Effects of Cryopreservation on Morphology and Viability of Sperm and Larvae of Atlantic Cod, Gadus morhua L.
Abstract
The effects of cryopreservation on the viability, morphology and capability of spermatozoa in Atlantic cod, Gadus morhua L., were studied. The sperm was cryopreserved in straws using Hanks' balanced salt solution, hens' egg yolks and glycerol in the vapor of liquid nitrogen. Straws of cryopreserved sperm were stored in liquid nitrogen and thawed in seawater (35 C) for 8 sec before use. The motility of cryopreserved sperm was low (range 8–19%) compared to motility before freezing (range 69–76%). The fertilization rate (range 94–95%) in control groups using fresh sperm was significantly higher (P < 0.05) than in test groups (range 48–72%). In cryopreserved sperm, a relatively high percentage (range 82–93%) of the spermatozoa had changes in morphology. Many spermatozoa had no mitochondria; when mitochondria were present, the observed number varied from one and five in cryopreserved spermatozoa, and from two and seven in noncryopreserved spermatozoa. In groups where cryopreserved sperm was used, the hatching rate was lower (range 18–38%) than in control groups (range 41–63%), indicating higher mortality during embryonic development. Paternal effects on progeny performance were noted in the proportion of abnormalities but no negative effects were identified in newly hatched larvae produced using cryopreserved sperm.
In aquaculture, the cryopreservation of fish sperm could simplify broodstock maintenance by reducing the number of males required and restricting spawning season manipulation to only females (Rana 1995; Suquet et al. 2000). Further, because the gametes would be easier to transport, the genetic variability in stocks would be ensured (Chao and Liao 2001). The ease of transportation would also facilitate maintenance of the genetic diversity of endangered species by the collection of sperm in gene banks (Labbe et al. 2001). These benefits also define the profitability and use of cryopreservation from an economic perspective (Caffey and Tiersch 2000). Butts et al. (2011b) developed a large scale sperm cryopreservation protocol for Gadus morhua that may prove to be important for the development of this species in aquaculture in the future.
The spermatozoa of G. morhua, as in other teleosts, are composed of three major components: the head, the midpiece, and the flagellum (Morrison 1990). The head is elongated and the flagellum consists of the typical nine paired microtubules with a surrounding plasma membrane (Morrison 1990). The mitochondria are located in the midpiece of the spermatozoa (Gwo 1995; Lu and Wu 2005b). The number of mitochondria in spermatozoa varies among species. However, in many species there is no clear link between the number of mitochondria and the viability of spermatozoa (Lahnsteiner and Patzner 2008).
Cryopreservation may have a significant impact on the quality of spermatozoa. In the black grouper, Mycteroperca bonaci, the fine structure of the head region was intact in only 10% of cryopreserved spermatozoa, whereas in puffer fish, Fugu niphobles, 20% of the spermatozoa showed changes in the ultrastructure of the spermatozoa (Gwo et al. 1993). In the spermatozoa of turbot, Scophthalmus maximus, 7% had changes in plasma membranes and 20% in the mitochondria, whereas in rainbow trout, Oncorhynchus mykiss (Walbaum), the plasma membrane was not intact in 55% of the spematozoa (Ogier de Baulny et al. 1999). In G. morhua, cryopreservation did not influence sperm head size; however, it had a negative effect on tail morphology (Butts et al. 2010a). Because of the complexity of the biological systems, the exact mechanism of cell damage caused by cryopreservation has not been fully elucidated (Karlsson and Toner 1996). Glycerol is often used as a cryoprotectant in the cryopreservation of marine fish spermatozoa (Suquet et al. 2000) and the addition of hens' egg yolks as a cryoprotectant has shown positive effects in a number of species (Caylor et al. 1994; Lahnsteiner et al. 2002; Liu et al. 2006).
A key issue in aquaculture is the quality of the spermatozoa as this has a great influence on the rates of fertilization (Gage et al. 2004; Casselman et al. 2006; Butts et al. 2011a) and the further development of the embryo and larvae (Ottesen and Babiak 2007). The quality of sperm is often defined according to spermatozoan motility, morphology, enzymatic activity and ATP concentration or seminal plasma osmolality, pH, and chemical composition (Kime et al. 2001; Rurangwa et al. 2004). In G. Morhua, many of the characteristics of fresh sperm can be used to determine the extent of the effect of cryopreservation on sperm quality (Butts et al. 2010b).
There are seasonal variations in sperm characteristics of G. morhua (Butts et al. 2010b) with quality decreasing at the end of the spawning season (Rideout 2004). In photo-manipulated broodstocks of cod, it has been noted that the males often stop producing sperm before egg production ceases in females (Rosenlund and Halldórsson 2007). In such cases, cryopreservation offers the possibility of storing sperm from genetically superior males for use during the spawning season, particularly during the later stages.
The effects of cryopreservation must be distinguished from the effects of the specific breeding conditions of the broodstock (Bonnet et al. 2007) on the eggs and larvae (Kamler 2005) or parental influences (Ottesen and Babiak 2007) that may induce specific malformations in the offspring. Deformities in the larvae of the African catfish, Clarias gariepinus, were related to damaged spermatozoa caused by cryopreservation (Horvath and Urbanyi 2000), whereas no effects were seen in the offspring of rainbow trout (Labbe et al. 2001). Recently, the genetic integrity of cryopreserved spermatozoa (Hsu et al. 2008) and the effects of cryopreservation on the further development of embryos and larvae have received increased attention (Pérez-Cerezales et al. 2010).
The high prevalence of malformations at hatching in the production of G. morhua (Puvanendran et al. 2009) causing reduced quality of larvae, lower growth rates and production losses, is undesirable. The effects of cryopreserved spermatozoa on the viability and ultrastructure of G. morhua are largely unknown. Also unknown are the possible consequences for embryo- and larval development when using cryopreserved sperm for fertilization. The aim of this study was to investigate the effects of cryopreservation on sperm morphology, fertilization, hatching, and larval development.
Material and Methods
Broodstock
Gadus morhua were captured in the coastal area near Bodø (67°14′48′′ N, 14°24′18′′ E) and held in broodstock tanks at the Marine Research Centre of the University of Nordland. Fish size ranged from 3.5 to 10 kg. The diameter of the tanks was 6 m, with flow-through seawater taken from a depth of 50 m and filtered through a 200 µm filter. The temperature during the spawning season ranged between 6.2 and 7.3°C. Spawning and spawning behavior were observed from mid-March to mid-May. Spermiation was observed from February to the end of May. The fish were hand-fed ad libitum with commercial dry pelleted feed (7, 9, and 13 mm Skretting Europa Marin S 18 and 17 mm Skretting Vitalis Repro Cod; Skretting AS, Norway) every second day. Females and males were held together in the tanks and identified by T-shaped plastic tags (Floy-tags) injected into the base of the dorsal fin.
Sampling of Gametes
On May 5, 2006, sperm was stripped from 10 individual males to study the effects of cryopreservation and thawing of sperm on spermatozoan morphology, motility, fertilization, and early development of cod larvae. Eggs were stripped from a single female, weighing 4.5 kg, on May 6, 2006, after observation of courtship behavior, that is, male and female swimming sporadically, belly to belly. The urine bladder and the gut were cleared by abdominal pressure to avoid urine, blood, and faecal contamination. The urogenital area was dried with a paper towel before the sperm was stripped into 5 mL syringes and the eggs into 250 mL glass beakers. Sperm was diluted to a concentration of 1:3 in HBSS.1 Fresh sperm which was to be used the next day was prepared for chilled storage (Babiak et al. 2006a). All samples were labelled and placed in a styrofoam box in a cooling room (6 C) until experimental use.
Cryopreservation of Spermatozoa
Sperm was cryopreserved on the same day that it was stripped, using 0.8 mL sperm and 1.944 mL of HBSS, 0.216 mL hen's egg yolk (EY) and 0.240 mL glycerol (Gly) as the cryoprotectant. Sperm samples were frozen in the vapor of liquid nitrogen (−196 C, LN2) as described by Babiak et al. (2008). A floating freezing rack was placed in an insulated styrofoam box (inner dimensions: 35 × 27 × 10 cm) with a lid, which was filled with LN2 that maintained the straws at approximately 4 cm above the surface regardless of LN2 level. Samples were kept on a trough of crushed ice (0–1 C) during all operations prior to freezing. French-type 0.5 mL straws (CRYO-VET) were marked and filled with diluted samples. The straws were sealed using PVA sealing powder (Minitüb) or a heated pair of nippers (ERSA®, SMD 1500 S, Minitüb, Germany). The straws were placed horizontally on the freezing rack, held in the LN2 vapor for approximately five minutes and thereafter were placed directly in the LN2. Straws for use in the fertilization trials were transferred, one by one, from the cryotank to a styrofoam box (1 L) which had been filled with water of approximately 35 C. The straws were thawed for eight seconds. The contents were discharged into test tubes filled with 1 mL of HBSS and kept on crushed ice (0–1 C) until use. Dilution lowers toxicity of the cryoprotectant and thawed sperm does not need to be used immediately after thawing (I. Babiak, unpublished).
Motility of Fresh and Cryopreserved Sperm
The motility parameters of spermatozoa from fresh samples taken from six males and cryopreserved samples taken from four males were assessed on May 6, 2006. Spermatozoa, both fresh and after cryopreservation, were activated and video-recorded using a CCD black-and-white video camera (XC-ST50CE PAL, Sony, Tokyo, Japan) connected to a negative phase-contrast microscope (Olympus CH30, Olympus, Tokyo, Japan) with 10× magnification. A 0.12 µL aliquot of sperm was placed on a cooled (6–8 C) standard counting chamber (Art.no SC 20-01-C, Leja products BV, Nieuw-Vennep, The Netherlands) and activated with 4.5 µL filtered seawater (5 µm, 34 ppt). Counts were excluded from analyses where the number of motile spermatozoa was lower than three. Three to five straws were measured for each sample from males 1, 2, 3, and 4. Between two and six measurements were taken to assess their repeatability and reliability. Computer-assisted sperm analysis (CASA) (HTM-CEROS sperm tracker, CEROS Version 12, Hamilton Thorne Research, Beverly, MA, USA) was used to measure and analyze motility as described by Babiak et al. (2006c). The percentage of motile spermatozoa was quantified for each sample 30 sec post-activation. The settings for the image analyzer were: frame rate, 50 Hz; number of frames, 25; minimum contrast, 7; and minimum cell size, 11 pixels.
Morphology of Spermatozoa – Electron Microscopy
To study the effects of cryopreservation on spermatozoa morphology, samples of fresh and cryopreserved sperm from four males (males 5, 6, 7, and 8) were fixed in 2.5% glutaraldehyde with 2.5% paraformaldehyde in a 0.05 M cacodylate buffer, adjusted to 330 mOsM/kg (Ottesen 1997). Samples were washed in a phosphate buffer prior to post-fixation in 1% aqueous OsO4 for 2 h at 4 C for transmission electron microscope (TEM). The specimens were then stained in 2% aqueous uranyl acetate for 1.5 h at 20 C, dehydrated in a series of graded ethanol and subsequently embedded in Epon/Araldite. Semi- and ultra-thin sections were cut on an ultramicrotome (RMC MT-7, RMC, Tucson, AZ, USA). Ultra-thin sections were examined and photographed with a Jeol JEM1010 TEM operated at 80 kV. Samples were washed in a 0.1 M cacodylate buffer prior to post-fixation in 1% aqueous OsO4 for 1.5 h at 20 C followed by dehydration in a series of graded ethanol for scanning electron microscope (SEM). Samples were dried in a CO2 critical point dryer (Baltzer Union CPD 020), mounted on aluminium stubs and coated with gold in a Polaron SEM Coating Unit E5000. Specimens were examined and photographed using a Jeol JSM-6300 SEM operated at 5 kV. Further, 109 TEM and SEM micrographs of fresh and cryopreserved spermatozoa were subjectively assessed using the software Cell P (Olympus, Hamburg, Germany). Spermatozoan morphology was classified as either normal or abnormal. An abnormal classification was given when one or more of three abnormalities were observed in SEM images (deformed head, no flagellum or no mitochondria), when the membrane of the head was partly or totally disintegrated or a spongiotic/disintegrated nucleus was observed in the TEM images. The orientation of spermatozoa on the SEM micrographs varied and in the preparation for TEM studies, not all mitochondria were sectioned at the same level. Thus, it was not always possible to determine the exact number of mitochondria in each spermatozoan.
Fertilization and Larval Viability
Portions of approximately 200 eggs were placed in beakers (30 mL). Eggs were fertilized with sperm from four individual males (males 1, 2, 3, and 4) which had been cryopreserved, as described above, and two controls (males C1 and C2) with fresh sperm. Each cross was replicated five times. Prior to fertilization, the spermatozoa concentration from each of the males was estimated by direct counting of 10 × 2 squares for individual samples in a Bürker counting chamber (Marienfield, Paul Marienfield GmbH and Co. KG, Lauda-Königshofen, Germany) using an invert light microscope (400×) (Babiak et al. 2006b). Motility was initiated by adding seawater (salinity 34‰, temperature 7 C) to the sperm sample. Sperm (6 × 105 spermatozoa per egg) and filtered autoclaved seawater (2.5 mL) were added simultaneously to beakers with eggs and swirled for three seconds. Between five and fifteen minutes post-insemination, eggs were washed in a circular stainless steel tea-strainer using 60 mL of autoclaved seawater to flush out the redundant sperm and ovarian fluid. Washed eggs were transferred to Petri dishes with 40 mL seawater that was filtered, autoclaved, and supplemented with antibiotics (Terramycin®vet, 100 mg/mL, Pfizer, New York, NY, USA) to a final concentration of 0.25 mL Terramycin/L seawater. The remaining eggs were washed out of the sieve with water from the Petri dish using a pipette. Dishes were held in a styrofoam box which was stored in a dark cooling room. Fertilization rates was determined approximately 14–18 hours (16–32 cell stages) after fertilization. During the incubation period, dead eggs were removed every second day and counted. Every fourth day, or when contamination was detected, seawater was exchanged with newly filtered and autoclaved water containing antibiotics. Once hatching started, dishes were tended every second day; dead larvae and eggshells were removed and the water replenished. All experimentation was performed in a temperature-controlled room at 5–6 C.
At between five and eight days post-hatch, 50 larvae were randomly sampled. This was to assess the effects of cryopreservation on the sperm from each of the test males on variability and performance of the progeny. Larvae were anaesthetized for three minutes in 0.2–0.3 mL of sedative (tricaine methanesulfonate, MS-222, ARGENT, Redmond, WA, USA) which had been added to each Petri dish and then preserved in a modified Karnowsky's fixative in the proportion of 18:2, Modified Karnowsky's fixative: 25% glutaraldehyde (Sigma-Aldrich, St. Louis, MO, USA) by volume. All the larvae were photographed and 30 images from each group were randomly selected for the analysis of standard length (SL), myotome height (MH) and deformities. Larvae were placed on a microscope slide in a drop of distilled water. Images of larvae in a horizontal, left-facing position were taken with a camera (U-TVO.5XC-3, Colour View IIIu, Soft Imaging System, Olympus, Tokyo, Japan) connected to a stereoscopic microscope (SZX 12, Olympus, Japan) at 32× magnification. Images were analyzed using the image software programme Cell A (Olympus, Japan). SL was measured from the posterior end of the notochord to the anterior tip of the nose. It was measured in rectilinear sequences along the notochord and to the tip of the nose for curved larvae. MH was measured vertically, above the urinary bladder, from the ventral to the dorsal edge of the myotome. Larval development was scored as normal (n) or abnormal (a) and malformations were assessed by subjective observation. Larvae were considered to be abnormal when they showed one or more of three types of malformations: (1) malformations of the notochord such as scoliosis, lordosis or lordosis/kyphosis complex; (2) swollen myotome: a considerable increase in the myotome volume in a limited area; (3) jaw deformity: the mandible in a diverging position or shortened.
Statistics
The percentage data were arcsine transformed (Zar 2010). The effects of cryopreservation on larval SL, MH, fertilization, and hatching rates in treatment and control groups were investigated using ANOVA. Tukey's post hoc test was used to identify differences between each of the males in treatment and control groups.
Results
Motility of Spermatozoa
The motility of fresh and cryopreserved spermatozoa was 76 and 13%, respectively, for male 1, 74 and 9% for male 2, 69 and 19% for male 3, and 73 and 8% for male 4. Motility of spermatozoa was 73% in each of the controls, males C1 and C2.
Morphology of Spermatozoa
The numbers of normal and abnormal spermatozoa based on TEM and SEM micrographs in fresh and cryopreserved spermatozoa samples are shown in Table 1.
| Male | TEM of cod spermatozoa | SEM of cod spermatozoa | ||||
|---|---|---|---|---|---|---|
| Cryopreserved | Fresh | Cryopreserved | Fresh | |||
| Total | Abnormal | Normal | Total | Abnormal | Normal | |
| 5 | 16 | 15 | 32 | 80 | 78 | 163 |
| 6 | 8 | 4 | 25 | 47 | 44 | 63 |
| 7 | 46 | 42 | 40 | 59 | 51 | 93 |
| 8 | 20 | 13 | 23 | 61 | 57 | 2 |
| Total | 90 | 74 | 120 | 247 | 230 | 319 |
- 1The total number of spermatozoa investigated, and number of abnormal spermatozoa in cryopreserved sperm are indicated. Spermatozoa were classified as abnormal when one or more of two abnormalities were observed in TEM images; partly or totally disintegrated membrane of the head or spongiotic/disintegrated nucleus, or when one or more of three abnormalities were observed in SEM images: deformed head, no flagellum or no mitochondria. The number of normal spermatozoa in fresh sperm before cryopreservation is shown. Spermatozoa classified as normal in cryopreserved and fresh sperm had none of the abnormalities classified above.
- 2Not assessed
Fresh spermatozoa had normal morphology as observed using TEM (Fig. 1A) and SEM (Fig. 1B) and none of the defined abnormalities were observed. Between two and seven mitochondria were observed in the midpiece region in fresh spermatozoa.
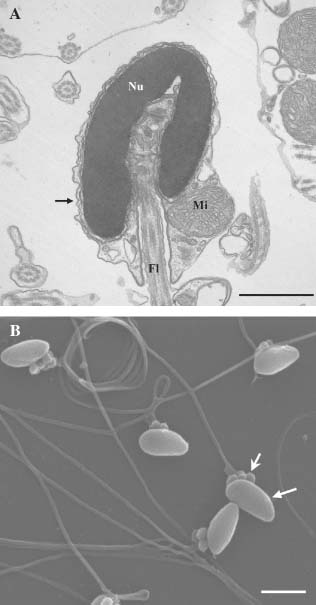
Fresh spermatozoa of Gadus morhua captured under TEM (A) and SEM (B). (A): Nucleus (Nu), mitochondria (Mi), flagellum (Fl), and plasma membrane (arrow) are indicated. Bar 1µm. (B): Spermatozoa head (arrow) and mitochondria are seen (stealth arrow). Bar 2µm.
Cryopreserved spermatozoa were characterized by severe damage to the plasma membrane of the head, deformed heads, disintegrated and spongiotic nuclei (Fig. 2A), cells without flagella (Fig. 2B) and cells without mitochondria in the midpiece (Fig. 2C). The lowest number of mitchondria observed was one and the highest was five.
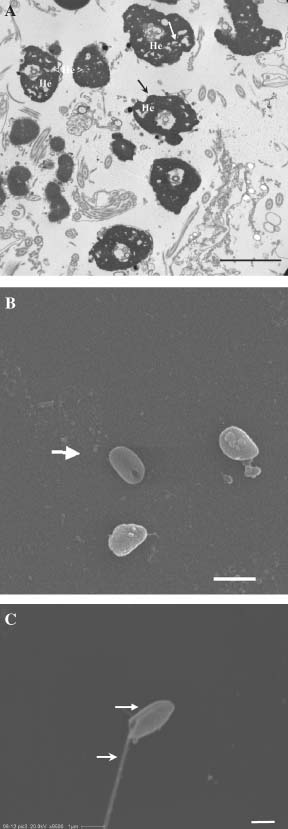
Cryopreserved spermatozoa of Gadus morhua captured under TEM (A) and SEM (B, C). (A): Spermatozoa heads (He). Note the spongiotic nucleus (arrow) and the lack of plasma membrane (stealth arrow) in the heads. Bar 2µm. (B): Spermatozoa (arrow) without flagellum and mitochondria. Bar 2µm. (C): Spermatozoa (arrow) with flagellum (stealth arrow) and without mitochondria. Bar 1µm.
Fertilization and Larval Viability
The fertilization and hatching rates of eggs, and the SL and MH of the progenies of eggs fertilized with cryopreserved sperm from males 1, 2, 3, and 4 as well as that of the fresh sperm from males C1 and C2 are shown in Table 2.
| Males | Fertilization (%) | Hatching (%) | SL (mm) | MH (mm) | Deformed larvae (%) |
|---|---|---|---|---|---|
| C1 | 94 ± 4a | 41 ± 5ab | 5.0 ± 0.1a | 0.27 ± 0.02ab | 33 |
| C2 | 95 ± 2a | 63 ± 14a | 4.9 ± 0.1a | 0.27 ± 0.01ab | 57 |
| 1 | 59 ± 9bc | 32 ± 5bc | 5.0 ± 0.2a | 0.27 ± 0.01ab | 23 |
| 2 | 72 ± 2b | 34 ± 4bc | 4.9 ± 0.1a | 0.27 ± 0.02b | 30 |
| 3 | 48 ± 4c | 18 ± 1c | 5.0 ± 0.2a | 0.28 ± 0.02a | 17 |
| 4 | 72 ± 7b | 38 ± 7bc | 4.9 ± 0.1a | 0.27 ± 0.01ab | 40 |
- 1Standard length (SL) ± standard deviation, myotome height (MH) ± standard deviation and percentage of deformed larvae hatched from eggs fertilized with either fresh (males C1 and C2) or cryopreserved sperm (Males 1, 2, 3, and 4). Significant differences between males are denoted by different letters.
The differences in fertilization and hatching rates in control and treatment groups were significant, F = 38.73, P < 0.05, DF = 12, and F = 12.76, P < 0.05, DF = 12, respectively.
The differences in the SL of the progeny of the six different males (males 1, 2, 3, 4, C1, and C2) were not significant (F = 1.89, P > 0.05, DF = 116), but differences in the MH were significant (F = 2.28, P < 0.05, DF = 174).
The percentage of larvae with visible malformations (Fig. 3A–D) varied between 17 and 40% in the larvae produced with cryopreserved sperm, whereas in the control groups C1 and C2, 33 and 57%, respectively, of the larvae had deformities (Table 2).
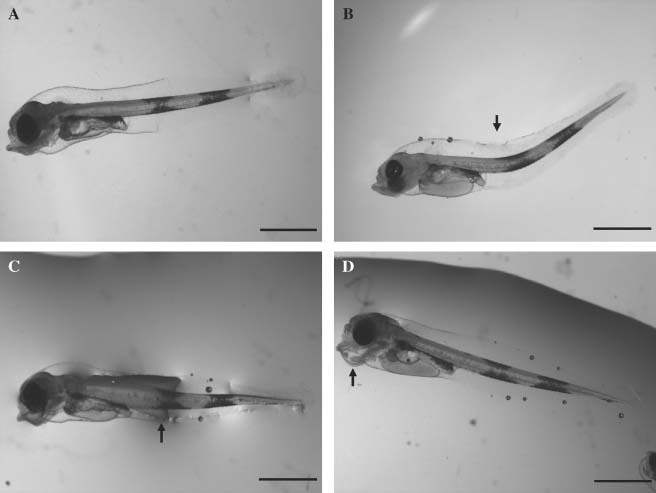
Larvae of Gadus morhua. (A): Normal larva (B): malformed (lordosis) notochord (arrow). (C): swollen notochord (arrow). (D): jaw deformity (arrow). Bar 1 mm.
Discussion
Cryopreservation severely compromised the viability of the spermatozoa as shown by the low percentage of motile spermatozoa (8–19%) and the large numbers of damaged sperm cells. Values for sperm motility reported in other studies are higher than our observations. Mounib (1978) found post-thaw motility of 35% in the spermatozoa of G. morhua; 23% was found in Siberian sturgeon, Acipenser baerii (Billard et al. 2000), and 25% in Macrozoarces americanus L. (Yao et al. 2000). Recently, Butts et al. (2011a) found high fertilization rates, with values ranging from 18.5 to 90.2%, in G. morhua eggs using cryopreserved sperm with relatively high activity. Similarly, in the present study the fertilization rates were relatively high (48–72%) using sperm with low motility. However, the observed effects of cryopreservation on spermatozoa and fertilization ability could be masked by the fact that sperm was used in excess for fertilization. The lowest motility recorded was 8%, which means that, per egg, approximately 4.8 × 104 spermatozoa were motile. Butts et al. (2009) found that sperm to egg ratios ranging from 1 × 103 to 1 × 105 per egg increased fertilization rates from 20 to 78% in G. morhua. Accordingly, the ratio of viable spermatozoa to eggs in the present study may have been sufficient to result in a high fertilization rate when cryopreserved sperm was used. Thus, to increase fertilization when using cryopreserved sperm, an increase in the spermatozoa to egg ratio may offset the effect of a low percentage of motile spermatozoa.
In some species, the spermiating season ends later than egg production, and this is often associated with reduced sperm production, motility, and fertilization (Bobe and Labbe 2010). A decline in motility, storage capacities, and alterations in the plasma membrane in turbot were observed during the spermiation season (Suquet et al. 1998a, 1998b). Senescence of sea bass and turbot spermatozoa resulted in a decrease in freezing ability, and a low cryoresistance of sea bass spermatozoa was recorded at the end of the spawning season (Dreanno et al. 1999). Sperm was stripped late in the spawning season in the present study. However, whether reduced cryoresistene of the sperm may explain the relatively low motility after cryopreservation, remains to be confirmed in future studies.
A total of 82 and 93% of cryopreserved spermatozoa had structural changes, as observed by SEM and TEM, respectively. This is somewhat higher than, but comparable to, findings in Siberian sturgeon (Billard et al. 2000) and rainbow trout (Lahnsteiner et al. 1996) where 60 and 90%, respectively, of the cryopreserved spermatozoa had morphological changes. One to five mitochondria and, in many cases, spermatozoa without mitochondria were observed in the mid-piece of cryopreserved spermatozoa. In European catfish, Silurus glanis (Mattei 1991) and in ocean pout, Macrozoarces americanus L., (Yao et al. 1995), less than 10 mitochondria and more than nine mitochondria, respectively, were observed in fresh spermatozoa. Blenniidae and Labridae have up to six mitochondria (Lahnsteiner and Patzner 2008). Some variability in the number of mitochondria may be attributed to normal variation in G. morhua, as is the case in many other species (Lahnsteiner and Patzner 2008). A reduction in post-thaw motility compared to fresh sperm was related to disturbances in the midpiece of the spermatozoa in ocean pout (Yao et al. 1995, 2000). It was also suspected that reduced spermatozoa motility in turbot (Chauvaud et al. 1995) and carp Cyprinus carpio L. (Perchec et al. 1995) was related to an interruption of energy transfer from the mitochondria to the flagella (Billard et al. 2000). Nevertheless, the deleterious effect of cryopreservation on mitochondria in the spermatozoa of cod may have a significant effect on fertilization; mitochondria provide energy for the spermatozoan's flagella and therefore play a crucial role in determining sperm motility (Lu and Wu 2005a). Disruption of the plasma membrane may expose the DNA to environmental influences, including the possible risk of contamination by microorganisms (Bielanski et al. 2000). A loss of flagella and severe effects on the nuclei were observed. However, the affected spermatozoa, depending on the severity of the damage, may still have the capacity to fertilize eggs. This is indicated by the relatively high fertilization rate observed in the present study and agrees with the findings of Gatti et al. (1990) and Tsvetkova et al. (1996). The comparatively low hatching rate of eggs fertilized with cryopreserved sperm indicates that a high proportion of the embryos in the fertilized eggs died during development. However, many factors influence embryo development and hatching rates. Whether the relatively low hatching rate observed in the present study was caused only by the effects of cryopreservation on spermatozoa viability and structures should be elucidated in more detail in future studies.
In the present study there were no differences in the standard length, and only small differences in the myotome height of larvae produced using cryopreserved sperm compared with fresh sperm. These results agree with a study by Tiersch et al. (1994), who found no differences in growth between progeny produced with cryopreserved and fresh sperm of channel catfish, Ictalurus punctatus. However, relatively large numbers of deformed larvae were observed in all experimental groups, including the control group, suggesting the influence of other unknown factors. These observations are supported by those of Fitzsimmons and Perutz (2006) who noted that 61% of G. morhua had vertebral malformations. No evidence was found in the present study to indicate that the morphology of larvae produced with frozen–thawed spermatozoa is different from those produced with fresh spermatozoa. This concurs with Labbe et al. (2001) who found that DNA was quite stable in the cryopreserved spermatozoa of rainbow trout. Further, the survival of 10-day-old turbot larvae were not significantly different between groups produced using fresh or frozen–thawed sperm (Suquet et al. 1998a, 1998b). The use of cryopreserved sperm had no effect on the survival of, and development in burbot, Lota lota L. (Lahnsteiner et al. 2002), or on the morphology of G. morhua larvae (Mounib 1978), supporting our conclusions that using cryopreserved sperm to fertilize G. morhua eggs had no effect on the progeny.
Cryopreservation had severe effects on spermatozoa as confirmed by the observation of reduced motility and high levels of deformed spermatozoa. However, a severe impact on the fertilization rate when using cryopreserved sperm was avoided by using a sufficiently high ratio of viable spermatozoa per egg. Nonetheless, a comparatively low hatching rate was observed, indicating high mortality rates during embryo development and also that not all fertilized eggs were viable. It is likely that there is a relationship between the inferior quality of cryopreserved spermatozoa and low hatching rates, but the effects on embryonic development need to be confirmed in future studies. There were paternal effects on progeny performance in terms of the proportion of abnormalities but there were no negative effects of cryopreservation on the performance of newly hatched larvae. Possible effects of senescence on viability and morphology of G. morhua spermatozoa remains to be confirmed. The methods for freezing and thawing should be optimized to increase the number of viable spermatozoa after cryopreservation.
Acknowledgments
We thank Jim Treasurer for editing the language, Bjørnar Eggen and Steinar Johnsen, University of Nordland, for technical assistance, Geir Rudolfsen and Randi Olsen, University of Tromsø, for help with the CASA analyses and electron microscopy studies, respectively. This research was partly supported by the project NorthCod, NPP, EU structural funds.




