves of tumor responses to PDT with high vs low fluence rate (a) and at inverse fluence rate × photosensitizer dosing (b). Treatment conditions are abbreviated as fluence rate (mW) × photosensitizer dose (mg) for fluence rate units of mW cm−2 and photosensitizer dose units of mg kg−1. Illumination (630 nm) was for 30 min at ∼24 h after i.v. Photofrin injection; light controls received 225 mW cm−2 illumination, but no Photofrin injection. Solid symbols indicate regimens of inverse fluence rate × photosensitizer dosing, calculated based on fluence rate and photosensitizer dose range that produced an ∼nine-fold difference in delivered light intensity and tumor drug uptake, respectively. Open symbols indicate treatment regimens utilizing lower photosensitizer doses. n = 7–13 animals/condition.
Studies were expanded to include intermediate fluence rates and photosensitizer doses because similar tumor response was found for PDT at 25 mW × 10 mg and 225 mW × 2.5 mg. Based on the fact that 25 mW × 10 mg and 225 mW × 2.5 mg were both efficacious, a three-fold increase in fluence rate was paired with a two-fold decrease in photosensitizer dose. In this manner, intermediate conditions of 75 mW × 5 mg and 150 mW × 3.5 mg were designed. Kaplan–Meier curves (Fig. 1b) demonstrate that tumor responses at 75 mW × 5 mg and 150 mW × 3.5 mg were very similar to those found at the lowest fluence rate (25 mw × 10 mg) and highest fluence rate (225 mW × 2.5 mg) regimen. Among these four conditions median time-to-400 mm3 ranged from 12.5 to 19 days with 0–1 animal cured per treatment group (0–11% cure rates); no significant difference was detected between the condition with the longest median time-to-400 mm3 (75 mW × 5 mg) and either of the conditions with the shortest median time-to-400 mm3 (225 mW × 2.5 mg and 25 mW × 10 mg).
PDT effect on tumor hemoglobin oxygen saturation
The rationale behind pairing increasing fluence rate with decreasing photosensitizer dose was to facilitate maintenance of tumor oxygenation during high fluence rate PDT. The above tumor-response studies found high fluence rate × low photosensitizer dose pairs to be equally efficacious as low fluence rate × high photosensitizer dose in treatments of equivalent length. Next, we evaluated whether these isoeffective treatment regimens enabled conservation of tumor oxygenation during PDT.
In vivo optical spectroscopy was used to measure tumor hemoglobin oxygen saturation (SO2) immediately before and after PDT, and the effect of treatment on tumor oxygenation was reported as relative-SO2, which is the normalized change in SO2 (SO2 after PDT/SO2 before PDT) (Fig. 2). Thus, a relative-SO2 value of 1 indicates no change in oxygenation relative to baseline (pre-PDT) levels. The highest fluence rate regimen (225 mW × 2.5 mg) did lead to some oxygen depletion during illumination: median relative-SO2 (SE) was 0.86 ± 0.12, which was statistically different (P = 0.037) from a value of 1. At the lower fluence rate regimens of 150 mW × 3.5 mg, 75 mW × 5 mg and 25 mW × 10 mg, median relative-SO2 values were 1.12 ± 0.17, 1.26 ± 0.11 and 1.04 ± 0.21, respectively. These values suggest that hemoglobin oxygen saturation actually improved slightly at treatment conclusion, but only the 75 mW × 5 mg regimen was significantly (P = 0.040) greater than a value of 1. Animals that were cured by PDT both exhibited relative-SO2 values of ≥2 (see markers on the 150 mW × 3.5 mg and 25 mW × 10 mg plots). Two additional animals with relative-SO2 values of ≥2 demonstrated long times to tumor regrowth, 24 and 21 days after PDT at 75 mW × 5 mg and 25 mW × 10 mg, respectively.
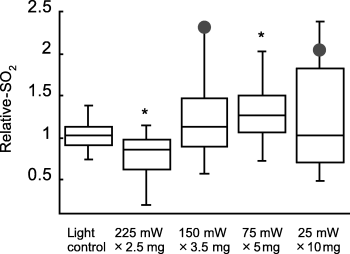
PDT effect on tumor hemoglobin oxygen saturation during treatment with inverse fluence rate × photosensitizer dosing (box plots). Relative-SO2 was measured as tumor SO2 after PDT normalized to the pre-PDT value in the same animal. Treatment conditions are abbreviated as fluence rate (mW) × photosensitizer dose (mg) for fluence rate units of mW cm−2 and photosensitizer dose units of mg kg−1. Illumination (630 nm) was for 30 min at ∼24 h after i.v. Photofrin injection; light controls received 225 mW cm−2 illumination, but no Photofrin injection. Markers indicate the relative-SO2 values of animals that were cured. n = 8–13 animals/condition; * indicates P < 0.05 (sign-rank test) for a difference from a ratio of 1.
Correlation between tumor response and PDT effect on hemoglobin oxygen saturation
We hypothesized that the therapeutic efficacy of inverse fluence rate × photosensitizer dosing was related to its effectiveness at maintaining tumor oxygenation during illumination. In order to test this hypothesis, we evaluated the association between response durability (time-to-400 mm3) and relative-SO2. Figure 3, panels a–d, plot time-to-400 mm3vs relative-SO2 for animals treated with 225 mW × 2.5 mg, 150 mW × 3.5 mg, 75 mW × 5 mg and 25 mW × 10 mg, respectively. Within all treatment regimens, a significant association (P < 0.048) was detected between response durability and the PDT-induced change in tumor hemoglobin oxygen saturation whereby increasing relative-SO2 predicted for a better tumor response. Furthermore, all four of these treatment regimens could be fit by a single model (Fig. 3e; P = 7.971 × 10−10).
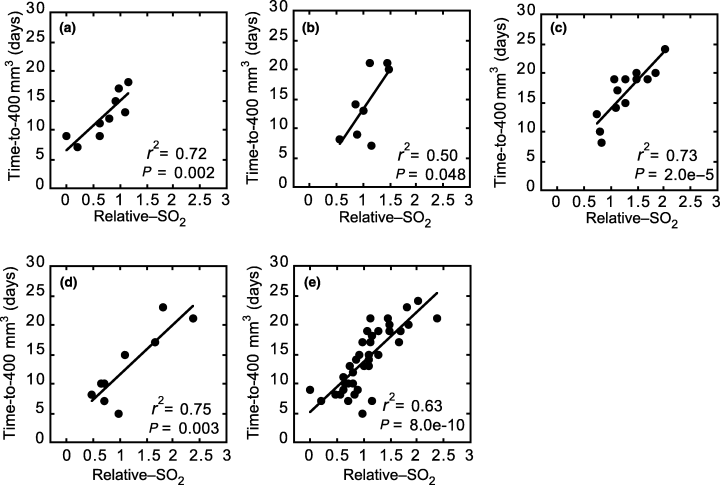
The association between tumor response and PDT effect on tumor hemoglobin oxygen saturation for treatment regimens of 225 mW cm−2, 2.5 mg kg−1 Photofrin (a); 150 mW cm−2, 3.5 mg kg−1 Photofrin (b); 75 mW cm−2, 5.0 mg kg−1 Photofrin (c); 25 mW cm−2, 10 mg kg−1 Photofrin (d); and all of the above (e). Tumor response was measured as the number of days after PDT until tumor regrowth to 400 mm3 (time-to-400 mm3). PDT effect on hemoglobin oxygen saturation was measured as tumor SO2 after PDT normalized to the pre-PDT value in the same animal (relative-SO2). Each data point indicates an individual animal. PDT illumination (630 nm) was for 30 min at ∼24 h after i.v. Photofrin injection. The correlation coefficient (r2) and P-value (by a Wald test) of a linear fit to the data is indicated for each plot.
Control animals receiving only illumination at 225 mW (no photosensitizer) demonstrated no association between relative-SO2 and time-to-400 mm3, which establishes that relative oxygen levels do not independently predict for tumor growth (data not shown). Therefore, as to be expected, we also found no association between relative-SO2 and time-to-400 mm3 for treatment regimens that failed to have any PDT effect (225 mW × 1 mg and 25 mW × 5 mg; data not shown).
Mechanisms of tumor damage
PDT-created tumor cell death at 25 mW × 10 mg vs 225 mW × 2.5 mg was studied using an in vivo/in vitro clonogenic assay. The data (Fig. 4) show PDT at 225 mW × 2.5 mg produced over one log (P < 0.021) of cell kill in tumors excised immediately after PDT, which suggests the presence of substantial direct singlet oxygen-mediated cell damage during illumination. In contrast, 25 mW × 10 mg led to only insignificant cell death immediately after PDT. However, cell kill gradually increased as a function of time after treatment conclusion, and a significant decrease in tumor clonogenicity was detectable by 17 h after 25 mW × 10 mg PDT. No differences in tumor clonogenicity were found among control conditions of untreated tumor, illumination only (no photosensitizer) at 225 mW cm−2, illumination only at 25 mW cm−2 and photosensitizer only (no illumination) at 10 mg kg−1 Photofrin.
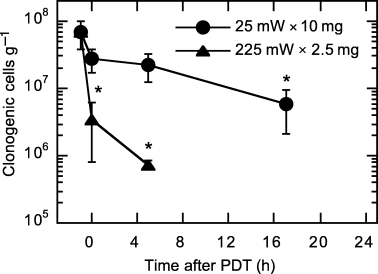
In vivo/in vitro clonogenic assay of tumor cell death after PDT at 25 mW cm−2, 10 mg kg−1 Photofrin (25 mW × 10 mg) and 225 mW cm−2, 2.5 mg kg−1 Photofrin (225 mW × 2.5 mg). Illumination (630 nm) was for 30 min at ∼24 h after i.v. Photofrin injection. Points indicate mean (±SE) clonogenic cells/g of tumor. Data point at −1 h represents an average of control conditions (untreated tumor, 10 mg kg−1 drug only, 25 mW cm−2 light only and 225 mW cm−2 light only). n = 3–5 animals/condition; * indicates P < 0.05 (rank-sum test) for a difference from control conditions.
In order to investigate how PDT effect on tumor blood flow may have contributed to differences in direct cell kill at 25 mW × 10 mg vs 225 mW × 2.5 mg, in vivo optical spectroscopy was used to measure THC in tumors prior to and immediately after PDT. During PDT at 25 mW × 10 mg, the median (±SE) change in THC among the individual tumors was a decrease of 28 ± 12 μM, from an average (±SE) pre-PDT value of 139 ± 18 μM to a post-PDT value of 114 ± 13 μM. This bordered on a significant change (P = 0.064) in hemoglobin concentration, suggesting the presence of PDT-created reductions in vascular perfusion during the 25 mW × 10 mg regimen. In contrast, minimal change in THC was found during PDT at 225 mW × 2.5 mg; a median (±SE) increase of 5 ± 8 μM was measured (pre-PDT value 122 ± 5 μM; post-PDT value 125 ± 9 μM).
Normal tissue toxicity to inverse fluence rate × photosensitizer dosing
Lastly, a toxicity assay was used to evaluate the normal tissue response to isoeffective regimens of inverse fluence rate × photosensitizer dose pairing. Figure 5 displays murine foot response to PDT at 25 mW × 10 mg and 225 mW × 2.5 mg. PDT at 25 mW × 10 mg clearly produced a strong normal tissue response, demonstrated by a maximum score (median ± SE) of 1.65 ± 0.07. A median (±SE) of 10 ± 2 days was needed for complete resolution of tissue damage after PDT at 25 mW × 10 mg. In contrast, 225 mW × 2.5 mg resulted in a maximum score (median ± SE) of 0.5 ± 0.13 and a median (±SE) of 4 ± 0.6 days to resolution. The difference in foot response to these regimens was significant (P < 0.0006) in terms of both the peak and duration of response. No response was observed in control feet exposed to only photosensitizer (10 mg kg−1 Photofrin), only light (225 mW cm−2) or untreated.
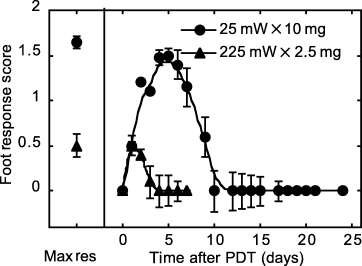
Normal tissue toxicity to PDT at 25 mW cm−2, 10 mg kg−1 Photofrin (25 mW × 10 mg) and 225 mW cm−2, 2.5 mg kg−1 Photofrin (225 mW × 2.5 mg). Illumination (630 nm) was for 30 min at ∼24 h after i.v. Photofrin injection. Foot-response scores are described in Table 1 of the Materials and Methods. Maximum response (Max res) indicates the median (±SE) of the highest score observed in each animal regardless of the day on which this response occurred. Line plots depict median (±SE) score as a function of day after PDT; the 0 day reading was a baseline observation before PDT. n = 10 animals/condition.
Although normal tissue toxicity between the extreme isoeffective regimens was strikingly different, an intermediate isoeffective regimen (75 mW × 5 mg) did not result in intermediate toxicity. Rather, at 75 mW × 5 mg normal tissue toxicity was reduced dramatically compared with toxicity at 25 mW × 10 mg. The median response at 75 mW × 5 mg (maximum score 0.45; 4 days to resolution) was indistinguishable from response to 225 mW × 2.5 mg.
Discussion
The data of this report demonstrate that high fluence rate PDT can be carried out under oxygen-conserving conditions by combining the high rate with low photosensitizer dose. Although complete preservation of pre-PDT oxygen levels was not achieved at the highest fluence rate tested, significant direct cell kill occurred in the absence of substantial normal tissue toxicity and tumor response was as durable as that found for the more oxygen-conserving regimens. The relevance of tumor oxygen levels to the high fluence rate × low photosensitizer dose regimens was confirmed by the presence of highly significant associations between PDT effect on tumor hemoglobin oxygen saturation (relative-SO2) and response durability (time-to-400 mm3).
High fluence rate PDT offers the clinical advantage of shorter illumination times, thus these data are of direct clinical relevance. The drug doses needed for oxygen-conserving yet efficacious high fluence rate PDT (e.g. 2.5–3.5 mg kg−1) are no lower than those already commonly applied in the clinic. These findings emphasize the need for measurement of tissue oxygenation and photosensitizer levels during clinical applications of PDT. In studies performed to date, highly variable levels of PDT-created hypoxia have been detected both within and among patients receiving identical PDT regimens (18,31,32). Intertissue variability in photosensitizer levels has been suggested as an explanation for these findings (31), but no attempts to correlate photosensitizer levels and PDT oxygen depletion in the same tumor sample have been made. We intend to study these variables in our upcoming clinical trials.
These data also demonstrate that time-conserving PDT can be carried out at low fluence rate by increasing the photosensitizer dose. In other words, higher photosensitizer dose facilitated the use of low fluence rate without a need to increase treatment time. Although some models have suggested that it may be possible to utilize oxygen-conserving low fluence rates without extension of treatment time (33), in preclinical applications longer treatment times are required at low fluence rate to compensate for the slower rate of light delivery compared with high fluence rate (6). Unfortunately, our data show that tumor response gained by increasing photosensitizer dose at low fluence rate came at a cost of greater damage to normal tissue. Therefore, this approach to controlling treatment time at low fluence rate is not clinically favorable.
Although therapeutic efficacy was similar at the high and low fluence rates we studied, these regimens evoked different mechanisms of action. During PDT at 25 mW × 10 mg direct cell kill was limited, as indicated by small decreases in tumor cell clonogenicity immediately after PDT. Ultimately, indirect mechanisms of cell kill, potentially including vascular, immune or other host effects were sufficient to create a durable tumor response. Indeed, a reduction in THC at the conclusion of PDT suggests that tumor perfusion decreased during PDT and the presence of a significant decrease in tumor clonogenicity by 17 h after PDT suggests that indirect PDT effects ultimately impact treatment response. However, substantial normal tissue toxicity was also created by 25 mW × 10 mg PDT, most likely as a result of a prominent vascular component to this treatment. Reducing the photosensitizer dose to 5 mg kg−1 at 25 mW cm−2 enabled better maintenance of THC at treatment conclusion (data not shown), but it also rendered the treatment ineffective, with a response durability similar to control response. Together these data suggest that vascular effects, which are likely a consequence of the high photosensitizer dose, are an essential component of the response to low fluence rate × high photosensitizer regimens.
Vascular effects during 25 mW × 10 mg PDT are expected to have limited direct cell kill. However, tumor hemoglobin oxygen saturation was not depleted during PDT (relative-SO2 > 1). Thus, although total tumor blood flow decreased during illumination, those vessels with flow remained well oxygenated. It is possible for vascular perfusion to decrease in the absence of an effect on SO2 because SO2 reflects the oxygenation status of vessels with blood flow without direct consideration of any change in the number of vessels with flow.
During PDT at 225 mW × 2.5 mg, a significant amount of direct tumor cell kill occurred. This was likely aided by preservation of blood flow during treatment as no PDT effect on THC was detected with this treatment protocol. A minor but statistically significant decrease in relative-SO2 occurred during 225 mW × 2.5 mg PDT. The depletion of tumor hemoglobin oxygen saturation suggests that some photochemical consumption of oxygen occurred during treatment. Nevertheless, SO2 was maintained at ∼85% of its baseline value, and the extent of consumption did not significantly alter response durability compared with other effective treatment protocols, nor did it prevent the occurrence of significant direct cell kill. When photosensitizer dose was lowered to 1.0 mg kg−1 tumor response was significantly abrogated, suggesting limitations in tumor drug levels.
This investigation is unique from other investigations of fluence rate effects because we used constant treatment time and varied photosensitizer and light dose, whereas others have typically used a constant photosensitizer and light dose and varied treatment time. Due to these differences in study design, we have found a different spectrum of fluence rate-dependent tissue responses than those previously reported. Our use of a high drug dose led to acute vascular effects and limited direct cell kill for our low fluence rate regimen. In published studies, low fluence rate is associated with substantial direct cell kill and delayed vascular effects (2,34–36). This suggests that increasing the photosensitizer dose may have altered the time frame of vascular damage development. When we used a low drug dose in combination with high fluence rate, minimal oxygen depletion, significant direct cell kill and a durable tumor response were found. In published studies, high fluence rate is associated with limited cell kill and abrogated tumor response (2,4,13). Therefore, decreasing the photosensitizer dose at high fluence rate may increase the contribution of direct cytotoxicity to tumor response.
PDT effect on tumor oxygenation was studied as a function of changes in tumor hemoglobin oxygen saturation (SO2). Higher SO2 at the conclusion of PDT predicted for a more durable tumor response; in fact all animals with relative-SO2 values ≥2 (n = 5) required at least 21 days for tumor regrowth to 400 mm3 (n = 2) or were cured (n = 3). This includes the one animal that was cured after PDT at 225 mW × 1 mg (SO2 data not shown). On average, only the 75 mW × 5 mg condition led to a significant increase in relative-SO2, which was likely a result of decreases in metabolic oxygen consumption as some direct cytotoxicity (data not shown) was found with this treatment. However, 225 mW × 2.5 mg was associated with both significant direct cytotoxicity and a modest decrease in relative-SO2. In this case, the decrease in SO2 could be attributed to photochemical oxygen consumption, which was likely attenuated by an increase in SO2 due to reduced metabolic oxygen consumption. Therefore, change in SO2 during PDT appears to represent a balance between increases due to cell death (reduced metabolic oxygen consumption) and decreases due to photochemical oxygen consumption.
Vascular effects can also be expected to play some part in determining SO2, but it is important to reiterate that SO2 reflects the oxygenation status of hemoglobin, so constricted vessels void of hemoglobin will not contribute to the measured signal. In other words, regional hypoxia created by the local shutdown of vessels during PDT will not be reflected by SO2 measurement unless vascular shutdown is extensive enough to lead to greater tumor-averaged extraction of O2 from the hemoglobin of perfused blood vessels. Therefore, PDT-induced changes in blood flow can occur independent of a corresponding effect on SO2 (32), which explains why 25 mW × 10 mg PDT demonstrated decreases in vascular perfusion and limitations in direct cell kill, but no affect on SO2.
The change in tumor SO2 during identical PDT conditions was variable, despite the fact that tumors from the same cell solution were implanted on inbred animals. This finding can perhaps be explained by intertumor heterogeneity in Photofrin uptake. Drug uptake could vary substantially among tumors, especially those exposed to the higher photosensitizer doses. The standard error in drug uptake was 3.7 ng mg−1 among mice receiving an injected dose of 10 mg kg−1 (average drug uptake 13 ng mg−1), whereas the standard error was 0.08 ng mg−1 among mice receiving an injected dose of 2.5 mg kg−1 (average drug uptake 1.3 ng mg−1). At the lower drug doses especially, photobleaching during PDT could further reduce intertumor variability in drug levels or even lead to complete drug depletion. Less variability in SO2 response during PDT was found for regimens using lower photosensitizer doses (compare panels a and d of Fig. 3), which suggests that SO2 response and intra- and/or intertumor variability in photosensitizer levels are related. A dedicated investigation of Photofrin uptake and photobleaching will be needed to confirm these expectations.
The highly significant correlations between relative-SO2 and response durability (time-to-400 mm3) as found in these studies are a valuable addition to previous publications by ourselves (17) and others (37), which identify PDT effect on tumor oxygenation as an individualized predictor of treatment outcome. In the present manuscript, we demonstrate that the PDT-induced change in hemoglobin oxygen saturation is predictive of tumor response over a range of Photofrin-PDT conditions, including those that create damage through different mechanisms of action. Data from all response-producing protocols were well fit by a single model relating relative-SO2 and response durability. This suggests that the utility of SO2 monitoring is not protocol specific among regimens of Photofrin-PDT. Not surprisingly, however, the data also show that SO2 monitoring is of little value for ineffectual PDT protocols: if the photosensitizer and illumination conditions are insufficient to elicit a PDT response then optimization of the physiological environment is to no avail, albeit one would also expect to no harm. Therefore, these results support continued development of SO2 monitoring during Photofrin-PDT as a method of real time dosimetry with the potential to individualize patient treatment toward the goals of reduced morbidity and increased efficacy. Such techniques are currently being pursued in the clinical setting by several groups, including ourselves (32,38–41).
In summary, this report presents the results of a unique investigation that identifies how manipulation of photosensitizer dose in combination with fluence rate can be used to control oxygen depletion and enhance therapeutic efficacy. Among the inverse fluence rate × photosensitizer dose pairs studied, we have found high fluence rate in combination with low photosensitizer dose to provide efficacious tumor response in the absence of significant normal tissue toxicity. Such a regimen is readily adaptable to the clinic where low drug dose and high fluence rate are generally considered desirable for the sake of reduced skin photosensitivity and more rapid treatment, respectively. The importance of oxygen maintenance to the success of inverse fluence rate × photosensitizer dose pairs, including high fluence rate × low photosensitizer dose, was confirmed by its highly significant correlation to tumor response. Therefore, the translation of these results to clinical applications is readily feasible through use of oxygen and photosensitizer monitoring technologies, which are under intense development.
Acknowledgments
Acknowledgements— This work was supported by NIH grants RO1 CA-85831 and PO1 CA-87971. We thankfully acknowledge Carmen Rodriquez for technical assistance with laser maintenance.




