Multiple Components of Photodynamic Therapy Can Phosphorylate Akt†
This invited paper is part of the Symposium-in-Print: Photodynamic Therapy.
Abstract
A growing number of clinically relevant molecular and cellular responses are observed following photodynamic therapy (PDT). PDT-mediated oxidative stress and PDT-induced tissue hypoxia can elicit the transcriptional and/or translational expression of genes associated with cellular stress, inflammation, angiogenesis, immuno-modulation, apoptosis and signal transduction. One of the signaling molecules activated by oxidative stress is Akt/protein kinase B. Phosphorylation of Akt/protein kinase B activates this signaling molecule and induces a survival response in effected cells and tissue. We hypothesized that PDT using Photofrin (PH) as the photosensitizer could also induce increased levels of Akt phosphorylation. Results from our initial set of experiments demonstrated that in vitro and in vivo PDT treatments induced Akt phosphorylation. Interestingly, incubation of mouse and human breast cancer cells with the porphyrin-based photosensitizer, PH, increased the expression of Akt phosphorylation in the absence of light. Exposure of the corresponding mouse and human-derived breast cancer tumors growing in mice to 630 nm light in the absence of PH administration also induced Akt phosphorylation. These results demonstrate that individual components of the PDT process, photosensitizer alone and light alone, as well as the complete PDT procedure can activate the Akt signaling pathway.
Introduction
Therapeutic applications of photodynamic therapy (PDT) in cancer treatment remain encouraging but improvement of long-term PDT responsiveness is needed to decrease tumor recurrence (1). Preclinical investigations show that PDT can induce expression and/or activation of pro-angiogenic molecules including vascular endothelial growth factor, cyclooxygenase-2 and prostaglandins, TNF-α, matrix metalloproteinases, integrins, IL-6 and IL-8 within the tumor microenvironment (2–8). Overexpression of these molecules is often associated with a survival phenotype and recent studies show that targeted therapy directed at these molecules can enhance the therapeutic effectiveness of PDT (9).
Akt/protein kinase B is a signaling molecule that plays a key role in integrating cellular responses to growth factors and extracellular signals (10). The Akt pathway plays a major role in tumor development as well as in the responsiveness of tumor cells to treatment (11). Phosphorylation of Akt activates this molecule and can provide cells with a survival signal that protects them from apoptotic stimuli. Numerous studies have shown that oxidative stress stimulates Akt phosphorylation and proteins activated by phosphorylated Akt promote cell survival (12,13). Singlet oxygen generated by rose bengal-mediated photosensitization elicited Akt phosphorylation in mouse NIH 3T3 fibroblasts (12). Additionally, inhibition of phosphatidylinositol-3-OH kinase (PI3-K) attenuated Akt phosphorylation and resulted in enhanced cell death.
In the current study we examined whether PH-mediated PDT phosphorylates Akt in mouse and human breast cancer cells cultured in vitro and growing as solid tumors in mice. As expected, we found that PH-mediated PDT induced strong Akt phosphorylation and that PI3-K inhibitors blocked this response. However, we also observed that PH alone, without any light exposure, induced measurable levels of Akt phosphorylation in cancer cells exposed to the photosensitizer in culture and that 630 nm light alone induced Akt phosphorylation in transplanted tumors growing in mice. These results indicate that individual components of the PDT process and not just PDT can play a role in activating the Akt pathway. The therapeutic relevance of these observations will require additional examination.
Materials and methods
Drugs and reagents. The photosensitizer PH was a gift from Axcan Scandipharm Inc. (Birmingham, AL) and was dissolved in 5% dextrose to make a 2.5 mg mL−1 stock solution. Cell lysis buffer was purchased from Cell Signaling Tech. (Beverly, MA). Wortmannin was purchased from Calbiochem (San Diego, CA) and dissolved in DMSO to make a stock solution of 1.0 mM. LY294002 was purchased from Sigma (St. Louis, MO) and dissolved in DMSO to make a stock solution of 29 mM.
Cell culture and tumor models. Mouse breast cancer (BA) and human breast cancer (BT-474) cells were grown as monolayers in DMEM culture medium supplemented with 10% fetal calf serum (FCS), 0.5%l-glutamine, and 1% penicillin/streptomycin. BA tumors were generated by subcutaneous trochar injection of 1 mm3 pieces of tumor into the hind flank of 8- to 10-week-old female C3H/HeJ mice. BT-474 tumors were generated by injecting 8 × 106 cells within 100 μL matrigel matrix (BD Biosciences, San Jose, CA) to the hind flank of 10- to 12-week-old female athymic mice.
In vitro and in vivo treatment protocols. For in vitro treatments, cells were seeded in Petri dishes and incubated for 24 h in complete growth medium. Cells were incubated in the dark at 37°C for 16 h in DMEM plus 5% FCS containing PH (25 μg mL−1) with or without Wortmannin (1 μM) or LY294002 (100 μM) and then exposed to broad-spectrum red light (570–650 nm, 0.35 mW cm−2). Additional treatment conditions for cells included controls (no treatment), PH alone (no light) and light alone (no photosensitizer). In vivo tumor treatments of lesions measuring 6–7 mm in largest diameter involved an i.v. injection of PH (5 mg kg−1) followed 24 h later with tumor irradiation using a 630 nm diode laser (Diomed Inc., Andover, MA). Light was delivered via a quartz fiber microlens (5). A light dose rate of 75 mW cm−2 and the total light dose 100 J cm−2 was used to treat tumors.
Western immunoblot analysis. Protein expression was documented by Western immunoblot analysis (4). Cells and tumor tissues were collected at designated times after treatment and sonicated in 1X cell lysis buffer from Cell Signaling Tech. Protein samples were size-separated on 10% polyacrylamide gels from Invitrogen Life Tech. (Carlsbad, CA) and transferred to nitrocellulose membranes. Filters were blocked with 5% nonfat milk for 1 h at room temperature and then incubated overnight with rabbit polyclonal antibody to phospho-Akt (Ser473) from Cell Signaling Tech. for 1 h with goat polyclonal antibody to Akt (Akt1/2, N-19) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), or for 1 h with mouse monoclonal anti-actin antibody (clone C-4) from MP Biomedicals, Inc., Aurora, OH. Filters were then incubated with either anti-rabbit or anti-mouse peroxidase conjugate obtained from Sigma Aldrich Inc., St. Louis, MO or anti-goat peroxidase conjugate from Santa Cruz Biotechnology, Inc. The resulting complexes were visualized by enhanced chemiluminescence autoradiography from Amersham Life Sci. (Chicago, IL).
Detection of apoptosis. Cellular apoptosis was evaluated using a Cell Death Detection ELISA Plus kit (Boehringer Mannheim, Indianapolis, IN) as previously reported (14). This assay quantifies mono- and oligo-nucleosomes from cell lysates using monoclonal antibodies targeting DNA and histones in a quantitative photometric sandwich enzyme immunoassay. Cells were analyzed 6 h after treatment. Apoptotic enrichment factors were calculated from absorbance ratios of treated versus control cells. Results were normalized for protein concentrations.
Statistics. Statistical analysis was performed using a two-tailed Student’s t-test to determine statistical differences for apoptosis indexes and for phospho-Akt densitometry. Differences with P < 0.05 were regarded as significant.
Results and discussion
A variety of cytotoxic and survival responses are observed following PDT (1). Oxidative stress, associated with the Type II photochemical generation of singlet oxygen, and hypoxia, generated by the photochemical depletion of oxygen and by direct vascular disruption, are effective at initiating numerous molecular responses including the activation of Akt (12,15). Akt is a target of PI3-K and reactive oxygen species as well as hypoxia can activate the Akt pathway and lead to increased cell survival (12,13,16). Activation of Akt requires phosphorylation of two conserved residues, Thr-308 and Ser-473 (10). When Akt is phosphorylated it relocalizes within the cell and phosphorylates proteins involved in anti-apoptotic signaling, including Forkhead transcription factors, BAD, and MDM2. Akt increases cell survival in a PI3-K-dependent manner and efforts to target or deregulate Akt are being pursued as means of enhancing conventional chemotherapy and radiation therapy (11). During studies designed to examine the possible biologic roles of Akt in PH-mediated PDT we observed that individual components of the PDT procedure were able to induce Akt phosphorylation in cancer cells and tumors. This report describes our findings that in vitro exposure of cells to the porphyrin-based photosensitizer in the absence of light and in vivo exposure of tumors to light alone could induce Akt phosphorylation.
In our first set of in vitro experiments we observed: (1) treatment of murine BA breast cancer cells and human BT breast cancer cells with PH-mediated PDT induced phosphorylation of Akt, (2) PH in the absence of light also induced Akt phosphorylation and (3) these actions could be attenuated with PI3-K inhibitors. Figure 1a,b shows the protein expression profiles for phospho-Akt, total Akt and actin in murine BA cells and human BT cells. Akt phosphorylation was examined at 2 and 6 h after PDT or after PH incubation. Two inhibitors of PI3-K, Wortmannin and LY294002, attenuated Akt-induced phosphorylation indicating that PDT and PH were acting through the PI3-K signaling pathway. These results agree with recent studies demonstrating that both the heme molecule and an extended porphyrin, Motexafin gadolinium, induce Akt phosphorylation (17,18). Interestingly, PH incubation, in the absence of light, also induces expression of HIF-1α as demonstrated by increased GFP levels in cells containing a GFP expression construct controlled by five copies of the hypoxic response element (19). In some studies Akt signaling can activate HIF-1 and therefore the increase in GFP expression may involve increased Akt levels following PH incubation (20). Figure 2 shows that exposure of the murine and human breast cancer cells to red light in the absence of exogenous photosensitizer did not induce Akt phosphorylation above control levels.
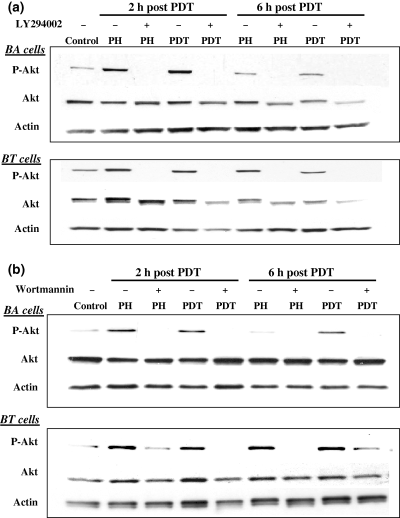
Photofrin-mediated PDT and PH alone induce Akt phosphorylation in murine and human breast cancer cells. Murine BA and human BT cells were incubated with PH (25 μg mL−1) along with or without LY294002 (100 μM) (a) or Wortmannin (1 μM) (b) for 16 h in the dark and then exposed to 315 J m−2 of broad-spectrum red light. Cells were then incubated in the dark for an additional 2 or 6 h in the presence or absence of LY294002 (100 μM) (a) or Wortmannin (1 μM) (b). Cell lysates from control, PH alone and PH-PDT cultures were collected and assayed for phospho-Akt, total Akt and actin. Experiments were performed three times. Results from one experiment are shown.
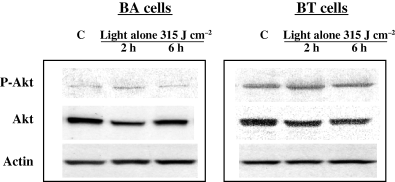
Exposure of murine or human breast cancer cells to red light alone does not increase Akt phosphorylation. Murine BA and human BT cells were incubated in the dark for 16 h, exposed to red light (315 J m−2) and then incubated in the dark for an additional 2 or 6 h. Cell lysates from control and light-treated cultures were collected and assayed for phospho-Akt, total Akt and actin. Experiments were performed three times. Results from one experiment are shown.
A major function of activated Akt is to promote cell survival and to block apoptosis (11). Phosphorylation of the pro-apoptotic effector molecule BAD by activated Akt decreases the ability of BAD to induce apoptosis. Likewise, caspase-9 acts as an initiator and an effecter of apoptosis and activated Akt can phosphorylate caspase-9 and attenuate the apoptotic response in human cells. We observed a role for PI3-K inhibitors in modulating cellular apoptosis following PH-mediated PDT. Figure 3a,b shows that Wortmannin or LY294002 induced increased levels of apoptosis in PDT-treated BA and BT cells while Fig. 1a,b shows that these inhibitors block Akt phosphorylation. The observed apoptosis involves direct toxicity by the inhibitors and could include a role for phospho-Akt. Additional experiments are needed to confirm a direct effect of Akt in modulating PDT response. Our results agree with a previous study showing Akt phosphorylation following exposure of mouse fibroblasts to singlet oxygen generated by rose bengal-mediated photosensitization (12). This study also reported that inhibition of Akt activation using Wortmannin and LY294002 resulted in increased cellular apoptosis. Interestingly, these PI3-K inhibitors can block autophagy in cells undergoing photosensitization. The increase in apoptosis observed in our study may also involve blockage of the pro-survival component of autophagy (21). Continued examination of methods to selectively attenuate Akt phosphorylation may prove useful in improving PDT responsiveness by enhancing treatment-related apoptosis.
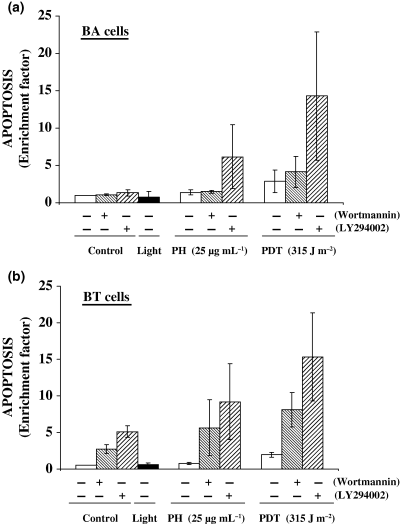
PI3-K inhibitors increase apoptosis in PDT-treated murine BA and human BT breast cancer cells. (a) BA cells and (b) BT cells were treated with PH-mediated PDT in the presence or absence of Wortmannin or LY294002 as described in Fig. 1. Apoptosis was measured as an enrichment factor 6 h following treatment using the Cell Death Apoptosis Detection ELISA Plus kit. Results are expressed as the mean ± SE of three separate experiments.
We next examined the in vivo effects of PH-mediated PDT on Akt phosphorylation using tumors generated from the same cell lines described above for in vitro experiments. Figure 4a,b shows Western immunoblots and the corresponding densitometry readings for murine BA tumors exposed to PDT, light alone, or untreated controls. Both PDT and light exposure induced phosphorylated Akt levels above background. Figure 5a,b shows Western immunoblots and densitometric readings for murine BA tumors exposed to PH alone, light alone or untreated controls. In these experiments we did not observe increased Akt phosphorylation in tumors receiving PH alone. The intracellular levels of PH are much higher for the in vitro experiments and this may be why Akt phosphorylation was observed following PH alone in cells but not in tumors (22). Figure 6a,b shows Western immunoblots and corresponding densitometry readings for human BT tumors and shows increased Akt phosphorylation following PH-mediated PDT. Administration of the photosensitizer PH without subsequent light exposure did not result in increased Akt phosphorylation while exposure of BT tumors to light alone resulted in a small increase in Akt phosphorylation over control levels.
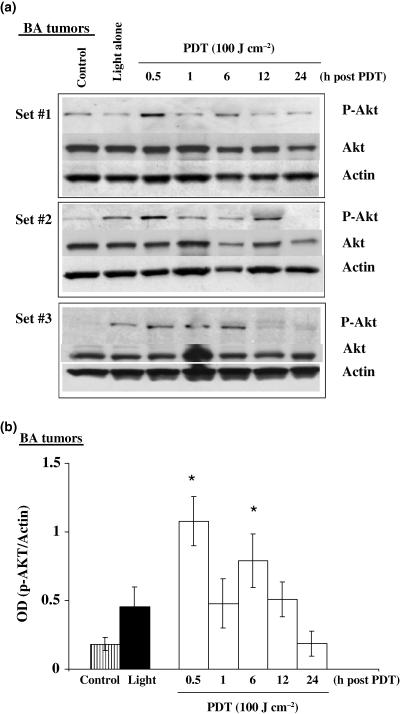
Photofrin-mediated PDT and light alone induce Akt phosphorylation in murine BA breast cancer tumors. C3H/HeJ female mice with subcutaneously transplanted BA tumors were injected with PH (5 mg kg−1) and then 24 h later the tumors were exposed to a 100 J cm−2 dose of 630 nm light. Additional tumor-bearing mice not injected with PH were also exposed to light alone. Tumor lysates were collected at various time intervals (0.5–24 h) after treatment and assayed for phospho-Akt, total Akt and actin. (a) Western immunoblot analysis for three separate experiments and (b) densitometric readings obtained from the immunoblots. *The increases in phospho-Akt above control were statistically significant (P < 0.05) at 0.5 and 6 h after PDT (n = 3).
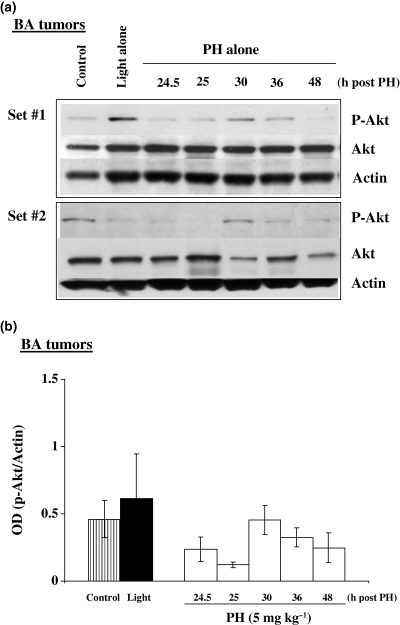
Photofrin alone does not induce Akt phosphorylation in murine BA breast cancer tumors. C3H/HeJ mice with subcutaneously transplanted BA tumors were injected with PH (5 mg kg−1) and then kept in the dark until assayed for protein expression. Tumor lysates were collected at various time intervals (24.5–48 h) after PH injection and assayed for phospho-Akt, total Akt and actin. (a) Western immunoblot analysis for two separate experiments and (b) densitometric readings obtained from the immunoblots.
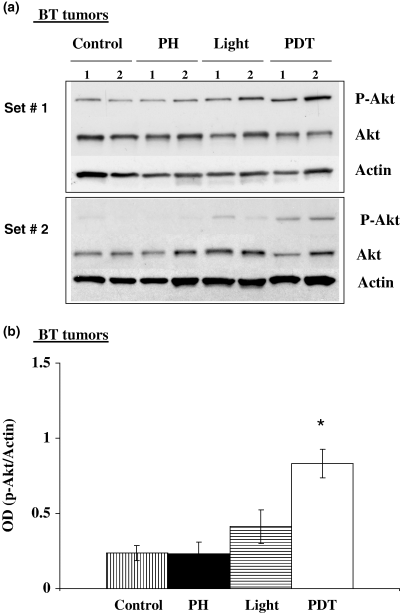
Photofrin-mediated PDT and light alone induce Akt phosphorylation in human BT breast cancer tumors. Athymic mice with subcutaneously transplanted BT tumors were injected with PH (5 mg kg−1) and then 24 h later the tumors were exposed to a 100 J cm−2 dose of 630 nm light. Additional mice were exposed to light alone or kept in the dark following PH administration. Tumor lysates were collected 1 h after treatment and assayed for phospho-Akt, total Akt and actin. (a) Western immunoblot analysis for two separate experiments and (b) densitometric readings obtained from the immunoblots. *The increase in p-Akt above control was statistically significant (P < 0.05) for PDT (n = 4).
In summary, we show that PH-mediated PDT induces Akt phosphorylation in murine and human breast cancer cells and tumors. These results are expected as previous reports have clearly shown that reactive oxygen species, including singlet oxygen, can phosphorylate and activate Akt (12,13). In addition, we demonstrated that blockage of Akt phosphorylation using PI3-K inhibitors can increase PDT-mediated apoptosis in treated cells and this also agrees with previous work (12). The observations of Akt phosphorylation following incubation of breast cancer cells with PH in the absence of light and following treatment of experimental breast cancer tumors with light alone suggest that individual components of PDT can activate signaling pathways. It is currently unclear exactly why PH alone or light alone can induce Akt phosphorylation. A variety of chromophores, including porphyrin-based molecules, are found within cells and these molecules may interact with light to elicit the photochemical generation of oxidative stress. The in vivo light dose was higher than the in vitro light dose and this could contribute to the observed increase in Akt phosphorylation in tumors treated with light alone but not in cells. In conclusion, the observation that PH-mediated PDT induces Akt phosphorylation and that inhibition of this process can increase apoptosis suggests that targeting the Akt pathway may improve PDT responsiveness and warrants additional investigation.
Acknowledgments
Acknowledgements— Grant support: NIH grants RO1-CA31230, RO1-CA98971, R21-CA119907, the Neil Bogart Memorial Fund of the Martell Foundation for Leukemia, Cancer and AIDS Research, and the Las Madrinas Endowment for Experimental Therapeutics.




