Ex Vivo Fluorescence Imaging of Normal and Malignant Urothelial Cells to Enhance Early Diagnosis
ABSTRACT
Urinary cytology is a noninvasive and unconstraining technique for urothelial cancer diagnosis but lacks sensitivity for detecting low-grade lesions. In this study, the fluorescence properties of classical Papanicolaou-stained urothelial cytological slides from patients or from cell lines were monitored to investigate metabolic changes in normal and tumoral cells. Time- and spectrally-resolved fluorescence imaging was performed at the single cell level to assess the spectral and temporal properties as well as the spatial distribution of the fluorescence emitted by urothelial cells. The results reveal quite different fluorescence distributions between tumoral urothelial cells, characterized by a perimembrane fluorescence localization, and the normal cells which exhibit an intracellular fluorescence. This is not caused by differences in the fluorescence emission of the endogenous fluorophores NAD(P)H, flavoproteins or porphyrins but by various localization of the EA 50 Papanicolaou stain as revealed by both the spectral and time-resolved parameters. The present results demonstrate that the use of single-cell endofluorescence emission of Papanicolaou-stained urothelial cytological slides can allow an early ex vivo diagnosis of low-grade bladder cancers.
Introduction
A major goal for enhancing early cancer diagnosis and treatment has involved the development of new methods to identify cancer signatures (1–3). In this context, many efforts were made to improve the early detection of bladder cancer (4). Over the last decade, many promising applications for fluorescence or autofluorescence spectroscopy have been expanded by physicists namely in the field of endoscopy and in the development and analysis of fluorescence imaging techniques (1,5–7).
Besides these clinical trials, only a few new approaches are systematically used for diagnosis and the current standard of diagnosis and follow-up consists of cystoscopy and essentially urine cytology (UC) every 3–4 months and transuretral resection of the bladder when a doubt is revealed. These approaches are costly, invasive and uncomfortable for the patients. In addition, especially for the low-grade lesions, UC is of limited value because of operator dependency and the low sensitivity (only 50% of sensitivity of UC for the highly differentiated transitional cell carcinoma) (8,9). For these reasons, many new urine-based tests for urothelial cell carcinoma detection have been developed. Among them, several techniques propose to research the specific bladder tumor antigen (BTA) using BTA-test, BTAstat or BTAtrak-test (10,11), to evaluate the nuclear matrix protein 22 as a tumoral marker: NMP-22 test (12,13), to assess the fibrin-fibrinogen degradation products: FDP-test (14), to use the ImmunoCyt-test as a fluorescence immunocytology method (15) or the immunocytology test for the Lewis X antigen and for 486p3/12 detection (16). In contrast to these protein based tests, the use of fluorescence in situ hybridization with centromeric probes was developed to detect chromosomal numerical aberrations on several chromosomes (chromosome 3, 7, 17, and 9p21) in the urine sediments of patients presenting urothelial carcinoma (UroVysion) (17–20). All these methods need meticulous and specific techniques and are often time-consuming in addition to their high price. Another problem is also the low sensitivity of these tests for low-grade tumoral lesions (15).
In parallel, other approaches were developed combining the principles of photodynamic diagnosis and urinary cytology. These methods used intravesical instillation of either 5-aminolevulinic acid (ALA) or hypericin before obtaining urinary cytology specimens on which the induced fluorescence of urothelial cells is detected by intensity microscopy imaging (5–7,21,22). ALA-induced fluorescence diagnosis is based on the fact that ALA is a precursor of heme biosynthesis and produces fluorescent endogenous porphyrins, mainly protoporphyrin IX (PPIX) which accumulates in tumors. Hypericin is a natural red-fluorescent pigment from the polycyclic aromatic quinone family, which selectively accumulates in urothelial carcinoma cells. The efficiency of these diagnosis and therapeutic methods was proved (86% for all different tumor lesions) (22), especially for highly differentiated and carcinoma in situ (efficiency of 82% for pTaG1 lesions or carcinoma in situ marked by ALA [22], and 94% for those marked by hypericin [5]). Although the effectiveness, reproducibility and usefulness of this method were shown, it remains invasive, traumatizing and constraining because of the drug injection.
The objective of this work was to access an original fluorescence screening method of urothelial cytological slides without changing either the sampling of the urinary specimens or the Papanicolaou-staining protocol, in order to allow a most robust diagnosis for pathologists in critical cases such as low-grade carcinoma.
To this aim, we have systematically characterized the differences in the fluorescence properties of normal and tumoral urinary cytologies. These included not only the high-grade and low-grade carcinoma cases from patients but also the T-24 and HUC-1 cell lines which are carcinogenic and normal reference cell lines, respectively. Fluorescence signatures from each cell type, in terms of intensity, spectral and time resolution were measured over the 400–800 nm spectral range to characterize the fluorescence of endogenous fluorophores, NAD(P)H, flavoproteins (FP) and porphyrins, and Papanicolaou stains. The fluorescence spectrum was investigated under one-photon excitation (1P) while two-photon excitation (2P) was used for the time-resolved analysis which combines the advantages of limiting photodamage when long-time acquisitions are required with the simplicity of only one excitation wavelength for all the fluorophores. A prerequisite for this investigation was the detailed study of the photophysical properties of the different dyes used for the Papanicolaou staining which is reported here for the first time.
Materials and methods
Preparation of cytological slides. Urinary cytological slides coming from patients were prepared as follows: urination urines were gathered in a sterile container. To 30 mL of urine, 4 mL formol 4% was added and this sample was transferred to the anatomo-pathological department for cytospinning and staining using the classical Papanicolaou technique (23). In this method, three stain mixtures are used for cell staining with different localization: Harris hematoxylin, specific of cell nucleus staining, orange G6 (OG 6) which interacts with cytoplasmic keratin and EA 50, a trichromatic stain containing light green, yellow eosin and Bismarck brown with nonspecific localization. For each slide, 10 to 25 cells were analyzed.
Cell line culture. In order to study unstained urinary cytological slides, urothelial cells were prepared from a human urinary bladder carcinoma T-24 cell line or from a normal human ureter uroepithelium SV40-immortalized cell line (HUC-1) provided by the American Type Culture Collection (Rockville, MD) and respectively used as carcinogenic cell or normal cell references. The T-24 cells were maintained in 15 mL (75 cm²) Falcon flasks (Becton Dickinson, Franklin Lakes, NJ) in Mac Coy 5A’s medium supplemented with 10% fetal calf serum, 100 U.mL−1 penicillin, 100 μg.mL−1 streptomycin, and 2 mM L-glutamine (all from Gibco/BRL Life Technologies, Grand Island, NY) at 37°C with 5% CO2. The HUC-1 cells grew in Ham’s F12 K medium with 2 mM L-glutamine adjusted to contain 1.5 gL−1 sodium bicarbonate and 10% fetal calf serum (all from Gibco/BRL Life Technologies) at 37°C with 5% CO2. The cells were re-seeded every 2–3 days to maintain them in asynchronous and exponential growth phase during 12 days for stabilization of the cell line.
In order to work similar to cytological slides obtained from patients (as previously described), the T-24 and HUC-1 cells were trypsinized, counted in an hemacytometer, washed in Mac Coy 5A’s medium or Ham’s F12 K medium, respectively, and re-suspended in phosphate-buffered saline at the appropriate concentration. The cells were then fixed with the same volume of formol 4% as patients’ samples and cytospined for 10 min at 71.5 g. About 5.104 cells were deposited on each slide and fixed with Labofix spray (Labonord, Templemars, France). Three types of slides were studied, unstained, stained according to the classical Papanicolaou technique and with a modified Papanicolaou method without using EA 50.
Steady-state absorption and fluorescence measurements. The steady-state absorption spectra of the different Papanicolaou stains were performed on a Cary 300 (Varian, Les Ulis, France) spectrophotometer using a 1-nm illumination spectral bandwidth; the corresponding fluorescence emission spectra were measured on a Perkin-Elmer MPF-3 L spectrofluorimeter using a 3-nm emission and excitation bandwidth and a quartz cell with a 1-cm optical path length. The absorbance of the samples at the excitation wavelength was maintained less than 0.1 making inner filter effects negligible. Rayleigh and Raman scattering from water were subtracted from the fluorescence of the sample.
Wavelength-resolved confocal laser scanning microscopy. Confocal fluorescence images were acquired with a commercial device (Leica TCS SP2 or SP5 AOBS, Mannheim, Germany). A Leica objective (63×/1.4–0.60 NA, oil immersion) was used for acquiring images in direct slow scanning mode. It was checked that the immersion oil (Cargille, Cedar Groove) did not fluoresce over all the spectral range 400–800 nm irrespective of the fluorescence excitation wavelength used. Fluorescence images of urothelial cells were obtained under excitation either with the 364-nm line and 488-nm line of argon lasers (average laser power ∼3 μW) or with the 633-nm line of a helium-neon laser (average laser power ∼90 μW). For spectral measurements, the epifluorescence was imaged on a photomultiplier with a 10-nm spectral slit which was moved by 2-nm steps.
Two-photon excitation laser scanning fluorescence imaging. The confocal setup previously described does not allow time-resolved fluorescence measurements. For this purpose, a home-made two-photon excitation laser scanning microscopy setup was used.
The two-photon microscope has been previously described (24,25). Briefly, a Zeiss axiovert-135 inverted microscope (Carl Zeiss, Jena, Germany) equipped with a Zeiss 63×/1.4 NA oil immersion objective was used. The immersion oil is identical to the one used in confocal microscopy. Samples under the microscope objective were excited by means of a Ti:sapphire laser (Verdi-MIRA combination; Coherent) providing 76-MHz mode-locked pulses with wavelength selections ranging from 700 to 900 nm with an incident power of less than 1 mW.μm−² at the sample level. An excitation wavelength of 800 nm was fixed for the 2P experiments described here taking advantage of the Ti-sapphire laser properties in terms of stability, energy and beam quality. Furthermore all the endofluorophores and Papanicolaou stains can be satisfactorily excited at this wavelength, while it was not the most efficient for some chromophores such as NADP(H) (26).
Epifluorescence was separated from the back-scattered excitation light and directed toward the external fast photomultiplier (Hamamatsu R3809U-52) by a dichroic mirror. Possible diffused infrared excitation light was filtered through appropriate short-pass filters. Interferential 40-nm band pass filters (Coherent) were placed in front of the microchannel plate-photomultiplier tube in order to select various fluorescence emission spectral bands, centered at 450, 550, 650 and 700 nm, respectively. The fluorescence signal detected by the photomultiplier was amplified with an amplifier-pulse discriminator (Amplifier and Timing Discriminator 9327; EG&G Ortec) and then directed to a photon counting card (SCB 68; National Instrument). To obtain 2D-fluorescence intensity images, the laser beam was scanned by 1.μm steps on the cell surface with galvanometric mirrors, which gave an acquisition mean-time of about 30 s for a 50 × 50 μm² image.
Two-photon excitation fluorescence lifetime imaging. To perform fluorescence lifetime imaging measurements (FLIM), the laser beam was scanned by 1-μm steps and generally remained 1 s per point (defined as the ∼0.8-μm² excitation surface) on delimited regions of interest in order to achieve sufficient photon statistics for fitting. Laser power was adjusted to give average photon counting rates of the order 104–105 photon/s (10−4–10−3 photons/excitation event). The collected fluorescence signal was amplified in a similar way to fluorescence-intensity measurements and directed to a picosecond time analyzer (PTA 9308; EG&G Ortec). The data acquisition is based on the time-correlated single-photon counting method (27), which allows a detailed analysis of the fluorescence kinetics at different points of the image.
To obtain mean fluorescence lifetime in real time, we used a qualitative method called maximum a posteriori lifetime estimator (MLE, maximum likelihood estimator corrected for background signal) (P. Pernot, unpublished): it consisted in a single exponential fitting in the decays, adjusted to 60 ps after the excitation pulse in order to overpass the convolution of the fluorescence signal with the instrumental function. A MLE treatment led to a time resolution of about 100 ps. Furthermore, it allowed the representation of mean fluorescence-lifetime images as a pseudo color map.
Nevertheless, the fluorescence decays were also analyzed by the quantitative classical multiexponential reference model (MEM) with a 20-ps time resolution after signal deconvolution of the instrumental response (28–30): this method was much more precise but generally required longer acquisition times per point. It is important to mention that the relative differences in the mean fluorescence lifetimes calculated with both the analytical methods at the same point of the cell were less than the experimental precision (10%).
Results and discussion
Photophysical properties of the Papanicolaou stains
Absorption spectra of the Papanicolaou stains hematoxylin, OG 6 and EA 50 were acquired in pure water at pH 6.5 (Fig. 1a): Harris hematoxylin and OG 6 presented absorption maxima at 560 and 478 nm, respectively. EA 50 displayed a strong band centered at 518 nm with a shoulder at 485 nm and a minor band (about 25 times less intense) at 633 nm. Using steady-state fluorescence setup, only EA 50 appears fluorescent (Fig. 1a) with a maximum emission centered at 535 nm.
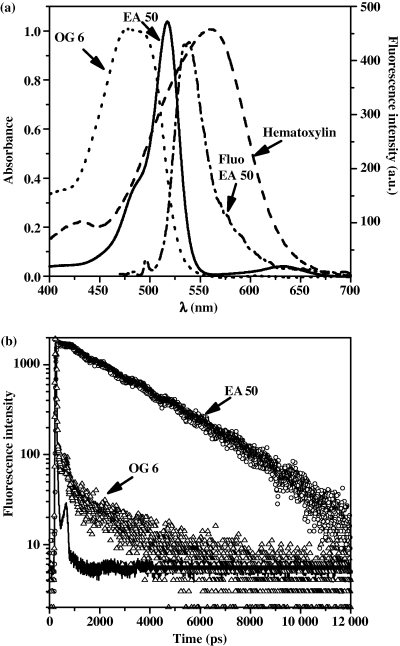
(a) Normalized absorption spectra of Papanicolaou stains, hematoxylin (dashed line), OG 6 (dotted line) and EA 50 (solid line). Fluorescence emission spectrum of EA 50 under 488-nm one-photon excitation (dash-dot-dashed line). (b) Fluorescence decays of EA 50 (circle) and OG 6 (triangle) and instrumental response (solid line) under 800-nm two-photon excitation.
The time-resolved fluorescence properties of EA 50, OG 6 and hematoxylin in solution were also investigated under 2P excitation (Fig. 1b). Hematoxylin emission cannot be distinguished from the instrumental response confirming that this dye does not contribute at all to the fluorescence of the Papanicolaou staining. The fluorescence decay profile of EA 50 shows a monoexponential behavior corresponding to a fluorescence lifetime of 2.8 ± 0.2 ns. A very short decay time at the limit of the instrumental resolution is almost completely dominating the emission of OG6 (Fig. 1b) which is in keeping with the absence of any stationary fluorescence detection. Nevertheless, the deconvolution of the OG 6 residual fluorescence decay trace using the MEM method resulted in a sum of two exponential components with corresponding lifetimes of 0.5 and 2.9 ns, respectively.
When different fluorescence emission spectral ranges were selected with the band-pass filters (see Materials and Methods), EA 50 fluorescence decay can be analyzed in the wavelength range 480–670 nm in agreement with the steady-state fluorescence spectrum of the dye (Fig. 1a) while OG 6 weakly fluoresced in the spectral range 430–650 nm. This fluorescence had to be considered for the measurements in cellular medium (see below).
Fluorescence localization and identification in urothelial cells
Figure 2A,B shows representative global fluorescence images for the normal and tumoral Papanicolaou-stained-urothelial cells performed either under 488-nm 1P excitation or 800-nm 2P excitation. Regardless of the experimental setup used, identical fluorescence images were observed, revealing that there was no effect of the different excitation pathways (i.e. 1P and 2P) on the endofluorescence of the cells, confirming early findings of the literature (26). These intensity images revealed that the tumoral cells can be distinguished from the normal urothelial cells on the basis of their fluorescence localization: in normal cells, fluorescence intensity was found inside the cell with a higher intensity measured in the cytoplasm than in the nucleus (Fig. 2A) whereas in malignant cells, the fluorescence was essentially localized on the perimembrane surface (Fig. 2B). By contrast, the fluorescence localization of unstained T-24 high-grade malignant cell line is intracellular similar to that of the normal stained cells (Fig. 2C). These results reveal that both the Papanicolaou stains and the endofluorophores of the urothelial cells contribute to the fluorescence emission.
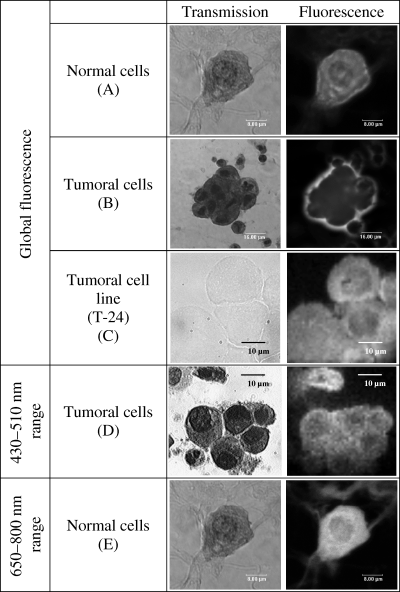
Transmission and fluorescence intensity images of Papanicolaou-stained urothelial cells isolated from healthy donors (A) or from pTaG3 cancer (B) and unstained T-24 tumoral cell line (C).Transmission images of cells were obtained under white light illumination and the corresponding fluorescence-intensity images were performed under 800-nm two-photon excitation. (D) Fluorescence intensity images in the 430–470-nm spectral range obtained under 800-nm two-photon excitation and fluorescence collection through a 40-nm interferential filter centered at 450 nm. (E) Fluorescence intensity images in the 650–800 nm spectral range obtained under 633-nm one-photon excitation.
The key endofluorophores which must be considered include NAD(P)H, FP and porphyrins. To improve the understanding of their individual contributions to the fluorescence images, a detailed analysis of their intensity, spectral and time-resolved fluorescence properties was performed in three distinct spectral bands (400–510, 510–650 and 650–800 nm) corresponding to the emission range of NAD(P)H, FP and porphyrins, respectively. To evaluate the contribution of the Papanicolaou stains in the different fluorescence patterns, three types of cell preparations were studied: unstained, stained according to the classical Papanicolaou technique and stained with a modified Papanicolaou method without using the EA 50 dye. The images have been recorded and analyzed on ∼40 HUC-1 cells, ∼100 T-24 cells, ∼100 normal cells from patients or healthy donors and ∼300 tumoral cells coming from 20 patients. It must be noted that the normal and tumoral pattern of the cells was established by the pathologist by means of white light transmission microscopy.
The NAD(P)H fluorescence images recorded in the spectral range below 510 nm, revealed for each cell type, an intracellular distribution of the fluorescence with a higher intensity in the cytoplasm than in the nucleus as highlighted from Fig. 2D. This was consistent with previous results which have shown that NAD(P)H was distributed throughout the cytosol and that the fluorescence signal originates predominantly from the mitochondria (26).
In this spectral range, the emission of the stains does not contribute to the steady-state fluorescence intensity. This was confirmed by the emission spectra (Fig. 3a) which coincides well with the in vitro spectrum of NAD(P)H (26,31) with a maximum at ∼ 450 nm. No changes were found in the emission spectral shape between the normal and tumoral cells.
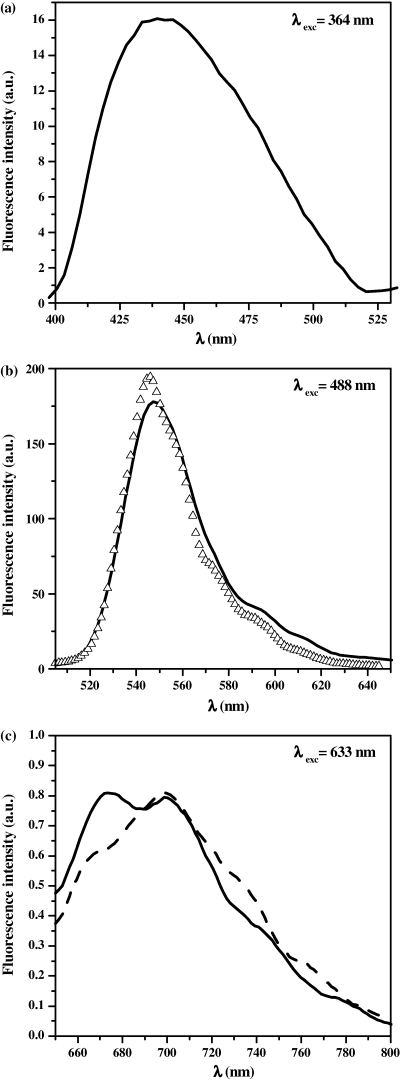
Typical fluorescence emission spectrum (solid lines) of Papanicolaou-stained normal and tumoral cells isolated from patients’ urinary samples obtained under one-photon excitation at (a) 364 nm, (b) 488 nm and (c) 633 nm. The spectrum obtained under 488-nm one-photon excitation was compared with that obtained for unstained HUC-1 (Δ) cell line (the spectra are normalized to the same area). The typical variations observed in spectra from one cell to another are illustrated in part (c) (dashed-line).
The variations in the NAD(P)H fluorescence intensity can be used as a probe to reveal changes in the cellular activity through redox status between normal and tumoral cells (32–34). Indeed, the balance between NAD(P)H (highly fluorescent) and its oxidized counterpart (not fluorescent) changes upon the metabolic activity of the cell (35). To evaluate variations in the NAD(P)H fluorescence between normal and tumoral Papanicolaou urothelial cells, we calculated the mean fluorescence intensity from each cell and then we averaged these values from a total of 31 normal cells and 24 clusters of malignant cells from cytological slides from patients. The ratio r of the average fluorescence intensity of the normal (In) and the tumoral (It) cells was then calculated and equal to r = In/It = 1.2 ± 0.2. It was thus considered that the NAD(P)H fluorescence signal remained at a relatively constant level between normal and tumoral cells which is supported by the similarity of the time-resolved fluorescence properties.
Due to the low fluorescence signal from NAD(P)H (stationary fluorescence intensity is approximately 10 times higher than the background signal), the decays required acquisition times from 30 to 120 s per point. Thus, to avoid major degradations of the cytological slides, we collect image sets for lines of only ∼10 points (∼10 μm) through the cytoplasm and the nucleus. For each cell type studied, the mean fluorescence lifetime is homogeneous over the whole cell (cytoplasm and nucleus) but depends if cells are stained or not. NAD(P)H fluorescence lifetime imaging of unstained cells is characterized by a nanosecond mean fluorescence lifetime for both normal and tumoral cells (Table 1). The more precise analysis of the decays by the MEM method (Fig. 4a) was well represented by two components leading the same mean fluorescence lifetime as obtained with the MLE method (Table 1). These lifetime values are in good agreement with early published data (36,37) on the free and bound NAD(P)H inside the cell. Unfortunately, there is no different contribution of bound and free forms of the chromophore between normal and tumoral urothelial cells.
| λ max (nm)* | MLE | MEM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <τ> (ns) | α 1§ | A 1¶ | τ 1 (ns) ** | α 2 | A 2 | τ 2 (ns) | α 3 | A 3 | τ 3 (ns) | <τ> (ns) | χ 2 | ||
| Unstained cells | |||||||||||||
| Normal | 450 | 2.4 | 0.42 | 0.82 | 3.1 | 0.58 | 0.18 | 0.484 | – | – | – | 2.6 | 1.03 |
| 550 | 2.6 | 0.70 | 0.91 | 3.0 | 0.30 | 0.09 | 0.700 | – | – | – | 2.8 | 1.04 | |
| 650 | 2.1 | 0.04 | 0.74 | 3.5 | 0.07 | 0.17 | 0.444 | 0.89 | 0.09 | 0.016 | 2.7 | 1.11 | |
| Malignant | 450 | 2.4 | 0.44 | 0.85 | 3.3 | 0.56 | 0.15 | 0.457 | – | – | – | 2.8 | 1.11 |
| 550 | 2.5 | 0.79 | 0.95 | 2.7 | 0.21 | 0.05 | 0.480 | – | – | – | 2.6 | 1.06 | |
| 650 | 1.8 | 0.04 | 0.69 | 3.3 | 0.08 | 0.18 | 0.393 | 0.88 | 0.13 | 0.022 | 2.3 | 1.08 | |
| Papanicolaou-stained cells | |||||||||||||
| Normal | 450 | 0.85 | 4 × 10−4 | 0.31 | 2.4 | 0.048 | 0.37 | 0.220 | 0.94 | 0.32 | <10−2 | 0.84 | 4.7 |
| 550 | 2.5 | 0.11 | 0.96 | 2.6 | – | – | – | 0.89 | 0.04 | 0.014 | 2.5 | 1.10 | |
| 650 | 0.33 | 6 × 10−3 | 0.25 | 0.96 | 0.064 | 0.36 | 0.134 | 0.93 | 0.39 | <10−2 | 0.29 | 1.32 | |
| Malignant | 450 | 0.83 | 1 × 10−4 | 0.26 | 2.5 | 1 × 10−4 | 0.27 | 0.370 | 0.99 | 0.47 | <10−2 | 0.75 | 1.05 |
| 550 intra† | 2.5 | 0.12 | 0.90 | 2.7 | – | – | – | 0.88 | 10 | 0.021 | 2.4 | 1.11 | |
| 550 extra‡ | 2.9 | 1 | 1 | 2.9 | – | – | – | – | – | – | 2.9 | 1.03 | |
| 650 | 0.37 | 5 × 10−3 | 0.21 | 1.2 | 0.075 | 0.46 | 0.166 | 0.92 | 0.33 | <10−2 | 0.32 | 1.37 | |
- *Central wavelength of 40-nm band-pass interferential filters localized on the emission beam. †“Intra” for decays performed inside the cell. ‡“Extra” for decays performed in the perimembrane region of the cell. §αi is the population of molecules fluorescing with the lifetime τi. ¶Ai represents the contribution of the molecules with the lifetime τi to the whole fluorescence. **τi is the fluorescence lifetime.
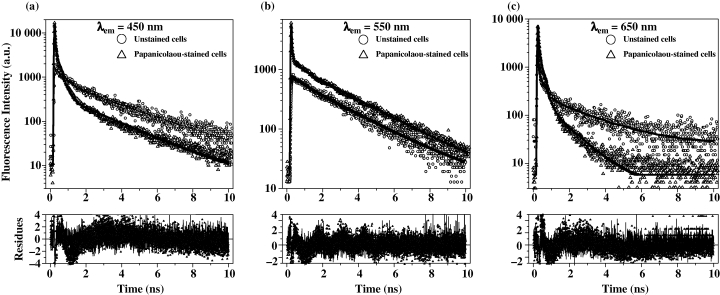
Normalized fluorescence decays of stained or unstained T-24 high-grade malignant cell line obtained under 800-nm two-photon excitation using band-pass filters centered at 450 nm (a), 550 nm (b) and 650 nm (c). Fitting residuals are presented below each layer.
The fluorescence emission of NAD(P)H is influenced by the presence of cell staining. This is characterized by an additional faster decay (<10 ps) visible on Fig. 4a and overall lower values of the amplitude and decay time of the long-lived components (Table 1). This fast component was also measured in the case of cells only stained with OG 6 and hematoxylin (without EA 50), and in the different studied spectral range from 430 to 650 nm (see below) in good agreement with the OG6 emission range. The molecular origin of the OG6 effect on the NAD(P)H fluorescence emission has not been further detailed. The main result is that normal and tumoral cells can not be distinguished by the time-resolved properties of NAD(P)H, both for stained and unstained cells.
Fluorescence properties of the endoporphyrins of urothelial cells have been detailed in the wavelength range 650–800 nm; in this spectral range there is no overlap with the emission of both NAD(P)H and FP. The 2P intensity images of porphyrins excited at 800 nm (Soret band of porphyrins) were found to be identical to their 1P counterparts excited at 633 nm (Q bands of porphyrins). They revealed for each cell type (normal or tumoral cell lines or cells isolated from patients’ samples, stained and unstained cells) an intracellular pattern for the fluorescence intensity with a prevailing cytoplasmic localization as expected (Fig. 2E).
The corresponding steady-state spectra obtained under 633-nm 1P excitation are represented on Fig. 3c: irrespective of the cell type, normal or tumoral, the emission was characterized by two main bands centered at about 675 and 700 nm with a shoulder around 740 nm. It was observed that the ratio between the two emission maxima fluctuated from one cell to another. This was probably due to the coexistence of different porphyrin populations and/or porphyrin conformations with varied concentrations depending on the maturation of the cell as confirmed by the fluorescence lifetime analysis (see below). Nevertheless, the spectrum is characteristic of general spectral profile of porphyrins (38,39) but not specifically to the PPIX (40). No differences were observed between the unstained cell lines (HUC-1 and T-24) and the stained ones confirming the absence of Papanicolaou dye involvement on the stationary fluorescence in this spectral range. Unfortunately, the fluorescence level of porphyrins is too low (∼5 times higher than the background signal), to quantify intensity variations in correlation with cellular activity as previously reported for NAD(P)H.
Time-resolved fluorescence properties of porphyrins of urothelial cells are summarized in Table 1 and illustrated in Fig. 4c together with the typical residual function obtained with MEM analysis of the data. As already reported, a low fluorescence level restricted the area of FLIM data acquisitions, typically less than 10 points for the porphyrins. However, moving from cytoplasm to nucleus, no change was observed in the mean fluorescence lifetime, which depends only on the cell nature, stained or unstained (Table 1). All data obtained for the unstained cells were best fitted with three lifetimes from picosecond to nanosecond time scale. Normal and tumoral cells show similar behavior while they behave differently from stained cells. OG6 stain also contributes to porphyrin fluorescence as previously described for NAD(P)H. Nevertheless, due to the lower signal from porphyrins, the contribution of OG6 emission became more significant and a quasi-disappearance of the longer nanosecond component were observed therefore decreasing the mean lifetime to ∼0.3 ns by comparison with ∼2 ns for the unstained cells. Normal and tumoral stained urothelial cells cannot be any more discerned by their time-resolved fluorescence properties.
Under illumination with the 488-nm Ar laser line of the confocal device, the fluorescence intensity images recorded in the spectral range 510–650 nm of the Papanicolaou-stained malignant and normal urothelial cells isolated from patients’ samples or cell lines superimposed that of the global cell fluorescence represented on Fig. 2A,B. Interestingly, the images obtained for unstained cells or partially stained with OG 6 and hematoxylin do not show any surrounding fluorescence intensity pattern. This result demonstrated that the perimembrane fluorescence localization observed for a malignant urothelial cell was a typical signature of the EA 50 stain. This was confirmed by the spectrally and time-resolved analysis which revealed that the perimembrane emission peaks at ∼550 nm (data not shown) and corresponds to a lifetime of 2.9 ns (Table 1) as measured for the free dye in solution.
The intracellular fluorescence detected in this spectral range was characterized using unstained cells. It is preferentially emitted from the cytoplasm and presented a spectral maximum at about 550 nm (Fig. 3b) in good agreement with the fluorescence properties of FP (41,42). The FLIM data (also obtained on a minimum number of acquisition points due to the low signal) revealed a nanosecond mean fluorescence lifetime over the whole cell, independently of its normal or tumoral character (Table 1). The decay analysis was well represented by two nanosecond and picosecond components in agreement with the literature data (43,44) corresponding to the coexistence of two FP conformations, bound and free forms, within the cell.
In stained normal cells, the intracellular fluorescence was about 10 times more intense than for the unstained ones revealing that the FP fluorescence is practically totally hidden by the EA 50 emission. Thus, the spectral (Fig. 3b) and FLIM (Fig. 5B-a) representations, issued either from cell lines (HUC-1) or from negative controls, correspond predominantly to the homogenous intracellular lifetime distribution of the EA 50 stain. The decay values and the average lifetimes measured in each point of the images are summarized in Table 1. The fast component (<10 ps), also observable on the fluorescence decay (Fig. 4b), corresponds to the contribution of OG6 emission but is too weak by comparison to EA 50 signal to influence the mean fluorescence lifetime.
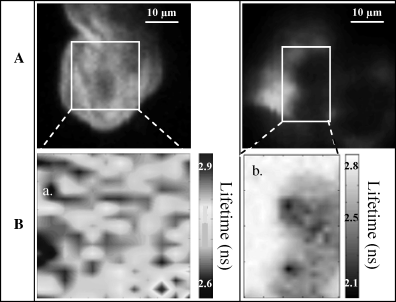
Fluorescence intensity (A) and mean fluorescence-lifetime (B) images of normal and malignant Papanicolaou-stained cells isolated from patients’ urinary samples. Fluorescence intensity images are squares of 50-μm side, performed under 800-nm two-photon excitation: fluorescence was collected in the 530–570 nm spectral range by means of a band-pass filter centered at 550 nm. Mean fluorescence lifetime images were performed on the area materialized by a white rectangle on the fluorescence intensity images. B-a and B-b correspond to FLIM images for normal and tumoral cell, respectively.
In the case of malignant cells (T-24 or isolated from patients), fluorescence lifetime images were heterogeneous (Fig. 5B-b) with a mean fluorescence lifetime τ = 2.9 ± 0.2 ns on the area surrounding the tumoral cells corresponding to EA 50 emission and a shorter intracellular average lifetime τ = 2.0 ± 0.2 ns corresponding to FP and probably also to a low concentration of EA 50 inside the cells.
In summary, no statistically significant differences per cell were observed in the fluorescence intensity, spectral and lifetime properties of each of the endofluorophore NAD(P)H and porphyrin of the normal cells and any of the malignant cell types. These results exclude the possibility to use these chromophores as biomarkers for the cytological detection of urothelial carcinomas, which can appear inconsistent with some data of the literature. Indeed, clinical studies have reported differences in the NAD(P)H fluorescence between urothelial tumoral and normal cell lines (31,35,45–48). Similar findings have been reported concerning accumulation of PPIX induced by ALA injection in urothelial tumor cells (48,49). The absence of stimulation in our experiments should preclude any difference in the porphyrin fluorescence between normal and tumoral urothelial cells due to their too weak concentration and fluorescence emission. However, the sample preparation could be more probably questioned: cytospinning, fixation and staining treatment required for the preparation of cytological slides leads to important modifications in the cell functionality by comparison with cell suspensions or tissues. Endofluorescence will be influenced not only by environment such as intermolecular binding between proteins (that can modify bound-free NAD(P)H ratio in the cell for example) but also by the oxygen supply available in the cells, a probably modified aspect when cells are fixed.
The more interesting feature of the study is the fluorescent specific halo observed for carcinoma cells due to the binding of the EA 50 Papanicolaou stain on the outer face of the plasma membrane which induces a perimembrane accumulation. This seems to suggest that a plasma membrane remodeling occurs from normal to tumoral cells. For pathological cells (50) or for cells under various stimulations as pro-inflammatory, pro-apoptosis, or pro-coagulating signals (51–56), the membrane phospholipid bilayer is restructured: a loss of the membrane asymmetry is observed and correlated to the externalization of phosphatidylserines (57–59). This results in the rush of negative charges at the outer leaflet of the plasma membrane, which offers the possibility of molecular binding sites as already observed for annexin V used to quantify cell apoptosis (55).
To verify such process, two other pathologic cases often encountered by the urologists, urinary lithiasis and urothelial cells infected by BK virus, were studied. In both cases, the perimembrane fluorescence halo pattern can be observed, as well for viral and inflammatory pathologies as for cancerous cells. Thus two different fluorescence patterns of urothelial cells from cytological slides can be defined: the “normal” intracellular fluorescence profile for resting cells which preserve a non-modified phospholipidic bilayer allowing the penetration of the EA 50 stain and the “tumoral-like” fluorescence perimembrane halo, linked to an activated status of the cells. This activation could be an inflammation (lithiasis for example), an infection (BK virus infection) or more generally could be consistent with a mitotic activity or a neoplastic cellular proliferation. These various stimulations lead to membrane remodeling promoting the preferential binding of the EA 50 dye to outer membrane sites as externalized phosphatidylserines.
Validations of the fluorescence aided diagnosis method
The two singular fluorescence patterns between normal and tumoral cells could be discriminating enough to be used as a method to help the pathologists in their early stage diagnosis of urothelial carcinoma.
For this purpose, different validation tests were performed. We first performed serial fluorescence blind tests on 26 Papanicolaou-stained slides and compared our interpretations of the results with the medical diagnosis (based on cytology and biopsy). Among these specimens, 11 were classified as normal and 15 as cancerous by our fluorescence diagnosis method in perfect agreement with the pathologist diagnosis revealing the sensitivity of the fluorescence detection method. Moreover this preliminary study was also specific regarding the fact that no benign cells were found to be positive according to the fluorescence detection criteria and that all malignant cells were detected.
A second approach consisted in the analysis of cells called “atypical” isolated from patients’ urine samples. These kinds of cells are a real problem to diagnose a malignant pathology by classical pathologist observation methods. They could present an increase of the nuclear-to-cytoplasmic ratio, a very heterogeneous chromatin, a variation of the nuclear size or shape, typical characteristics of carcinoma cells, but remain relatively isolated on cytological slides as can be observed on Fig. 6A-c. Other cells can be morphologically close to normal cells but could cluster as their carcinoma counterpart (Fig. 6A-a, b). Using the fluorescence method previously described, we studied 10 atypical slides according to the pathological criteria, which represents a total of about 200 cells. As observed on Fig. 6B, it was possible to classify these atypical cells in the normal cell group (Fig. 6B-a) or their malignant cell counterpart (Fig. 6B-b, c) according to the autofluorescence emission. Moreover on each slide, malignant cells were identified. This result was confirmed by further histological analysis of biopsies which established the low malignant grade pTaG1 of these samples.
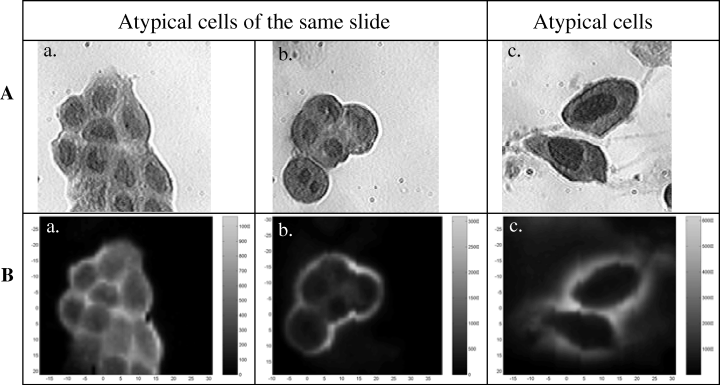
Transmission (A) and fluorescence intensity (B) images of Papanicolaou-stained cells isolated from patient’s urinary samples and called “atypical” by the pathologist. a: normal cluster of cells; b: tumoral cluster of cells; c: malignant cells. Transmission images of cells were obtained under white light illumination and the corresponding fluorescence-intensity images were performed under 800-nm two-photon excitation by collecting the fluorescence over the whole spectral range.
Conclusion
The fluorescence analysis method described in this study is thus able to evidence activated or proliferating cells in a resting or desquamating environment. Indeed an intense perimembrane fluorescence emission was observed around these cells whereas the fluorescence emission was only inside resting cells. We have also proved that fluorescence intensity images of Papanicolaou-stained urothelial cells allow the detection of early neoplastic lesions. This study has grounded the foundation for a novel diagnosis method which can easily be applied without any changes in the Papanicolaou staining protocol. Using a classical epifluorescence microscope, a direct screening of cytological slides by physicians needs only a few minutes to switch from the white light illuminator to the fluorescence mode to observe the characteristic halo of malignant cells. This detection protocol is now in clinical progress on various cell types in an inflammatory or tumoral context.
Acknowledgments
Acknowledgements— Karine Steenkeste thanks the Association pour la Recherche sur le Cancer for its financial support during her post-doctoral position and Ariane Deniset the French Ministry of Research for its research grant during her doctoral position. The Centre Laser de l’Université Paris Sud is acknowledged for its support.




